Nematophagous Fungi Occurrence: Prediction Using Bioclimatic Variables
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Period
2.2. Isolation and Identification of Nematophagous Fungi
2.3. Pathogenicity of Nematophagous Fungi to Entomopathogenic Nematode Larvae
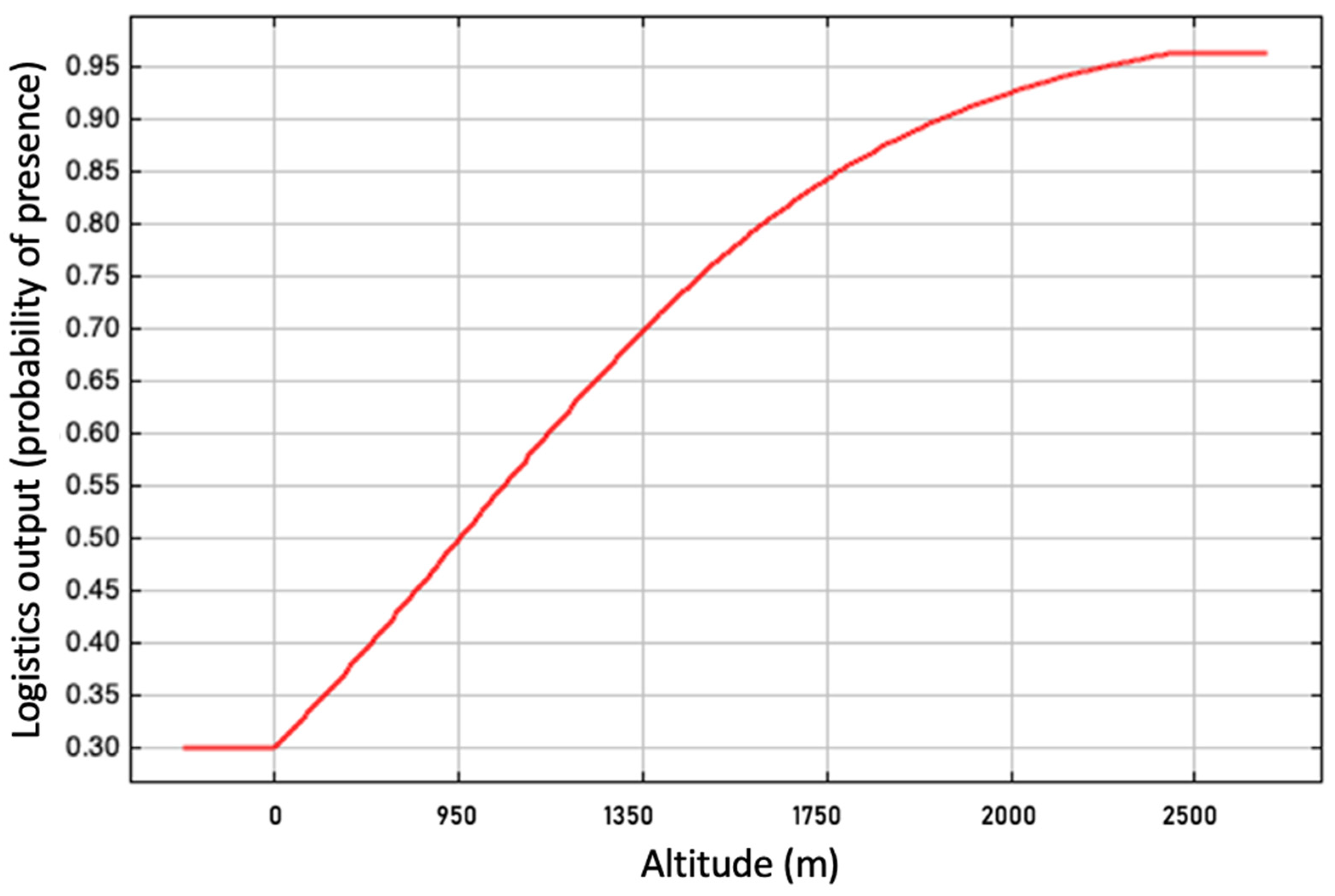
2.4. Environmental Suitability Model
3. Results and Discussion
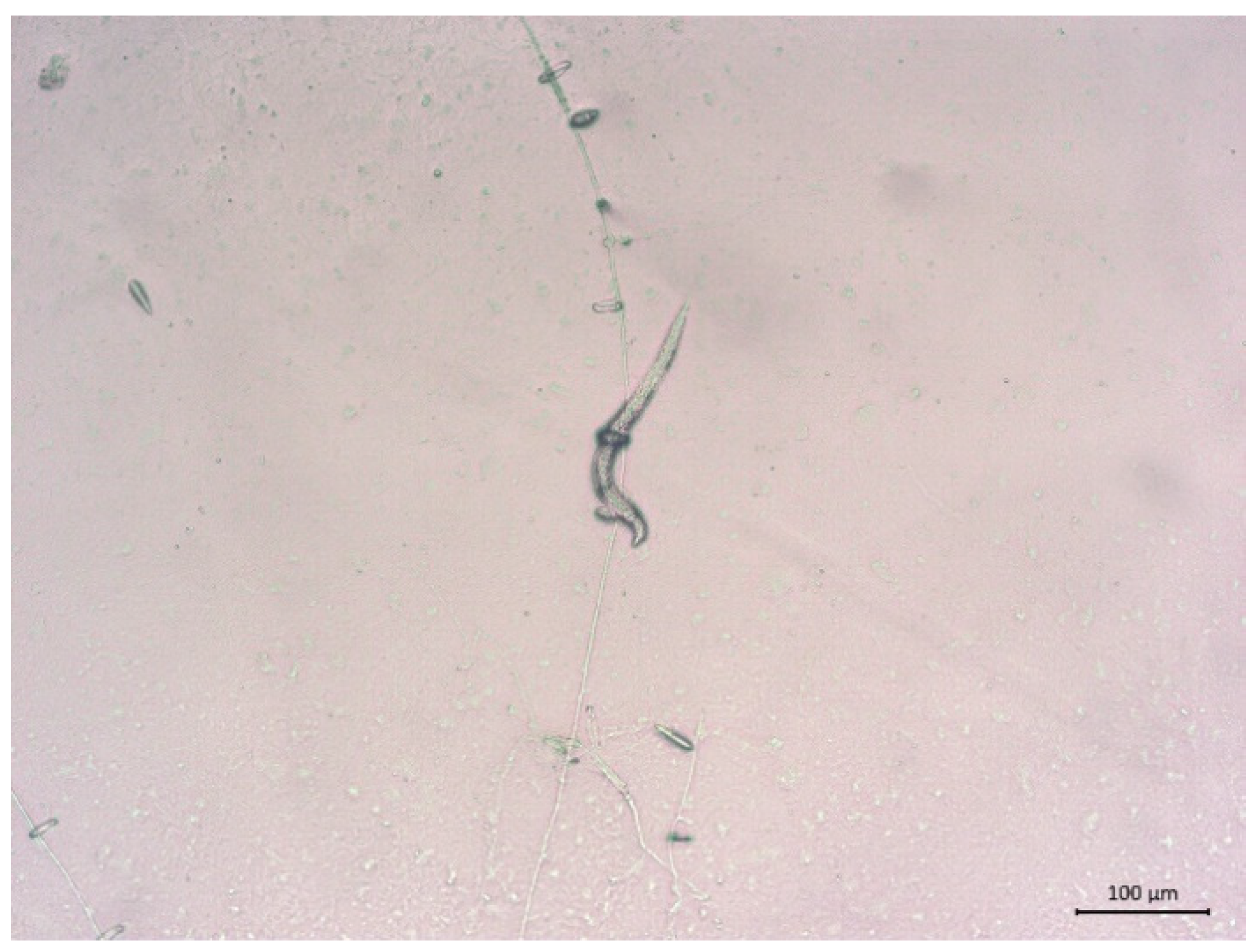
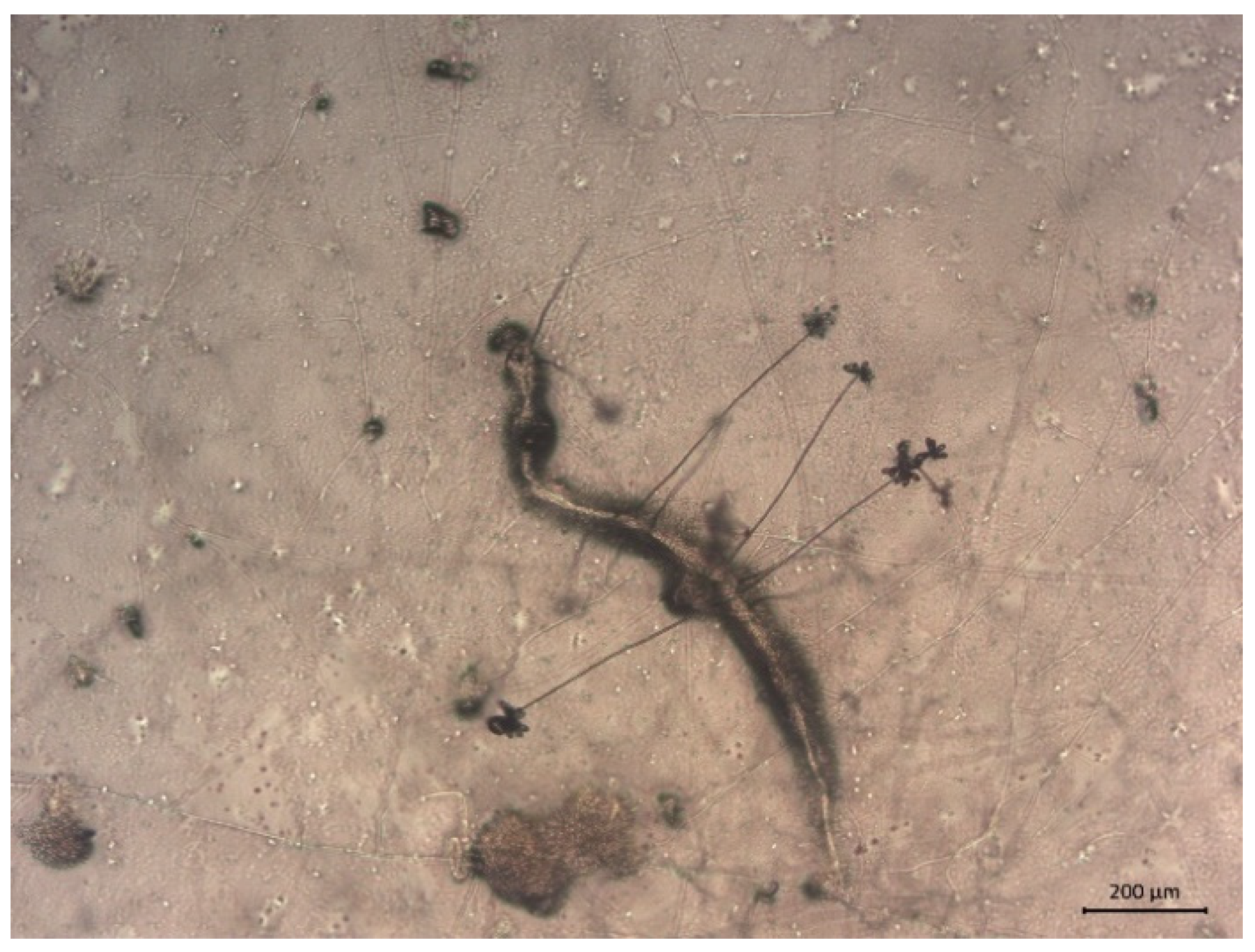
3.1. Isolation, Identification, and Pathogenicity of Nematophagous Fungi
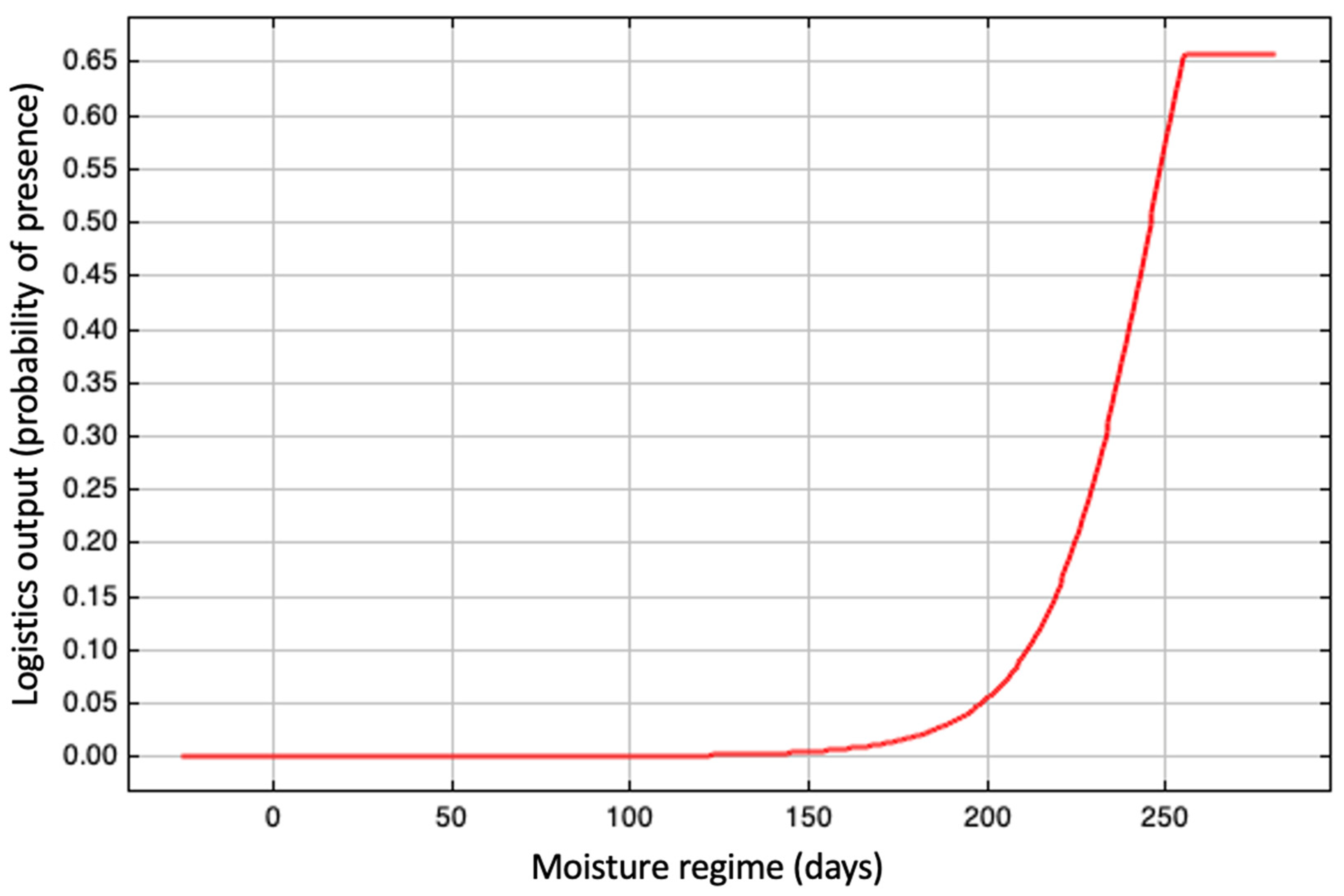
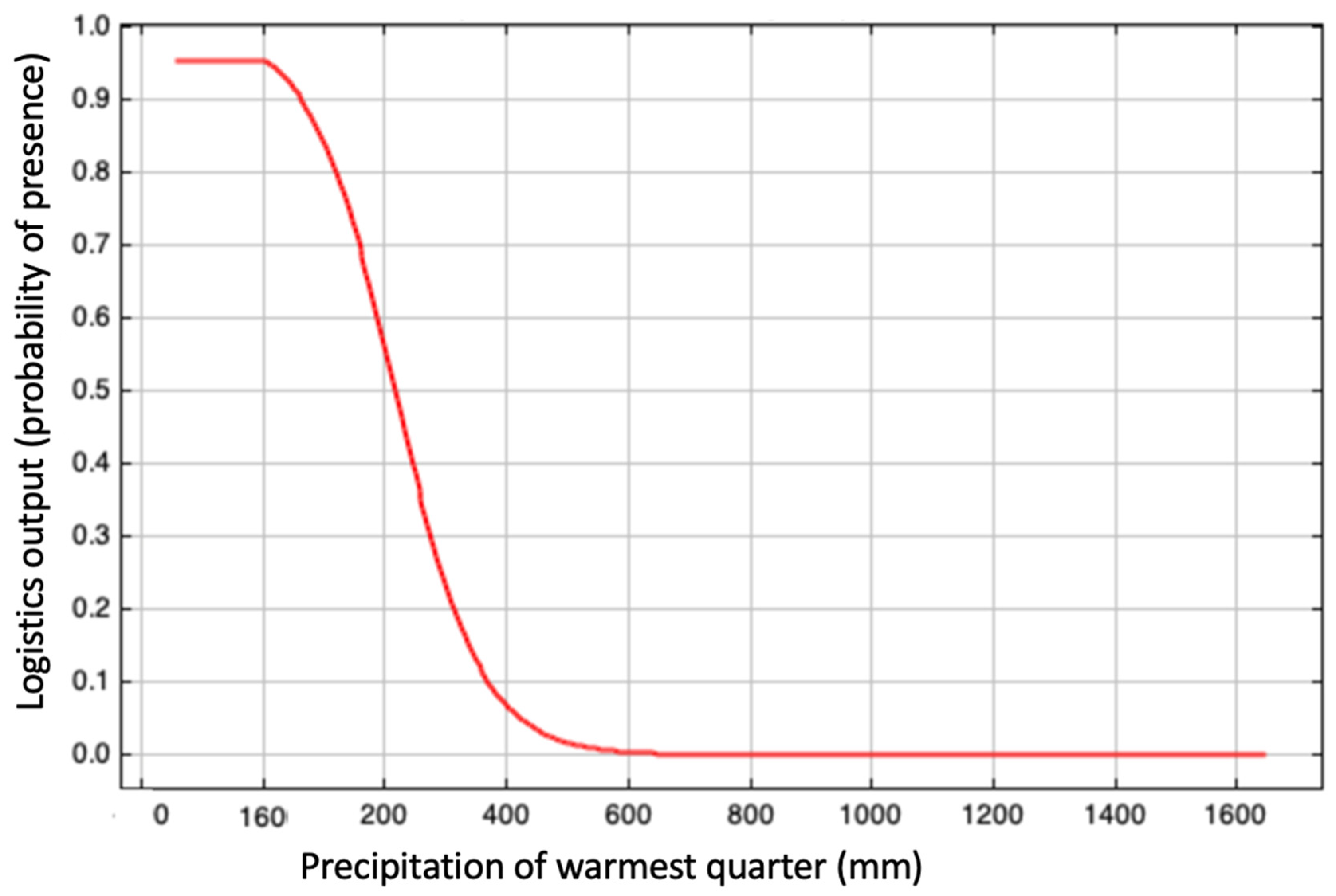
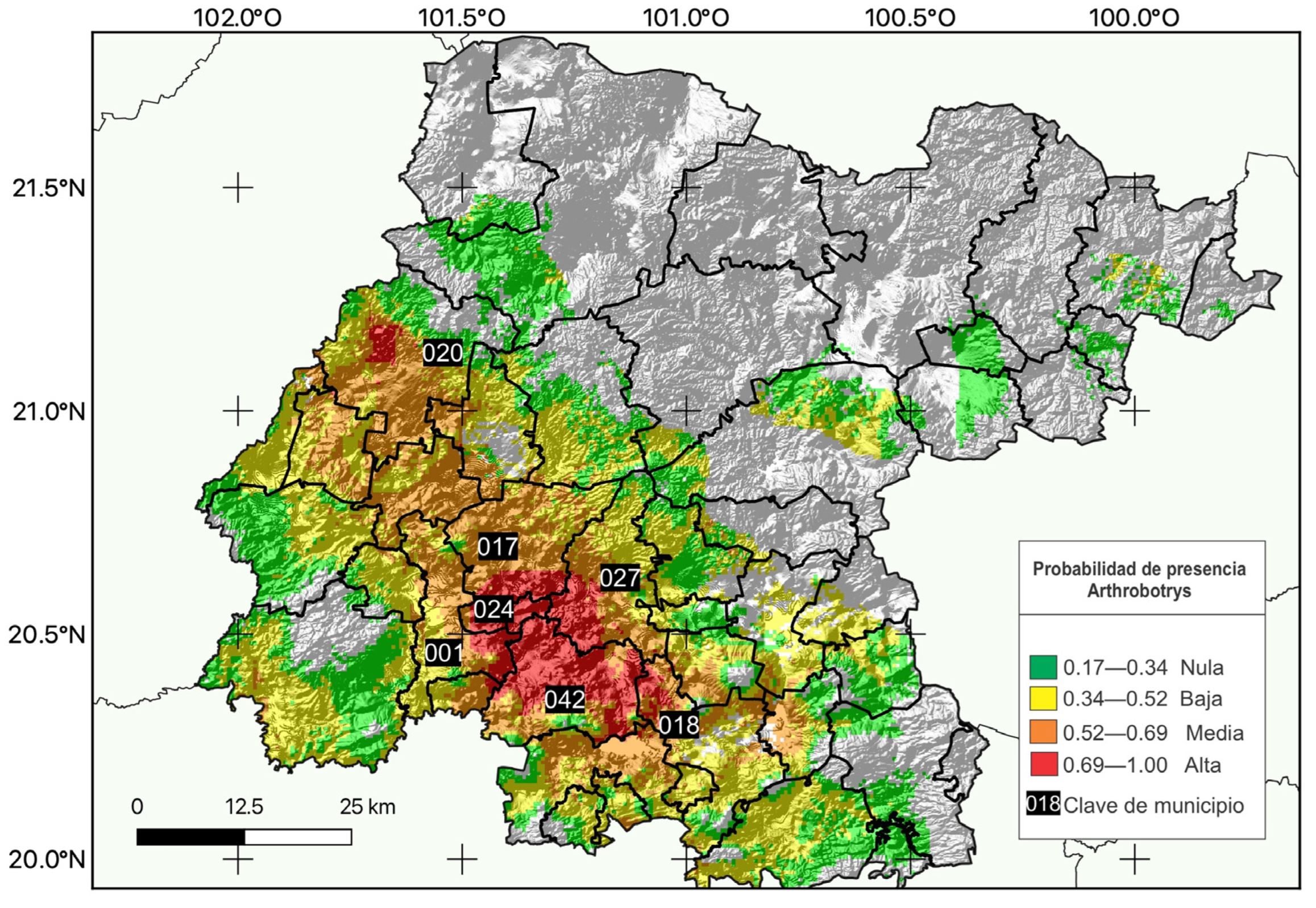
3.2. Evaluation of the Environmental Suitability Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jørgensen, B.B. Mineralization of organic matter in the sea bed the role of sulphate reduction. Nature 1982, 296, 643–645. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Tláskal, V.; da Rocha, U.N.; Baldrian, P. Global Distribution of Carbohydrate Utilization Potential in the Prokaryotic Tree of Life. Msystems 2022, 7, e0082922. [Google Scholar] [CrossRef] [PubMed]
- Sutela, S.; Poimala, A.; Vainio, E.J. Viruses of fungi and oomycetes in the soil environment. FEMS Microbiol. Ecol. 2019, 95, fiz119. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Rossman, A.Y. Where are all the undescribed fungi? Phytopathology 1997, 87, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Pacasa-Quisbert, F. Recursos genéticos de hongos. J. Selva Andin. Biosph. 2018, 6, 22–25. [Google Scholar] [CrossRef]
- Sun, S.; Hoy, M.J.; Heitman, J. Fungal pathogens. Curr. Biol. 2020, 30, R1163–R1169. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 6213. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.C.; Arroyo, T.L. Development, distribution, and host studies of the fungus Macrobiotophthoira vermicola (Entomophthorales). J. Nematol. 1990, 22, 89–94. [Google Scholar] [PubMed]
- Jiang, X.; Xiang, M.; Liu, X. Nematode trapping fungi. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, X.; Liu, X.; Xiang, M. Genome Studies on Nematophagous and Entomogenous Fungi in China. J. Fungi. 2016, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, N.; Rao, W.; Zhang, Y. Recent Advances in Life History Transition with Nematode-Trapping Fungus Arthrobotrys oligospora and Its Application in Sustainable Agriculture. Pathog. 2023, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Banihashemian, S.N.; Jamali, S.; Golmohammadi, M.; Noorizadeh, S.; Atighi, M.R. Reaction of Commercial Cultivars of Kiwifruit to Infection by Root-knot Nematode and Its Biocontrol Using Endophytic Bacteria. J. Nematol. 2023, 55, 20230020. [Google Scholar] [CrossRef] [PubMed]
- Zwart, R.S.; Thudi, M.; Channale, S.; Manchikatla, P.K.; Varshney, R.K.; Thompson, J.P. Resistance to plant-parasitic nematodes in chickpea: Current status and future perspectives. Front. Plant Sci. 2019, 10, 966. [Google Scholar] [CrossRef]
- Morgan, M.; Behnke, J.M.; Lucas, J.A.; Peberdy, J.F. In vitro assessment of the influence of nutrition, temperature and larval density on trapping of the infective larvae of Heligmosomoides polygyrus by Arthrobotrys oligospora, Duddingtonia flagrans and Monacrosporium megalosporum. Parasitology 1997, 115, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.H. Orbilia fimicola, a nematophagous discomycete and its Arthrobotrys anamorph. Mycologia 1994, 86, 451–453. [Google Scholar] [CrossRef]
- Tzean, S.; Liou, J. Nematophagous resupinate basidiomycetous fungi. Phytopathology 1993, 83, 1015–1020. [Google Scholar] [CrossRef]
- Berhanu, M.; Waktole, H.; Mamo, G.; Terefe, G. Isolation of nematophagous fungi from soil samples collected from three different agro-ecologies of Ethiopia. BMC Microbiol. 2022, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Allard, G. Los Impactos del Cambio Climático en la Sanidad Forestal; Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAO): Roma, Italy, 2009; pp. 154–196. Available online: https://www.fao.org/3/k3837s/k3837s.pdf (accessed on 18 September 2023).
- López-Rocha, E.; Mireles-Arriga, A.I.; Hernández-Ruiz, J.; Ruiz-Nieto, K.E.; Rucoba-García, A. Áreas potenciales para el cultivo de girasol en condiciones de temporal en Guanajuato, México. Agron. Mesoam. 2018, 29, 305–314. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schaphire, R.E. Maximum entropy modeling of specie geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- INEGI. Instituto Nacional de Estadísticas, Geografía e Informática. Geografía y Medio Ambiente, Climatología. Available online: https://www.inegi.org.mx/temas/climatologia/ (accessed on 18 September 2023).
- Cimen, H.; Půža, V.; NermuŤ, J.; Hatting, J.; Ramakuwela, T.; Hazir, S. Steinernema biddulphi n. sp., a New Entomopathogenic Nematode (Nematoda: Steinernematidae) from South Africa. J. Nematol. 2016, 48, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Peraza-Padilla, W.; Orozco-Aveces, M.; Esquivel-Hernández, A.; Rivera-Coto, G.; Chaverri-Fonseca, F. Aislamiento e identificación de hongos nematófagos nativos de zonas arroceras de Costa Rica. Agron. Mesoam. 2011, 22, 233–243. [Google Scholar] [CrossRef]
- Barron, G.L. The Nematode-Destroying Fungi; Canadian Biological Publications: Ottawa, ON, Canada, 1977; p. 140. ISBN 0920370004. [Google Scholar]
- Baral, H.O.; Weber, E.; Marson, G. Monograph of Orbiliomycetes (Ascomycota) Based on Vital Taxonomy. Part II; National Museum of Natural History: Luxembourg, 2020; p. 1539. ISBN 978-2-919877-24-9. [Google Scholar]
- Cooke, R.C.; Godfrey, B.E.S. A key to the nematode-destroying fungi. Trans. Brit. Mycol. Soc. 1964, 47, 61–74. [Google Scholar] [CrossRef]
- Swe, A.; Jeewon, R.; Hyde, K.D. Nematode-Trapping fungi from mangrove hábitats. Cryptogam. Mycol. 2008, 29, 333–354. [Google Scholar]
- Muñoz, R.V.; Cisterna, O.V.; France, I.A. Aislamiento de microorganismos fitopatógenos. Inst. De Investig. Agropecu. 2020, 428, 77–91. Available online: https://hdl.handle.net/20.500.14001/67 (accessed on 10 September 2023).
- Arroyo-Balán, F.; Landeros-Jaime, F.; González-Garduño, R.; Cazapal-Monteiro, C.; Arias-Vázquez, M.S.; Aguilar-Tipacamú, G.; Esquivel-Naranjo, E.U.; Mosqueda, J. High predatory capacity of a novel Arthrobotrys oligospora variety on the ovine gastrointestinal nematode Haemonchus contortus (Rhabditomorpha: Trichostrongylidae). Pathogens 2021, 10, 815. [Google Scholar] [CrossRef]
- Cohen, J. The Evolution of Koch’s Postulates. Infect. Dis. 2017, 1, 1–3.e1. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Software Maxent Software for Modeling Species Niches and Distributions (Versión 3.4.1). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 24 March 2024).
- CONABIO. Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad. Uso de Suelo y Vegetación de INEGI Agrupado por CONABIO. Escala 1:1,000,000. Modificado de: Instituto Nacional de Estadística, Geografía e Informática. Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 18 May 2024).
- Maples-Vermeersch, M. Regímenes de Humedad del Suelo en Hidrogeografía IV.6.2 Atlas Nacional de México; Escala 1:4000000; Instituto de Geografíay UNAM: Ciudad de México, México, 1992; Volume II. [Google Scholar]
- Gortari, C.; Cazau, C.; Hours, R. Hongos nematófagos de huevos de Toxocara canis en un paseo público de La Plata, Argentina. Rev. Iberoamer. Micol. 2007, 24, 24–28. [Google Scholar] [CrossRef]
- Caicedo-Rosero, L.C.; de Jesús Méndez-Ávila, F.; Gutiérrez-Zeferino, E.; Flores-Cuautle, J.D.J.A. Soil Moisture Measurement: Review of Methods and Characteristics. Pädi Boletín Científico De Cienc. Básicas E Ing. Del ICBI 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Larsen, M.; Wolstrup, J.; Henriksen, S.A.; Gronvold, J.; Nansen, P. In vivo passage through calves of nematophagous fungi selected for biocontrol of parasitic nematodes. J. Helminthol. 1992, 66, 137–141. [Google Scholar] [CrossRef]
- Gray, N.F. Ecology of nematophagous fungi: Effect of soil moisture, organic matter, pH and nematode density on distribution. Soil Biol. Biochem. 1985, 17, 499–507. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 4, 35–47. [Google Scholar] [CrossRef]
- Jaffee, B.A. Soil cages for studying how organic amendments affect nematode-trapping fungi. Appl. Soil Ecol. 2002, 21, 1–9. [Google Scholar] [CrossRef]
- García, R.A.J.; Gómez, D.S.; Santoyo, L.F.R.; Nieto, J.E.R.; González, J.P.; Ruiz, J.H. Áreas geográficas susceptibles a Fusarium oxysporum en el cultivo de fresa en Guanajuato, México. Bioagro 2021, 33, 51–58. [Google Scholar] [CrossRef]
- Soto-Barrientos, N.; de Oliveira, J.; Vega-Obando, R.; Montero-Caballero, D.; Vargas, B.; Hernández-Gamboa, J.; Orozco-Solano, C. In-vitro predatory activity of nematophagous fungi from Costa Rica with potential use for controlling sheep and goat parasitic nematodes. Rev. Biol. Trop. 2011, 59, 37–52. [Google Scholar] [CrossRef]
- Sánchez, P.J.F.; Lugo, G.G.A.; Mundo, O.M.; Reyes, O.A.; Ley, T.I.D.; Ole, B.J. Búsqueda y aislamiento de hongos nematófagos vs Meloidogyne spp. en el norte de Sinaloa, México. Rev. Mex. de Cienc. Agric. 2016, 7, 1829–1839. [Google Scholar] [CrossRef]
| Code | Variable | Unit |
|---|---|---|
| Bio1 | Annual mean temperature | °C |
| Bio2 | Mean diurnal range | °C |
| Bio3 | Isothermality | Dimensionless |
| Bio4 | Temperature seasonality | CV |
| Bio5 | Maximum temperature of warmest month | °C |
| Bio6 | Minimum temperature of coldest month | °C |
| Bio7 | Temperature annual range | °C |
| Bio8 | Mean temperature of wettest quarter | °C |
| Bio9 | Mean temperature of driest quarter | °C |
| Bio10 | Mean temperature of warmest quarter | °C |
| Bio11 | Mean temperature of coldest quarter | °C |
| Bio12 | Annual precipitation | mm |
| Bio13 | Precipitation of wettest month | mm |
| Bio14 | Precipitation of driest month | mm |
| Bio15 | Precipitation seasonality | CV |
| Bio16 | Precipitation of wettest quarter | mm |
| Bio17 | Precipitation of driest quarter | mm |
| Bio18 | Precipitation of warmest quarter | mm |
| Bio19 | Precipitation of coldest quarter | mm |
| Bio20 | Altitude | m |
| Bio21 | Moisture regime | ° |
| Bio22 | Vegetation | 23 types |
| Sample | Municipality | Species | Pathogenicity Test |
|---|---|---|---|
| S00-AO | Valle de Santiago | Arthrobotrys oligospora | + |
| S02-AM | Salamanca | Arthrobotrys musiformis | + |
| S03-AT | Valle de Santiago | A. musiformis | + |
| S08-AM | Huanimaro | A. musiformis | + |
| S09-AO | Jaral del progreso | A. oligospora | + |
| S10-AO | Valle de Santiago | A. oligospora | + |
| S13-AO | Valle de Santiago | A. oligospora | + |
| S17-AT | Salamanca | Arthrobotrys thaumasia | + |
| S20-AM | Pénjamo | A. musiformis | + |
| S21-AT | Valle de Santiago | A. musiformis | + |
| S27-AM | Valle de Santiago | A. musiformis | + |
| S28-AT | Valle de Santiago | A. thaumasia | + |
| S29-AM | Valle de Santiago | A. oligospora | + |
| Variable | Contribution (%) |
|---|---|
| Arthrobotrys | |
| Moisture regime (Bio 21) | 35.1 |
| Precipitation of warmest quarter (Bio18) | 21.3 |
| Altitude (Bio 20) | 20.7 |
| Temperature seasonality (Bio4) | 9.9 |
| Precipitation of driest month (Bio14) | 2.8 |
| Precipitation seasonality (Bio15) | 2.6 |
| Minimum temperature of coldest month (Bio 6) | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Avalos, A.M.; Chagoya-Sánchez, M.; Ángel-Sahagún, C.A.; Mireles-Arriaga, A.I.; Maki-Díaz, G.; Loredo-Portales, R.; Hernández-Ruíz, J. Nematophagous Fungi Occurrence: Prediction Using Bioclimatic Variables. Microbiol. Res. 2025, 16, 98. https://doi.org/10.3390/microbiolres16050098
Cruz-Avalos AM, Chagoya-Sánchez M, Ángel-Sahagún CA, Mireles-Arriaga AI, Maki-Díaz G, Loredo-Portales R, Hernández-Ruíz J. Nematophagous Fungi Occurrence: Prediction Using Bioclimatic Variables. Microbiology Research. 2025; 16(5):98. https://doi.org/10.3390/microbiolres16050098
Chicago/Turabian StyleCruz-Avalos, Ana Martha, Montserrat Chagoya-Sánchez, César Andres Ángel-Sahagún, Ana Isabel Mireles-Arriaga, Griselda Maki-Díaz, René Loredo-Portales, and Jesús Hernández-Ruíz. 2025. "Nematophagous Fungi Occurrence: Prediction Using Bioclimatic Variables" Microbiology Research 16, no. 5: 98. https://doi.org/10.3390/microbiolres16050098
APA StyleCruz-Avalos, A. M., Chagoya-Sánchez, M., Ángel-Sahagún, C. A., Mireles-Arriaga, A. I., Maki-Díaz, G., Loredo-Portales, R., & Hernández-Ruíz, J. (2025). Nematophagous Fungi Occurrence: Prediction Using Bioclimatic Variables. Microbiology Research, 16(5), 98. https://doi.org/10.3390/microbiolres16050098






