Ellagitannins and Their Derivatives: A Review on the Metabolization, Absorption, and Some Benefits Related to Intestinal Health
Abstract
1. Introduction
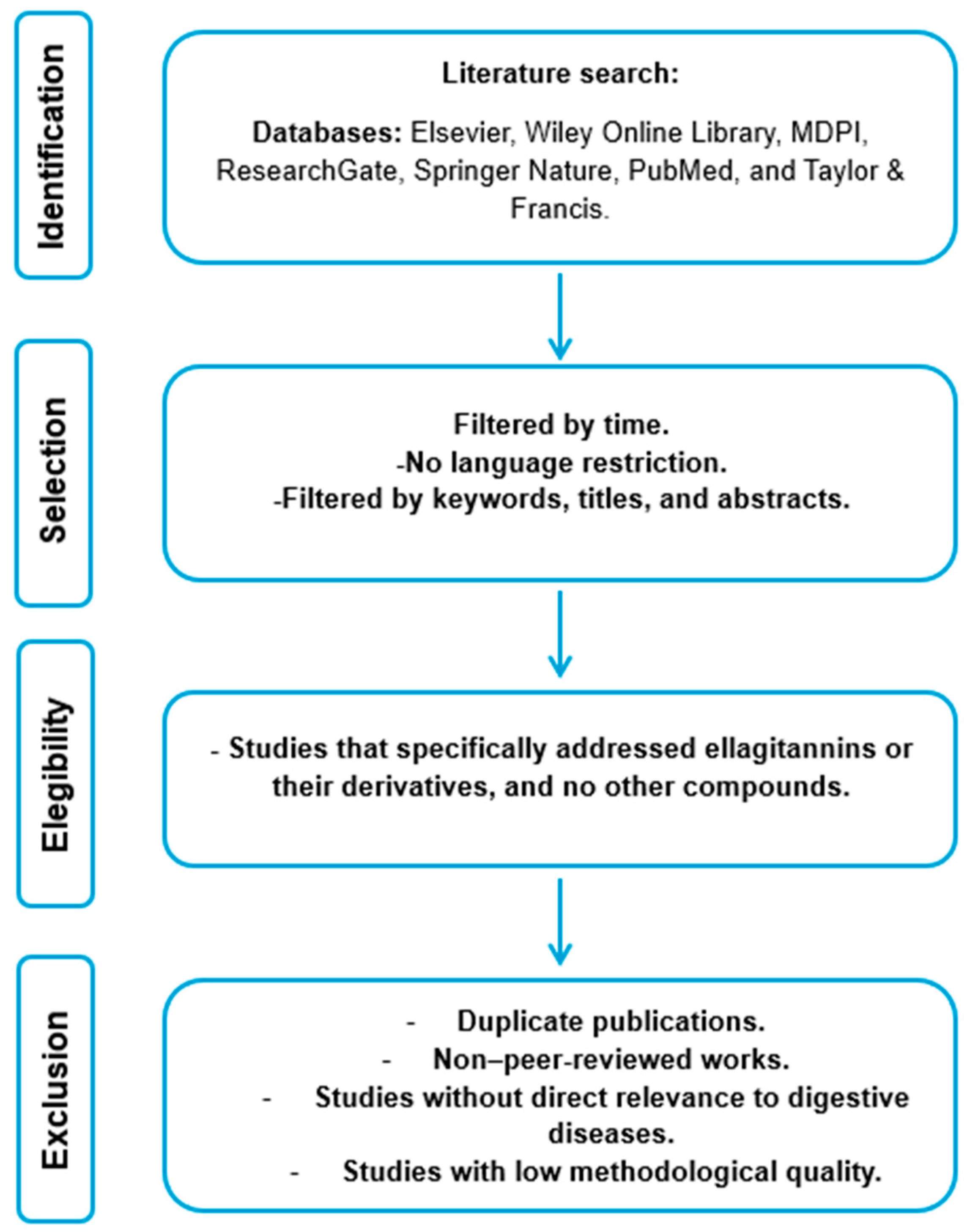
2. Research Methodology
3. Chemical Structure and Classification of Ellagitannins
| Type of Ellagitannin | Name | Chemical Structure | Molecular Formula | Molecular Weight (g/mol) | Reference |
| Monomeric | Punicalagin | 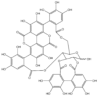 | C48H28O30 | 1084.71 | [21] |
| Corilagin |  | C27H22018 | 634.5 | [22] | |
| Geraniin |  | C41H28O27 | 952.6 | [23] | |
| Casuarictin |  | C41H28O26 | 936.6 | [24] | |
| Dimeric | Sanguin H-6 |  | C82H54O52 | 1871.27 | [25,26] |
| Coriariin A |  | C82H58O52 | 1875.3 | [27] | |
| Cornusiin E |  | C82H58O52 | 1875.3 | [28] | |
| Oenothein B |  | C68H48O44 | 1569.1 | [29] | |
| Oligomeric | Nupharin C |  | C82H58OH52 | 1875.307 | [30] |
| Lambertianin C |  | C123H80O78 | 2805.81 | [31] | |
| Agrimoniin |  | C82H54O52 | 1871.3 | [32] | |
| Hirtellin A |  | C82H58O52 | 1875.3 | [33] | |
| C-glycosidic | Castalagin |  | C41H26O26 | 934.63 | [34,35] |
| Vescalagin |  | C41H26O26 | 934.63 | [36] | |
| Casuarinin |  | C41H28O26 | 936.6 | [37] | |
| Grandinin |  | C46H34O30 | 1066.7 | [38] |
4. Sources of Ellagitannins
5. Biosynthesis of Ellagitannins
6. Degradation of Ellagitannins

6.1. Acid Hydrolysis
6.2. Microbial Biodegradation
6.3. Products of Degradation
6.3.1. Gallic Acid
6.3.2. Ellagic Acid
6.3.3. Urolithins
7. Action of the Gut Microbiota in the Degradation of Ellagitannins
7.1. Bacteria Capable of Degrading Ellagitannins into Ellagic Acid and Urolithins
| Family | Bacterial Strain | Urolithin Produced | Source of Insulation | Reference |
|---|---|---|---|---|
| Streptococcaceae | Streptococcus thermophilus FUA329 | Urolithin A | Human breast milk | [77] |
| Bifidobacteriaceae | Bifidobacterium pseudocatenulatum INIA P815 | Urolithin A and B | Human milk and feces and feces of infants | [78] |
| Streptococcaceae | Lactococcus garvieae FUA009 | Urolithin A | Human feces | [79] |
| Eggerthellaceae | Gordonibacter KGMB12511 T | Urolithin C | Human feces | [80] |
| Enterococcus | Enterococcus faecium FUA027 | Urolithin A | Human feces | [81] |
| Eggerthellaceae | Ellagibacter isourolithinifaciens DSM 104140 T | Urolithins M5, M6, C and isourolithin A | N/E | [82] |
| Eggerthellaceae | Gordinobzacter urolithinfaciens DSM 27213 T | Urolithin M5, M6 and C | N/E | [82] |
| Eggerthellaceae | CEBAS 4A1 | Urolithins M6, C and isourolithin A | Human feces | [83] |
7.2. Urolithin Metabotypes
8. Digestive System Diseases
9. Impact of Ellagitannins, Ellagic Acid, and Urolithins on Intestinal Health
9.1. Effect of Ellagitannins on Irritable Bowel Syndrome
9.2. Ellagitannins and Their Activity Against Gastric Ulcers
9.3. Ellagitannins Against Gastritis
9.4. Ellagitannins Against Colon Cancer
9.5. Ellagitannins Against Esophageal Cancer
9.6. Ellagitannins Against Pancreatic Cancer
9.7. Ellagitannins Against Liver Cancer
10. Food Products with Ellagitannins and Their Derivatives as Bioactive Ingredients
11. Future Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldhaleei, W.A.; Wallace, M.B.; Bi, Y.; Rusk, A.M.; Bhagavathula, A.S. Racial, Ethnic, and Geographic Disparities in Digestive Diseases Mortality in the United States, 2000–2019. Clin. Gastroenterol. Hepatol. 2025, 23, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zheng, X.; Lin, G.; Wang, X.; Lu, H.; Xie, P.; Jia, S.; Shang, Y.; Wang, Y.; Bai, P.; et al. Effects of air pollution on the development and progression of digestive diseases: An umbrella review of systematic reviews and meta-analyses. BMC Public Health 2025, 25, 183. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Chase, R.C.; Li, T.; Ramai, D.; Li, S.; Huang, X.; Antwi, S.O.; Keaveny, A.P.; Pang, M. Global Burden of Digestive Diseases: A Systematic Analysis of the Global Burden of Diseases Study, 1990 to 2019. Gastroenterology 2023, 165, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Barbara, G.; DuPont, H.L.; Mearin, F.; Gasbarrini, A.; Tack, J. New concepts on intestinal microbiota and the role of the non-absorbable antibiotics with special reference to rifaximin in digestive diseases. Dig. Liver Dis. 2018, 50, 741–749. [Google Scholar] [CrossRef]
- Su, Q.; Wang, F.; Chen, D.; Chen, G.; Li, C.; Wei, L. Deep convolutional neural networks with ensemble learning and transfer learning for automated detection of gastrointestinal diseases. Comput. Biol. Med. 2022, 150, 106054. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Et Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Márquez-López, A.; del Carmen Chavéz-Parga, M.; González Hernández, J.C. Aspectos generales sobre los elagitaninos y su conversión a ácido elágico. Cienc. Nicolaita 2019, 77, 36–58. [Google Scholar]
- Ebrahimi, P.; Lante, A. Polyphenols: A Comprehensive Review of their Nutritional Properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Aguilar-Zárate, P.; Wong-Paz, J.E.; Michel, M.; Buenrostro-Figueroa, J.; Díaz, H.R.; Ascacio, J.A.; Contreras-Esquivel, J.C.; Gutiérrez-Sánchez, G.; Aguilar, C.N. Characterisation of Pomegranate-Husk Polyphenols and Semi-Preparative Fractionation of Punicalagin. Phytochem. Anal. 2017, 28, 433–438. [Google Scholar] [CrossRef]
- Izábal-Carvajal, A.L.; Sepúlveda, L.; Chávez-González, M.L.; Torres-León, C.; Aguilar, C.N.; Ascacio-Valdés, J.A. Extraction of Bioactive Compounds via Solid-State Fermentation Using Aspergillus niger GH1 and Saccharomyces cerevisiae from Pomegranate Peel. Waste 2023, 1, 806–814. [Google Scholar] [CrossRef]
- Márquez, D.; Suárez, Á. El uso de taninos condensados como alternativa nutricional y sanitaria en rumiantes. Rev. De Med. Vet. 2008, 16, 87–110. [Google Scholar]
- Vega García, C.C.; García Niño, W.R. Efectos benéficos de los compuestos antioxidantes de la granada (Punica granatum L) en patologías asociadas con el estrés oxidante. Tequio 2022, 5, 67–94. [Google Scholar] [CrossRef]
- Bustamante, A.; García-Díaz, D.; Jiménez, P.; Valenzuela, R.; Pando, M.E.; Echeverría, F. Potential therapeutic effect for liver steatosis of polyphenols obtained from pomegranate peel. Rev. Chil. De Nutr. 2022, 49, 89–99. [Google Scholar] [CrossRef]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Józwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective effects of pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 1663–9812. [Google Scholar] [CrossRef]
- Aguilar-Zárate, P.; Gutiérrez-Sánchez, G.; Michel, M.R.; Bergmann, C.W.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Production of a Fungal Punicalagin-Degrading Enzyme by Solid-State Fermentation: Studies of Purification and Characterization. Foods 2023, 12, 903. [Google Scholar] [CrossRef]
- Mackie, A. The digestive tract: A complex system. Interdiscip. Approaches Food Dig. 2019, 1, 11–27. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, P.K.; Wong, H.C.; Zhao, D. Oral supplementation of Gordonibacter urolithinfaciens promotes ellagic acid metabolism and urolithin bioavailability in mice. Food Chem. 2024, 437, 137953. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut–Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 2005, 66, 2001–2011. [Google Scholar] [CrossRef]
- Klewicka, E.; Sójka, M.; Ścieszka, S.; Klewicki, R.; Milczarek, A.; Lipińska, L.; Kołodziejczyk, K. The antimycotic effect of ellagitannins from raspberry (Rubus idaeus L.) on Alternaria alternata. Eur. Food Res. Technol. 2020, 246, 1341–1349. [Google Scholar] [CrossRef]
- Xu, J.; Cao, K.; Liu, X.; Zhao, L.; Feng, Z.; Liu, J. Punicalagin regulates signaling pathways in inflammation-associated chronic diseases. Antioxidants 2022, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, A.K.; Kumar, R.; Ganguly, R.; Rana, H.K.; Pandey, P.K.; Sethi, G.; Bishayee, A.; Pandey, A.K. Corilagin in cancer: A critical evaluation of anticancer activities and molecular mechanisms. Molecules 2019, 24, 3399. [Google Scholar] [CrossRef] [PubMed]
- Ito, H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Medica 2011, 77, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Ceccoli, J.; Simon, S.R. Anti-melanogenic activity of ellagitannin casuarictin in B16F10 mouse melanoma cells. Nat. Prod. Res. 2021, 35, 1830–1835. [Google Scholar] [CrossRef]
- Davidson, M.; Louvet, F.; Meudec, E.; Landolt, C.; Grenier, K.; Périno, S.; Ouk, T.S.; Saad, N. Optimized Single-Step Recovery of Lipophilic and Hydrophilic Compounds from Raspberry, Strawberry, and Blackberry Pomaces Using a Simultaneous Ultrasound-Enzyme-Assisted Extraction (UEAE). Antioxidants 2023, 12, 1793. [Google Scholar] [CrossRef]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial Activities of Ellagitannins against Clostridium perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef]
- Feldman, K.S.; Lawlor, M.D. Ellagitannin chemistry. The first total synthesis of a dimeric ellagitannin, coriariin A. J. Am. Chem. Soc. 2020, 122, 7396–7397. [Google Scholar] [CrossRef]
- Niemetz, R.; Schilling, G.; Gross, G.G. Biosynthesis of the dimeric ellagitannin, cornusiin E, in Tellima grandiflora. Phytochemistry 2023, 64, 109–114. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic Potential of Polyphenols from Epilobium Angustifolium (Fireweed). Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef]
- Mora, J.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Regulation of Plant Tannin Synthesis in Crop Species. Front. Genet. 2022, 13, 1664–8021. [Google Scholar] [CrossRef]
- Kähkönen, M.; Kylli, P.; Ollilainen, V.; Salminen, J.P.; Heinonen, M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J. Agric. Food Chem. 2012, 60, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Skalicka-Wózniak, K.; Orhan, I.E.; Xiao, J.; Locatelli, M.; Jakub, P.P.; Granica, S.; Tomczyk, M. A comprehensive review of agrimoniin. Phytochem. Med. Food 2017, 1401, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Orabi, M.A.A.; Taniguchi, S.; Terabayashi, S.; Hatano, T. Hydrolyzable tannins of tamaricaceous plants. IV: Micropropagation and ellagitannin production in shoot cultures of Tamarix tetrandra. Phytochemistry 2011, 72, 1978–1989. [Google Scholar] [CrossRef] [PubMed]
- 34. Da Silva, R.D.C.V.; Bolda Mariano, L.N.; Bidinha, E.R.; Bueno De Almeida, C.L.; Cechinel-Filho, V.; Santos Zanuncio, V.S.; Silva, D.B.; Gasparotto Junior, A.; De Souza, P. Ethyl Acetate Fraction from Leandra dasytricha (A. Gray) Cong. Leaves Promotes Vasodilatation and Reduces Blood Pressure in Normotensive and Hypertensive Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 7203934. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.P.; Hoste, H. Ellagitannins Inhibit the Exsheathment of Haemonchus contortus and Trichostrongylus colubriformis Larvae: The Efficiency Increases Together with the Molecular Size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef]
- Araújo, A.R.; Camero, S.; Taboada, P.; Reis, R.L.; Pires, R.A. Vescalagin and castalagin reduce the toxicity of amyloid-beta42 oligomers through the remodelling of its secondary structure. Chem. Commun. 2020, 56, 3187–3190. [Google Scholar] [CrossRef]
- Wakamori, S.; Matsumoto, S.; Kusuki, R.; Ikeuchi, K.; Yamada, H. Total Synthesis of Casuarinin. Org. Lett. 2020, 22, 3392–3396. [Google Scholar] [CrossRef]
- Kaneshima, T.; Myoda, T.; Nakata, M.; Fujimori, T.; Toeda, K.; Nishizawa, M. Antioxidant activity of C-Glycosidic ellagitannins from the seeds and peel of camu-camu (Myrciaria dubia). LWT Food Sci. Technol. 2016, 69, 76–81. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions, and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Mateș, L.; Banc, R.; Zaharie, F.A.; Rusu MEy Popa, D.-S. Mechanistic Insights into the Biological Effects and Antioxidant Activity of Walnut (Juglans regia L.) Ellagitannins: A Systematic. Antioxidants 2024, 13, 974. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicka, E.; Sójka, M. Structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Gadrat, M.; Lavergne, J.; Emo, C.; Teissedre, P.L.; Chira, K. Validation of a mass spectrometry method to identify and quantify ellagitannins in oak wood and cognac during aging in oak barrels. Food Chem. 2021, 342, 128223. [Google Scholar] [CrossRef] [PubMed]
- Rasines-Perea, Z.; Jacquet, R.; Jourdes, M.; Quideau, S.; Teissedre, P.L. Ellagitannins and flavano-ellagitannins: Red wines tendency in different areas, barrel origin and ageing time in barrel and bottle. Biomolecules 2019, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Loredo, E.; Sepúlveda, L.; Wong-Paz, J.E.; Palomo-Ligas, L.; Rodríguez-Herrera, R.; Aguilar, C.N.; Ascacio-Valdés, J.A. Elagitaninos de Eucalyptus camaldulensis y su potencial uso en la industria alimentaria. Explor. Foods Foodomics 2024, 2, 83–100. [Google Scholar] [CrossRef]
- Karlińska, E.; Masny, A.; Cieślak, M.; Macierzyński, J.; Pecio, Ł.; Stochmal, A.; Kosmala, M. Ellagitannins in roots, leaves, and fruits of strawberry (Fragaria × ananassa Duch.) vary with developmental stage and cultivar. Sci. Hortic. 2021, 275, 109665. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Wall-Medrano, A.; González-Aguilar, G.A.; López-Díaz, J.A.; Álvarez-Parrilla, E.; De La Rosa, L.A.; Ramos-Jiménez, A. Taninos hidrolizables; bioquímica, aspectos nutricionales y analíticos y efectos en la salud. Nutr. Hosp. 2015, 31, 55–66. [Google Scholar] [CrossRef]
- Phane Quideau, S. Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; World Scientific: Singapore, 2009. [Google Scholar] [CrossRef]
- Yamada, H.; Wakamori, S.; Hirokane, T.; Ikeuchi, K.; Matsumoto, S. Structural revisions in natural ellagitannins. Molecules 2018, 23, 1901. [Google Scholar] [CrossRef]
- Feng, L.; Yin, Y.; Yang, X.; Tang, H.; Jiao, Q. Dynamic variations in punicalagin and related metabolic substances in pomegranate fruit and leaves during development periods. Hortic. J. 2019, 88, 444–454. [Google Scholar] [CrossRef]
- Aguilar-Zarate, P.; Wong-Paz, J.E.; Buenrostro-Figueroa, J.J.; Ascacio, J.A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Ellagitannins: Bioavailability, Purification and Biotechnological Degradation. Mini Rev. Med. Chem. 2018, 18, 1244–1252. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Gutiérrez-Sánchez, G.; Prado-Barragán, L.A.; Rodríguez-Herrera, R.; Aguilar-Zárate, P.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Tafolla-Arellano, J.C.; Aguilar, C.N. Influence of culture conditions on ellagitannase expression and fungal ellagitannin degradation. Bioresour. Technol. 2021, 337, 125462. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Zárate, P.; Wong-Paz, J.E.; Rodríguez-Duran, L.V.; Buenrostro-Figueroa, J.; Michel, M.; Saucedo-Castañeda, G.; Favela-Torres, E.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.C.; Aguilar, C.N. On-line monitoring of Aspergillus niger GH1 growth in a bioprocess for the production of ellagic acid and ellagitannase by solid-state fermentation. Bioresour. Technol. 2017, 247, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Palchetti, A.; Reniero, F.; Guillou, C.; Masuero, D.; Mattivi, F. Concentration and mean degree of polymerization of Rubus ellagitannins evaluated by optimized acid methanolysis. J. Agric. Food Chem. 2006, 54, 4469–4475. [Google Scholar] [CrossRef]
- De León-Medina, J.C.; Sepúlveda, L.; Buenrostro-Figueroa, J.J.; Mata-Gómez, M.A.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R.; Aguilar, C.N.; Ascacio-Valdés, J.A. Production and evaluation of ellagitannase activity using a pure geraniin substrate. Food Bioprod. Process. 2025, 149, 112–117. [Google Scholar] [CrossRef]
- Sójka, M.; Nowakowska, A.; Hejduk, A. Influencia de la clarificación enzimática, la filtración y la pasteurización en el contenido de elagitaninos y antocianinas en los jugos de frambuesa. Eur. Food Res. Technol. 2024, 250, 351–359. [Google Scholar] [CrossRef]
- De León-Medina, J.C.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Aguilar, C.N.; Ascacio-Valdés, J.A. Fungal biodegradation of ellagitannins extracted from rambutan peel. Food Bioprod. Process. 2023, 141, 81–90. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic acid and diabetes mellitus: Its association with oxidative stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef]
- Guzmán Martínez, O.; Isasmendi Cortés, M.; Biviano Pérez, C.; Dorantes Bautista, G.; Aguirre García, M.; González Pérez, M. Estudio In Silico de las Interacciones del Ácido Elágico y los Aminoácidos de los Tejidos Humanos usando Química Cuántica. Cienc. Lat. Rev. Científica Multidiscip. 2023, 7, 1354–1365. [Google Scholar] [CrossRef]
- Najmi, A.; Nikrad, N.; Ghaffari Sarghein, M.; Hosseinpour dogolsar, M.; Alizadeh, M. Ellagic acid supplementation on oxidative stress, antioxidative capacity and inflammation biomarkers: A systematic review and dose–response meta-analysis of randomized controlled trials. J. Funct. Foods 2024, 122, 106492. [Google Scholar] [CrossRef]
- McClements, D.J.; Öztürk, B. Utilization of Nanotechnology to Improve the Application and Bioavailability of Phytochemicals Derived from Waste Streams. J. Agric. Food Chem. 2022, 70, 6884–6900. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jiang, X. Effect of solubilization with surfactant on the antioxidant activity of ellagic acid. Tenside Surfactants Deterg. 2024, 61, 250–258. [Google Scholar] [CrossRef]
- Abd-Elghany, A.A.; Mohamad, E.A. Chitosan-coated niosomes loaded with ellagic acid present antiaging activity in a skin cell line. ACS Omega 2023, 8, 16620–16629. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Cortés-Martín, A.; Loria-Kohen, V.; Ramírez-de-Molina, A.; García-Mantrana, I.; Collado, M.C.; Espín, J.C.; Selma, M.V. Deciphering the Human Gut Microbiome of Urolithin Metabotypes: Association with Enterotypes and Potential Cardiometabolic Health Implications. Mol. Nutr. Food Res. 2019, 63, 1800958. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- Isla, K.K.Y.; Tanae, M.M.; de Lima-Landman, M.T.R.; de Magalhães, P.M.; Lapa, A.J.; Souccar, C. Vasorelaxant effects of ellagitannins isolated from Cuphea carthagenensis. Planta Medica 2024, 90, 276–285. [Google Scholar] [CrossRef]
- Álvarez, J.; Fernández Real, J.M.; Guarner, F.; Gueimonde, M.; Rodríguez, J.M.; Saenz de Pipaon, M.; Sanz, Y. Gut microbes and health. Gastroenterol. Y Hepatol. 2021, 44, 519–535. [Google Scholar] [CrossRef]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. The gut microbiota and gut disease. Intern. Med. J. 2021, 51, 1594–1604. [Google Scholar] [CrossRef]
- Espín, J.C.; Jarrín-Orozco, M.P.; Osuna-Galisteo, L.; Ávila-Gálvez, M.Á.; Romo-Vaquero, M.; Selma, M.V. Perspective on the Coevolutionary Role of Host and Gut Microbiota in Polyphenol Health Effects: Metabotypes and Precision Health. Mol. Nutr. Food Res. 2024, 68, 2400526. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. [Google Scholar] [CrossRef] [PubMed]
- Caballero, V.; Estévez, M.; Tomás-Barberán, F.A.; Morcuende, D.; Martín, I.; Delgado, J. Biodegradation of Punicalagin into Ellagic Acid by Selected Probiotic Bacteria: A Study of the Underlying Mechanisms by MS-Based Proteomics. J. Agric. Food Chem. 2022, 70, 16273–16285. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kishino, S.; Kudoh, M.; Yamamoto, H.; Ogawa, J. Evaluation of electron-transferring cofactor mediating enzyme systems involved in urolithin dehydroxylation in Gordonibacter urolithinfaciens DSM 27213. J. Biosci. Bioeng. 2020, 129, 552–557. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of urolithin production capacity from ellagic acid of two humans intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Ye, Q.; Hou, X.; Yang, G.; Lu, J.; Hai, Y.; Shen, J.; Fang, Y. Un nuevo Streptococcus thermophilus FUA329 aislado de leche materna humana capaz de producir urolitina A a partir de ácido elágico. Foods 2022, 11, 3280. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Álvarez, I.; Landete, J.M. Bifidobacterium pseudocatenulatum INIA P815: The first bacterium able to produce urolithins A and B from ellagic acid. J. Funct. Foods 2018, 45, 95–99. [Google Scholar] [CrossRef]
- Mi, H.; Liu, S.; Hai, Y.; Yang, G.; Lu, J.; He, F.; Zhao, Y.; Xia, M.; Hou, X.; Fang, Y. Lactococcus garvieae FUA009, una nueva bacteria intestinal capaz de producir el metabolito bioactivo urolitina A a partir del ácido elágico. Foods 2022, 11, 2621. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.S.; Suh, M.K.; Eom, M.K.; Do, H.E.; Lee, J.H.; Park, S.H.; Kang, S.W.; Lee, D.H.; Yoon, H.; et al. Gordonibacter faecis sp. nov., que produce urolitina C a partir de ácido elágico, aislado de heces de sujetos coreanos sanos. Arch. Microbiol. 2024, 206, 108. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Y.; Yang, G.; Hou, X.; Hai, Y.; Xia, M.; He, F.; Zhao, Y.; Liu, S. Isolation and characterization of a novel human intestinal Enterococcus faecium FUA027 capable of producing urolithin A from ellagic acid. Front. Nutr. 2022, 9, 1039697. [Google Scholar] [CrossRef]
- García-Villalba, R.; Beltrán, D.; Frutos, M.D.; Selma, M.V.; JCEspín, J.C.; Tomás-Barberán, F.A. Metabolism of different dietary phenolic compounds by the urolithin-producing human-gut bacteria Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens. Food Funct. 2020, 11, 7012–7022. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; García-Villalba, R.; Mira, A.; Espín, J.C.; Tomás-Barberán, F.A. Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andrés-Lacueva, C.; Alasalvar, C.; Örem, A.; Tomás-Barberán, F.A.; Espín, J.C. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome. Clin. Nutr. 2017, 37, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, D.; Romo-Vaquero, M.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Ellagibacter isourolithinifaciens gen. Nov., sp. Nov., a new member of the family Eggerthellaceae, isolated from human gut. Int. J. Syst. Evol. Microbiol. 2018, 68, 1707–1712. [Google Scholar] [CrossRef]
- Álvarez-Calatayud, G.; Guarner, F.; Requena, T.; Marcos, A. Diet and microbiota. Impact Health. Nutr. Hosp. 2018, 35, 11–15. [Google Scholar] [CrossRef]
- Lu, C.; Li, X.; Gao, Z.; Song, Y.; Shen, Y. Urolithins and intestinal health. Drug Discov. Ther. 2022, 16, 105–111. [Google Scholar] [CrossRef]
- Xia, M.; Hua, Z.; Zhao, Y.; Zhang, G.; Hou, X.; Yang, G.; Liu, S.; Fang, Y. Improvement of Urolithin A Yield by In Vitro Cofermentation of Streptococcus thermophilus FUA329 with Human Gut Microbiota from Different Urolithin Metabotypes. J. Agric. Food Chem. 2024, 72, 3008–3016. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Al-Harbi, S.A.; Abdulrahman, A.O.; Zamzami, M.A.; Khan, M.I. Urolithins: The Gut Based Polyphenol Metabolites of Ellagitannins in Cancer Prevention, a Review. Front. Nutr. 2021, 8, 647582. [Google Scholar] [CrossRef]
- Roa, I.; Meruane, M. Desarrollo del Aparato Digestivo. Int. J. Morphol. 2012, 30, 1285–1294. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Kumari, M.; Gond, D.P. Chapter 19—Basic overview of human physiology. In Smart Healthcare for Disease Diagnosis and Prevention; Academic Press: Cambridge, MA, USA, 2020; pp. 193–212. [Google Scholar] [CrossRef]
- Boland, M. Human digestion—A processing perspective. J. Sci. Food Agric. 2016, 96, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, Y.; Li, S.; Yi, X.; Shao, S.; Yu, W.; Li, E. Biological factors controlling starch digestibility in human digestive system. Food Sci. Hum. Wellness 2023, 12, 351–358. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Singh, H.; Rousseau, D. Destructuring and restructuring of foods during gastric digestion. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1658–1679. [Google Scholar] [CrossRef]
- Montoro-Huguet, M.A.; Belloc, B.; Domínguez-Cajal, M. Small and large intestine (I): Malabsorption of nutrients. Nutrients 2021, 13, 1254. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. In Gastrointestinal Pharmacology; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2017; Volume 239, pp. 1–16. [Google Scholar] [CrossRef]
- Mathews, S.C.; Izmailyan, S.; Brito, F.A.; Yamal, J.M.; Mijail, O.; Revere, F.L. Prevalence and Financial Burden of Digestive Diseases in a Commercially Insured Population. Clin. Gastroenterol. Hepatol. 2022, 20, 1480–1487. [Google Scholar] [CrossRef]
- Rose, T.C.; Pennington, A.; Kypridemos, C.; Chen, T.; Subhani, M.; Hanefeld, J.; Ricciardiello, L.; Barr, B. Analysis of the burden and economic impact of digestive diseases and investigation of research gaps and priorities in the field of digestive health in the European Region-White Book 2: Executive summary. United Eur. Gastroenterol. J. 2022, 10, 657–662. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional, and national burden of 10 digestive diseases in 204 countries and territories from 1990 to 2019. Front. Public Health 2023, 11, 2296–2565. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2018, 156, 254–272. [Google Scholar] [CrossRef]
- Chen, P.; Lei, J.; Chen, F.; Zhou, B. Ameliorative effect of urolithin A on D-gal-induced liver and kidney damage in aging mice via its antioxidative, anti-inflammatory and antiapoptotic properties. RSC Adv. 2020, 10, 8027–8038. [Google Scholar] [CrossRef]
- Katary, M.A.; Salahuddin, A. Gastroprotective Effect of Punicalagin Against Ethanol-Induced Gastric Ulcer: The Possible Underlying Mechanisms. Biomark. Journal. Biomark. J. 2017, 3, 1. [Google Scholar] [CrossRef]
- Franco, A.; Tocci, N.; Guella, G.; Dell’Agli, M.; Sangiovanni, E.; Perenzoni, D.; Vrhovsek, U.; Mattivi, F.; Manca, G. Myrtle Seeds (Myrtus communis L.) as a Rich Source of the Bioactive Ellagitannins Oenothein B and Eugeniflorin D2. Am. Chem. Soc. 2019, 4, 15966–15974. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, Y.J.; Kim, H.J. Determining the effect of ellagic acid on the proliferation and migration of pancreatic cancer cell lines. Transl. Cancer Res. 2021, 10, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, H.; Ni, J.; Liang, Z.; Wu, X.; Xue, J.; Wang, X. Geraniin selectively promotes cytostasis and apoptosis in human colorectal cancer cells by inducing catastrophic chromosomal instability. Mutagenesis 2018, 33, 271–281. [Google Scholar] [CrossRef]
- Peng, C.; Rong, W.; Jiexin, L.; Benhong, Z. Urolithin B protects mice from diet-induced obesity, insulin resistance, and intestinal inflammation by regulating gut microbiota composition. Food Funct. 2024, 15, 7518–7533. [Google Scholar] [CrossRef]
- Sebastián Domingo, J.J. Irritable bowel syndrome. Med. Clínica 2022, 158, 76–81. [Google Scholar] [CrossRef]
- Pontet, Y.; Olano, C. Prevalencia de síndrome de intestino irritable en América Latina. Rev. De Gastroenterol. Del Perú 2021, 41, 144–149. [Google Scholar] [CrossRef]
- Mirzaie, Z.; Bastani, A.; Hesami, S.; Pouryousefi, E.; Kavianpour, M.; Haghighian, H.K. Improving Effect of Ellagic Acid on Sleep Quality and Gastrointestinal Symptoms in Patient with Irritable Bowel Syndrome: Randomized Double-Blind Clinical Trial. Turk. J. Gastroenterol. 2021, 32, 937–944. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Ahluwalia, A. The critical role of growth factors in gastric ulcer healing: The cellular and molecular mechanisms and potential clinical implications. Cells 2021, 10, 1964. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, P.A.; Garduño-Siciliano, L.; Domínguez-Verano, P.; Balderas-Cordero, D.; Gorgua-Jiménez, G.; Canales-Álvarez, O.; Canales-Martínez, M.M.; Rodríguez-Monroy, M.A. Propolis and its Gastroprotective Effects on NSAID-Induced Gastric Ulcer Disease: A Systematic Review. Nutrients 2021, 13, 3169. [Google Scholar] [CrossRef]
- Al Sayed Haidy, E.; Michel, E.; Khattab, M.A.; El-Shazly, M.; Singab, A.N. Protective role of casuarinin from melaleuca leucadendra against ethanol-induced gastric ulcer in rats identification of casuarinin by spectroscopic data. Phytomedicine 2020, 86, 32–44. [Google Scholar] [CrossRef]
- Suwindri, S.; Tiranda, Y.; Cahya Ningrum, W. Faktor Penyebab Kejadian Gastritis di Indonesia: Literature Review. J. Keperawatan Merdeka 2021, 1, 209–223. [Google Scholar] [CrossRef]
- Rugge, M.; Sugano, K.; Sacchi, D.; Sbaraglia, M.; Malfertheiner, P. Gastritis: An Update in 2020. Curr. Treat. Options Gastroenterol. 2020, 18, 488–503. [Google Scholar] [CrossRef]
- Gasaly, N.; Riveros, K.; Gotteland, M. Phytochemicals: A new class of prebiotics. Rev. Chil. De Nutr. 2020, 47, 317–327. [Google Scholar] [CrossRef]
- Fumagalli, M.; Sangiovanni, E.; Vrhovsek, U.; Piazza, S.; Colombo, E.; Gasperotti, M.; Mattivi, F.; De Fabiani, E.; Dell’Agli, M. Strawberry tannins inhibit IL-8 secretion in a cell model of gastric inflammation. Pharmacol. Res. 2016, 111, 703–712. [Google Scholar] [CrossRef]
- Piazza, S.; Martinelli, G.; Fumagalli, M.; Pozzoli, C.; Maranta, N.; Giavarini, F.; Colombo, L.; Nicotra, G.; Vicentini, S.F.; Genova, F.; et al. Ellagitannins from Castanea sativa Mill. Leaf Extracts Impair, H. pylori Viability and Infection-Induced Inflammation in Human Gastric Epithelial Cells. Nutrients 2023, 15, 1504. [Google Scholar] [CrossRef]
- Yang, H.; Hu, B. Immunological Perspective: Helicobacter pylori Infection and Gastritis. Mediat. Inflamm. 2022, 2944156. [Google Scholar] [CrossRef]
- Ismail, T.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016, 8, 151. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Chen, Q.; Song, H.; Wang, Z.; Xing, W.; Jin, S.; Song, X.; Yang, H.; Zhao, W. The Gut Microbiota Metabolite Urolithin B Prevents Colorectal Carcinogenesis by Remodeling Microbiota and PD-L1/HLA-B. Oxidative Med. Cell. Longev. 2023, 6480848. [Google Scholar] [CrossRef]
- Cho, H.; Jung, H.; Lee, H.; Yi, H.C.; Kwak, H.K.; Hwang, K.T. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 2015, 6, 1675–1683. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, F.; Guo, Z.; Zhao, J.; Song, X.; Yang, H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol. Carcinog. 2018, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zambrano Zambrano, F.C.; Vélez Macías, M.A.; Chacha Suscal, N.R.; Basurto Macias, G.G.; Pesantez Durán, F.A. Sintomatología y tratamiento en cada etapa del paciente con cáncer de esófago. RECIAMUC 2020, 4, 263–272. [Google Scholar] [CrossRef]
- Pérez Pereyra, J.; Frisancho Velarde, O. Cáncer de esófago: Características epidemiológicas, clínicas y patológicas en el Hospital Rebagliati—Lima. Rev. Gastroenterol. Perú 2009, 29, 118–123. [Google Scholar]
- Shi, N.; Chen, T. Chemopreventive Properties of Black Raspberries and Strawberries in Esophageal Cancer Review. Antioxidants 2022, 11, 1815. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Wang, W.W.; Huang, J.; Zhu, W.G. Ellagic acid induces esophageal squamous cell carcinoma cell apoptosis by modulating SHP-1/STAT3 signaling. Kaohsiung J. Med. Sci. 2020, 36, 699–704. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Gašić, U.; Ćirić, I.; Pejčić, T.; Radenković, D.; Djordjević, V.; Radulović, S.; Tešić, Ž. Polyphenols as possible agents for pancreatic diseases. Antioxidants 2020, 9, 547. [Google Scholar] [CrossRef]
- Sudha, T.; Mousa, D.S.; El-Far, A.H.; Mousa, S.A. Pomegranate (Punica granatum) Fruit Extract Suppresses Cancer Progression and Tumor Angiogenesis of Pancreatic and Colon Cancer in Chick Chorioallantoic Membrane Model. Nutr. Cancer 2020, 73, 1350–1356. [Google Scholar] [CrossRef]
- Totiger, T.M.; Srinivasan, S.; Jala, V.R.; Lamichhane, P.; Dosch, A.R.; Gaidarski, A.A.; Joshi, C.; Rangappa, S.; Castellanos, J.; Vemula, P.K.; et al. Urolithin A, a novel natural compound to target PI3K/AKT/mTOR pathway in pancreatic cancer. Mol. Cancer Ther. 2019, 18, 301–311. [Google Scholar] [CrossRef]
- Chun-Yu Liu Kuen-Feng Chen, P.-J.C. Treatment of liver cancer. Liver Biol. Pathobiol. 2020, 61, 782–791. [Google Scholar] [CrossRef]
- Zhang Qin et, a.l. “Determining novel candidate anti-hepatocellular carcinoma drugs using interaction networks and molecular docking between drug targets and natural compounds of SiNiSan. PeerJ 2021, 9, e10745. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.H.; Khalifa, F.K. The protective role of ellagitannins flavonoids pretreatment against N-nitrosodiethylamine induced-hepatocellular carcinoma. Saudi J. Biol. Sci. 2014, 21, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Iranzo Pous, G.; Milán Navarro, S. Composiciones Antioxidantes de Un Producto Obtenido Del Fruto de Camu Camu. ES Patent ES 2445492B1, 12 December 2014. [Google Scholar]
- Blanco-Bose, W.; Andreux, P.; Rinsch, C. Composiciones Que Comprenden Compuestos de Urolitina. ES Patent ES 2832506T3, 10 June 2021. [Google Scholar]
- Alkayali, A. Ellagic Acid Food Supplement Prepared from Pomegranate Seed. U.S. Patent US 2006/0280819 A1, 14 December 2006. [Google Scholar]
- López Más, J.A.; Streitenberger, S.A.; Peñalver Mellado, M.; Pedreño López, Y.; Martinez Ortiz, P. Extractos de Granada, Productos Nutricionales Que Los Contienen y Sus Usos. ES Patent ES2343101T3, 15 July 2013. [Google Scholar]
- Cheshomi, H.; Bahrami, A.R.; Rafatpanah, H.; Matin, M.M. The effects of ellagic acid and other pomegranate (Punica granatum L.) derivatives on human gastric cancer AGS cells. Hum. Exp. Toxicol. 2022, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Aires, A.; Pinto, T.; Oliveira, I.; Vilela, A.; Gonçalves, B. Una revisión exhaustiva de taninos bioactivos en alimentos y bebidas: Propiedades funcionales, beneficios para la salud y cualidades sensoriales. Molecules 2025, 30, 800. [Google Scholar] [CrossRef]
- Ribeiro, M.; Alvarenga, L.; Cardozo, L.F.M.F.; Baptista, B.G.; Nascimento, D.; Esgalhado, M.; Mafra, D. Urolithin as a Metabolite of Ellagitannins and Ellagic Acid from Fruits and Nuts Produced by the Gut Microbiota: Its Role on Non-Communicable Diseases. Curr Nutr Rep 2025, 14, 55. [Google Scholar] [CrossRef]
- Hasheminezhad, S.H.; Boozari, M.; Iranshahi, M.; Yazarlu, O.; Sahebkar, A.; Hasanpour, M.; Iranshahy, M. A mechanistic insight into the biological activities of urolithins as gut microbial metabolites of ellagitannins. Phytother. Res. 2022, 36, 112–146. [Google Scholar] [CrossRef]





| Ellagitannin or Derivatives | Health Effects | Concentration | Type of Study | Reference |
|---|---|---|---|---|
| Urolithin A | Improvement in liver and kidney dysfunction. | 150 mg kg −1 | In vivo | [103] |
| Punicalagin | Gastroprotective effects against gastric ulcers. | 4 mg/kg | In vivo | [104] |
| Enothein B | Anti-inflammatory activity in gastric epithelial cells (potential support in anti-gastritis therapy). | 20 μM | In vitro | [105] |
| Ellagic acid | Inhibits the proliferation of pancreatic cancer cells. | 100–1000 µM | In vitro | [106] |
| Geraniin | Significantly reduces the nuclear division index, increases chromosomal instability homeostasis, and promotes apoptosis in colorectal cancer cells (Colo205 and Colo320). | 25, 50 o 100 μg/ml | In vitro | [107] |
| Urolithin B | Reduced intestinal inflammation. | 100 o 200 mg kg −1 | In vivo | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raya-Morquecho, E.M.; Aguilar-Zarate, P.; Sepúlveda, L.; Michel, M.R.; Iliná, A.; Aguilar, C.N.; Ascacio-Valdés, J.A. Ellagitannins and Their Derivatives: A Review on the Metabolization, Absorption, and Some Benefits Related to Intestinal Health. Microbiol. Res. 2025, 16, 113. https://doi.org/10.3390/microbiolres16060113
Raya-Morquecho EM, Aguilar-Zarate P, Sepúlveda L, Michel MR, Iliná A, Aguilar CN, Ascacio-Valdés JA. Ellagitannins and Their Derivatives: A Review on the Metabolization, Absorption, and Some Benefits Related to Intestinal Health. Microbiology Research. 2025; 16(6):113. https://doi.org/10.3390/microbiolres16060113
Chicago/Turabian StyleRaya-Morquecho, Erick M., Pedro Aguilar-Zarate, Leonardo Sepúlveda, Mariela R. Michel, Anna Iliná, Cristóbal N. Aguilar, and Juan A. Ascacio-Valdés. 2025. "Ellagitannins and Their Derivatives: A Review on the Metabolization, Absorption, and Some Benefits Related to Intestinal Health" Microbiology Research 16, no. 6: 113. https://doi.org/10.3390/microbiolres16060113
APA StyleRaya-Morquecho, E. M., Aguilar-Zarate, P., Sepúlveda, L., Michel, M. R., Iliná, A., Aguilar, C. N., & Ascacio-Valdés, J. A. (2025). Ellagitannins and Their Derivatives: A Review on the Metabolization, Absorption, and Some Benefits Related to Intestinal Health. Microbiology Research, 16(6), 113. https://doi.org/10.3390/microbiolres16060113









