Therapeutic Effects of Lycopene Alone or in Combination with Cephalexin on Chronic Prostatitis Caused by Staphylococcus aureus in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. The Chemicals and Reagents Used in This Study
2.2. Animals
2.3. CBP Model
2.4. Collection of Urine
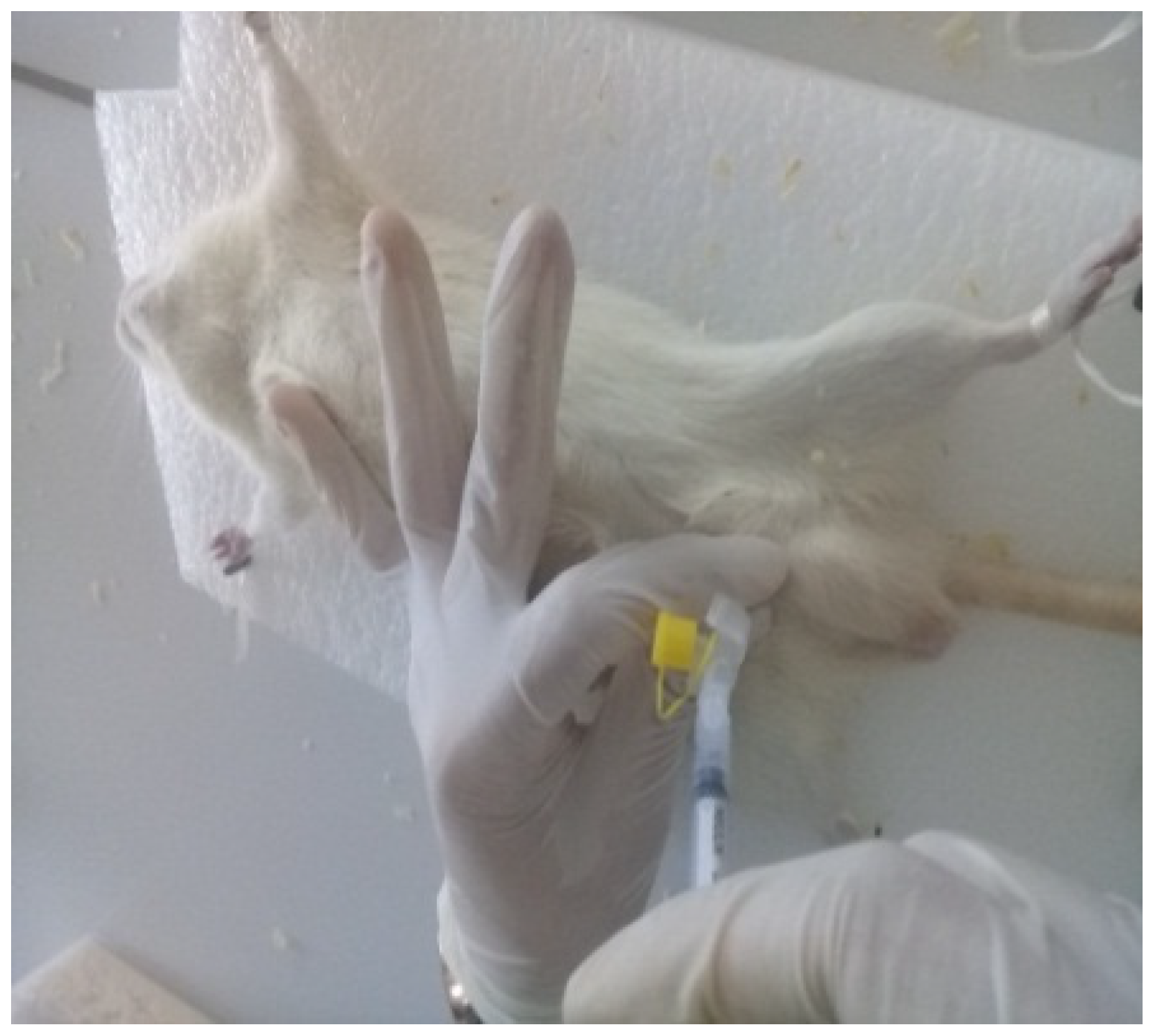
2.5. Sperm Collection
2.6. Animal Distribution
2.7. Animal Sacrifice and Specimen Collection
2.8. Prostate-Specific Antigen
2.9. Histological Study
2.10. Statistical Study
3. Results
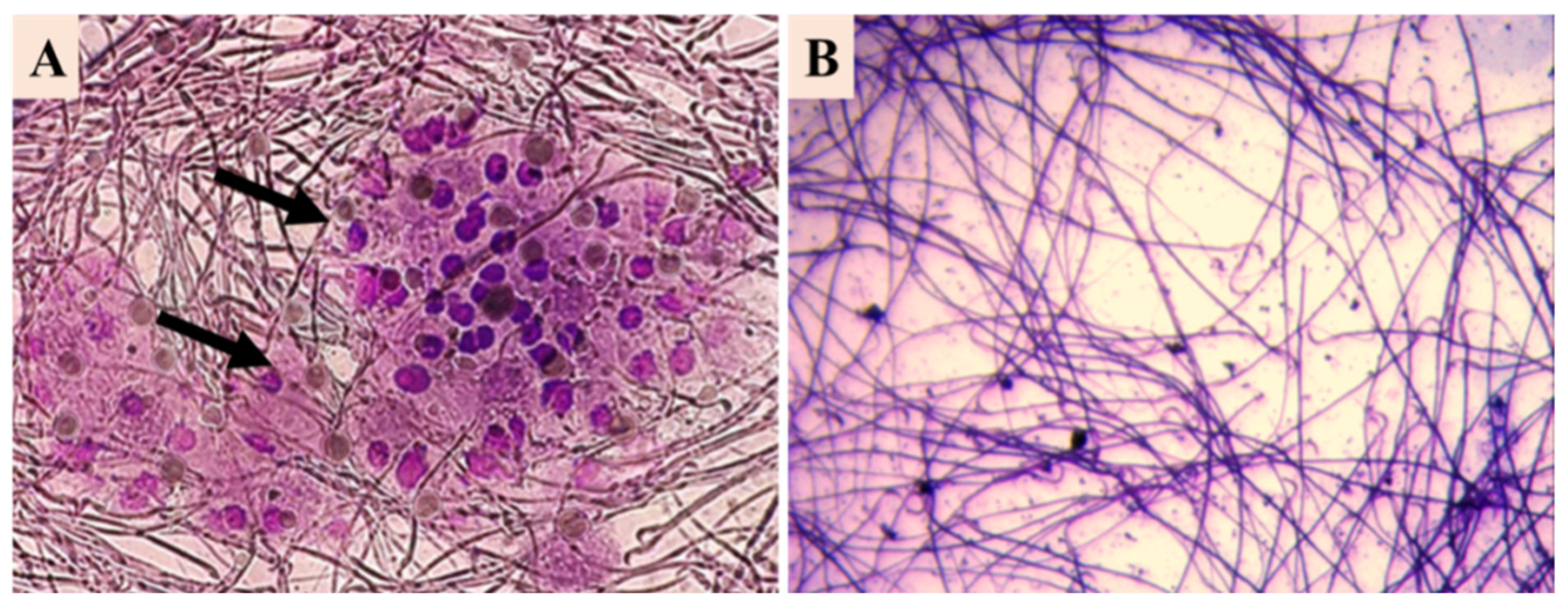
3.1. CBP Detection
3.2. Body Weight Evolution
3.3. Prostate-Specific Antigen Analysis
3.4. Preostate and Urine Cultures
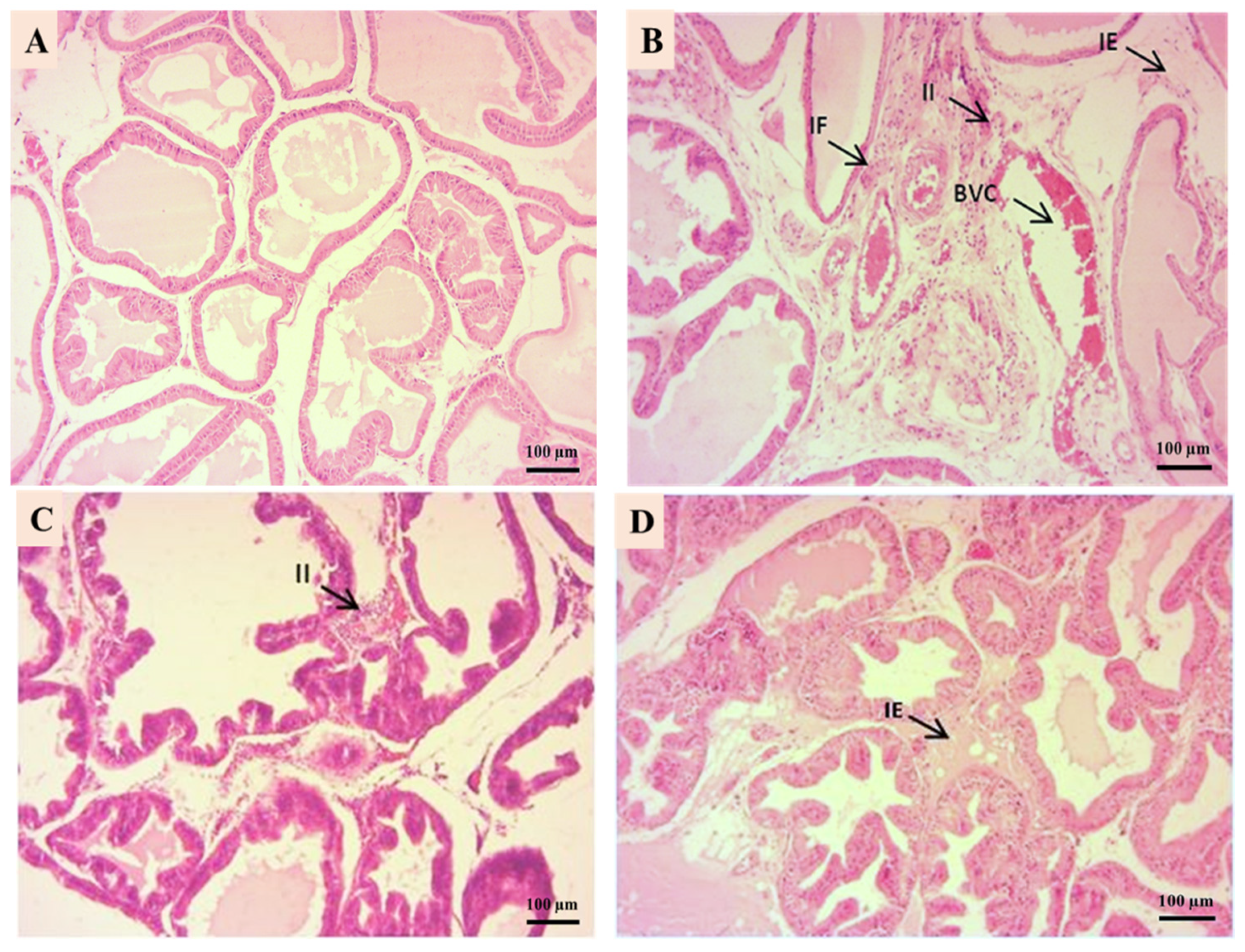
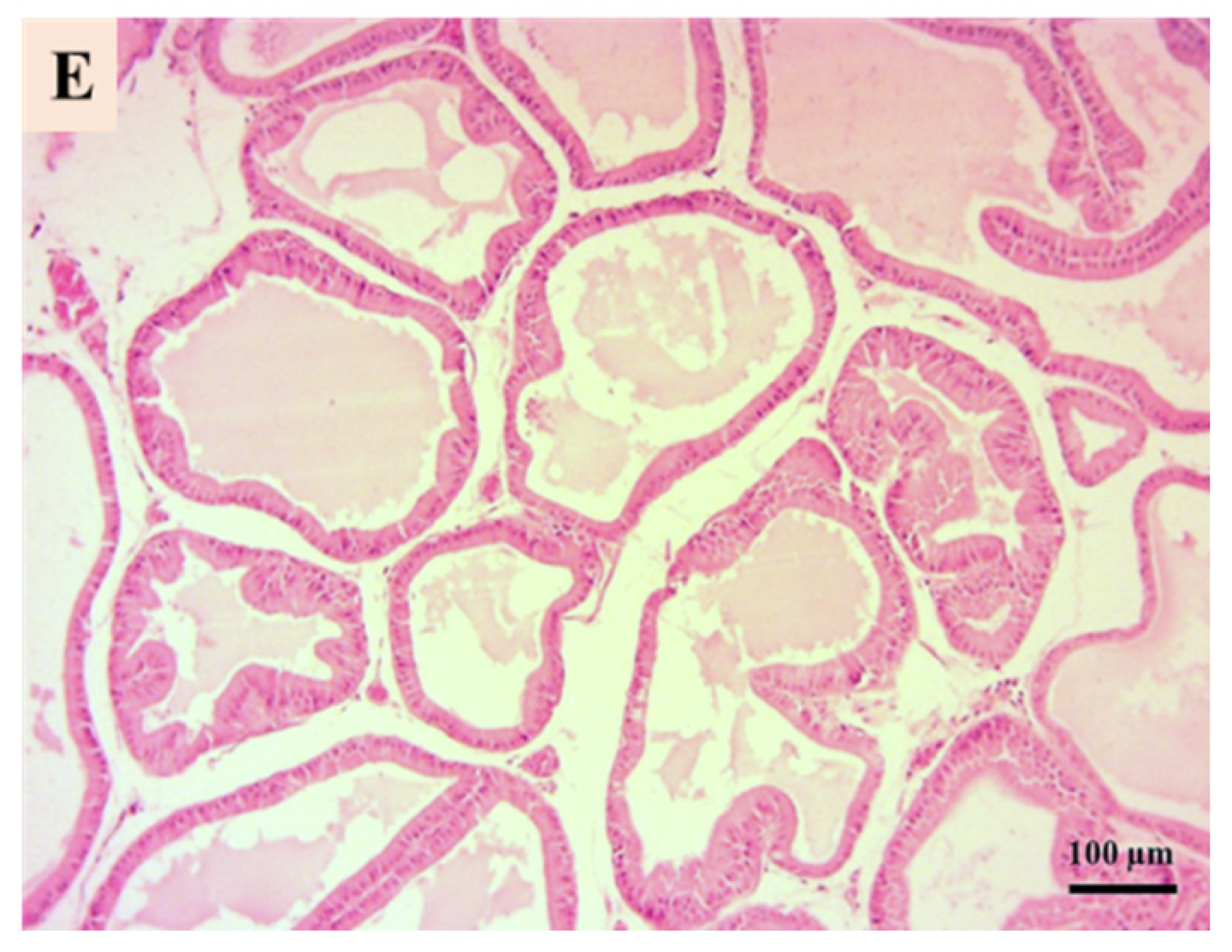
3.5. Prostate Histological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, X.; Luo, Y.; Ma, L.; Hu, X.; Simal-Gandara, J.; Wang, L.S.; Bajpai, V.K.; Xiao, J.; Chen, F. Recent trends and advances in the epidemiology, synergism, and delivery system of lycopene as an anti-cancer agent. Semin. Cancer Biol. 2021, 73, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Yang, C.H.; Sohn, D.W.; Kim, S.W.; Kang, S.H.; Cho, Y.H. Synergistic effect between lycopene and ciprofloxacin on a chronic bacterial prostatitis rat model. Int. J. Antimicrob. Agents 2008, 31, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Hong, S.; Cao, Y.; Liu, C. Comparative analysis of efficacy of different combination therapies of α-receptor blockers and traditional Chinese medicine external therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: Bayesian network meta-analysis. PLoS ONE 2023, 18, e0280821. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.G.; Silberman, M. Acute bacterial prostatitis. In Smith’s General Urology; McGraw-Hill: New York, NY, USA, 2017; pp. 210–215. [Google Scholar]
- Gerges, A.N.; Williams, E.E.; Hillier, S.; Uy, J.; Hamilton, T.; Chamberlain, S.; Hordacre, B. Clinical application of auricular transcutaneous vagus nerve stimulation: An exploratory review. Handicap. Réadapt. 2024, 46, 5730–5760. [Google Scholar]
- Lambertini, L.; Sandulli, A.; Salamone, V.; Bacchiani, M.; Giudici, S.; Massaro, E.; Cadenar, A.; Mariottini, R.; Coco, S.; Bardina, L.; et al. Efficacy and safety of a natural supplement containing Serenoarepens, Solanumlycopersicum, lycopene, and bromelain in reducing symptoms of chronic prostatitis/chronic pelvic pain syndrome: A prospective cohort study in 250 patients. Uro 2023, 3, 199–207. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The potential role of lycopene in the prevention and treatment of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Vrieling, A.; Voskuil, D.W.; Bonfrer, J.M.; Korse, C.M.; van Doorn, J.; Cats, A.; Depla, A.C.; Timmer, R.; Witteman, B.J.; Van Leeuwen, F.E.; et al. Lycopene supplementation elevates circulating insulin-like growth factor–binding protein-1 and -2 concentrations in persons at greater risk of colorectal cancer. Am. J. Clin. Nutr. 2007, 86, 1456–1462. [Google Scholar] [CrossRef]
- Diwadkar-Navsariwala, V.; Novotny, J.A.; Gustin, D.M.; Sosman, J.A.; Rodvold, K.A.; Crowell, J.A.; Bowen, P.E. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J. Lipid Res. 2003, 44, 1927–1939. [Google Scholar] [CrossRef]
- Carbone, L.; Austin, J. Pain and laboratory animals: Publication practices for better data reproducibility and better animal welfare. PLoS ONE 2016, 11, e0155001. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Elnasser, T.A.; El Haggar, S.; Mostafa, T.; Abdel-Malak, G.; Zohdy, W. May-Grunwald-Giemsa stain for detection of spermatogenic cells in the ejaculate: A simple predictive parameter for successful testicular sperm retrieval. Hum. Reprod. 2001, 16, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Stenman, U.H.; Leinonen, J.; Zhang, W.M.; Finne, P. Prostate-specific antigen. Semin. Cancer Biol. 1999, 9, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.D. Questionnaire survey of urologists and primary care physicians’ diagnostic and treatment practices for prostatitis. Urology 1997, 50, 543–547. [Google Scholar] [CrossRef]
- Arda, E.; Çakıroğlu, B.; Arıkan, M.G.; Gözüküçük, R. Chronic bacterial prostatitis in a Turkish population: The microbiological etiology and distribution. J. Acad. Res. Med. 2018, 8, 153–156. [Google Scholar] [CrossRef]
- Tetreault, A.; Crook, J.M.; Hamm, J.; Pickles, T.; Keyes, M.; McKenzie, M.; Pai, H.H.; Bachand, F.; Morris, W.J. Long-term prostate-specific antigen stability and predictive factors of failure after permanent seed prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, S137. [Google Scholar] [CrossRef]
- Neal, D.E.; Moon, T.D.; Clejan, S.; Sarma, D. Prostate specific antigen and prostatitis I. Effect of prostatitis on serum PSA in the human and nonhuman primate. Prostate 1992, 20, 105–111. [Google Scholar] [CrossRef]
- Baker, S.D.; Horger, D.C.; Keane, T.E. Community-acquired methicillin-resistant Staphylococcus aureus prostatic abscess. Urology 2004, 64, 808–810. [Google Scholar] [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef]
- Palozza, P.; Krinsky, N.I. β-Carotene and α-tocopherol are synergistic antioxidants. Arch. Biochem. Biophys. 1992, 297, 184–187. [Google Scholar] [CrossRef]
- Nickel, J.C.; Nyberg, L.M.; Hennenfent, M. Lignes directrices de recherche sur la prostatite chronique: Rapport de consensus du premier réseau international de collaboration sur la prostatite des National Institutes of Health. Urologie 1999, 54, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.; Cytron, S.; Sevadio, C.; Block, C.; Rosenfeld, J.B.; Pitlik, S.D. Prostatic abscess in the antibiotic era. Rev. Infect. Dis. 1988, 10, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radic. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Panizai, M.; Tareen, M.; Ortega-Martinez, S.; Doreulee, D. Review article: An overview on novel antioxidant and anti-cancer properties of lycopene: A comprehensive review. GMJ Med. 2018, 2, 45–50. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, F.; Meng, L.; Chen, D.; Wang, M.; Lu, X.; Chen, D.; Jiang, Y.; Xing, N. Lycopene attenuates chronic prostatitis/chronic pelvic pain syndrome by inhibiting oxidative stress and inflammation via the interaction of NF-κB, MAPKs, and Nrf2 signaling pathways in rats. Andrology 2019, 7, 747–755. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Naber, K.G.; Weidner, W. Prostatitis: An update. Eur. Urol. 2000, 37, 503–512. [Google Scholar]
- Wu, Z.; Zhang, X.; Li, Z.; Wen, Z.; Lin, Y. L’activation de l’autophagie contribue aux effets protecteurs du lycopène contre l’apoptose induite par le stress oxydatif dans les chondrocytes de rat. Recherche en phytothérapie 2021, 35, 4032–4045. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhao, Y.; Cheng, Y.; Li, Y.; Guo, S. Lycopene alleviates chronic prostatitis/chronic pelvic pain syndrome in rats by inhibiting inflammation and oxidative stress. Biomed. Pharmacother. 2019, 111, 791–798. [Google Scholar] [CrossRef]
- Hamed, D.; Keddari, S.; Boufadi, M.Y.; Bessad, L. Purification du lycopène avec l’anti-solvant DMSO: Optimisation à l’aide de la conception expérimentale de Box–Behnken et évaluation de l’effet synergique entre le lycopène et Ammi visnaga L. Chem. Pap. 2022, 76, 6335–6347. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Synergisticeffects of natural compounds and antibiotics against pathogenic bacteria. Microb. Pathog. 2017, 112, 308–313. [Google Scholar] [CrossRef]
| Groups | Body Weight (g) | Prostate Weight (g) |
|---|---|---|
| Negative control group | 276.24 ± 11.47 b | 0.54 ± 0.05 |
| Positive control group | 171.4 ± 38.66 | 0.34 ± 0.01 a |
| Lycopene group | 218.94 ± 25.95 a | 0.47 ± 0.12 a |
| Cephalexin group | 222.5 ± 11.46 a | 0.53 ± 0.05 |
| Lycopene/cephalexin | 275.34 ± 11.47 b | 0.55 ± 0.11 |
| Groups | PSA(×10−2 ng/mL) |
|---|---|
| Negative control group | 2.83 ± 0.002 |
| Positive control group | 2.64 ±0.008 |
| Lycopene group | 2.66 ± 0.003 |
| Cephalexin group | 2.62± 0.004 |
| Lycopene/cephalexin | 2.84 ± 0.001 |
| Groups | log10 CFU/g of Prostate Tissue | log10 CFU/100 µL of Urine |
|---|---|---|
| Negative control group | 00.00 | 00.00 |
| Positive control group | 6.43 ± 0.40 | 5.21 ± 0.16 |
| Lycopene group | 5.48 ± 0.36 | 4.66 ± 0.75 |
| Cephalexin group | 3.48 ± 0.37 a | 2.92 ± 0.89 a |
| Lycopene/cephalexin | 1.19 ± 0.39 a | 1.08 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keddari, S.; Hamed, D.; Bouhend, A.; Boufadi, M.Y.; Mokhtar, M.; Benbouziane, B.; Touzout, N.; Lekmine, S.; Zhang, J.; Amrane, A.; et al. Therapeutic Effects of Lycopene Alone or in Combination with Cephalexin on Chronic Prostatitis Caused by Staphylococcus aureus in a Rat Model. Microbiol. Res. 2025, 16, 114. https://doi.org/10.3390/microbiolres16060114
Keddari S, Hamed D, Bouhend A, Boufadi MY, Mokhtar M, Benbouziane B, Touzout N, Lekmine S, Zhang J, Amrane A, et al. Therapeutic Effects of Lycopene Alone or in Combination with Cephalexin on Chronic Prostatitis Caused by Staphylococcus aureus in a Rat Model. Microbiology Research. 2025; 16(6):114. https://doi.org/10.3390/microbiolres16060114
Chicago/Turabian StyleKeddari, Soumia, Djahira Hamed, Abla Bouhend, Mokhtaria Yasmina Boufadi, Meriem Mokhtar, Bouasria Benbouziane, Nabil Touzout, Sabrina Lekmine, Jie Zhang, Abdeltif Amrane, and et al. 2025. "Therapeutic Effects of Lycopene Alone or in Combination with Cephalexin on Chronic Prostatitis Caused by Staphylococcus aureus in a Rat Model" Microbiology Research 16, no. 6: 114. https://doi.org/10.3390/microbiolres16060114
APA StyleKeddari, S., Hamed, D., Bouhend, A., Boufadi, M. Y., Mokhtar, M., Benbouziane, B., Touzout, N., Lekmine, S., Zhang, J., Amrane, A., & Tahraoui, H. (2025). Therapeutic Effects of Lycopene Alone or in Combination with Cephalexin on Chronic Prostatitis Caused by Staphylococcus aureus in a Rat Model. Microbiology Research, 16(6), 114. https://doi.org/10.3390/microbiolres16060114








