1. Introduction
Root canal treatment (RCT) is a commonly performed dental procedure aimed at saving a tooth that has become infected or is irreversibly damaged [
1,
2]. However, despite the advancements in techniques and materials, RCT failure remains a significant challenge in clinical practice [
3,
4]. Failure can occur due to incomplete cleaning of the root canal system, microbial reinfection, or poor sealing of the canal [
5,
6]. Among the reasons for RCT failure, the persistence of microbial pathogens such as predominant
Enterococcus faecalis and
Candida albicans within the root canal is a prominent factor [
7]. These microorganisms can form biofilms and resist conventional treatments, complicating the healing process [
8,
9].
E. faecalis, a facultative anaerobe Gram-positive coccus, is frequently identified in secondary endodontic infections (77%) [
10]. It is also present, though in smaller quantities (40%), in the bacterial microbiota of primary endodontic infections [
3,
10,
11,
12]. This bacterium is found in large quantities both before and after mechanical preparation, indicating its resistance to endodontic procedures [
12].
C. albicans, a type of fungus that can switch between yeast and hyphal forms, may become pathogenic under certain circumstances. This transformation can lead to endodontic treatment failure. It is often identified in about 20% of the cases of secondary endodontic infections, especially in situations associated with periradicular lesions and persistent symptoms [
13,
14].
Gutta–percha (GP) is the most widely used material for root canal obturation due to its biocompatibility, ease of handling, and long-term stability [
15]. However, in some cases, retreatment is necessary. The endodontic retreatment procedure includes the removal of the existing obturation GP material by using organic solvents and retreatment rotary instruments and disinfecting the root canal to eliminate any remaining contaminated tissue, bacteria, or fungi responsible for the endodontic failure before restoring the canals once more [
16,
17].
Various solvents are used to dissolve GP during root canal retreatment [
18,
19]. Chloroform has traditionally been one of the most effective solvents for GP due to its ability to quickly dissolve the material [
20,
21]. Chloroform is a volatile organic compound, and prolonged exposure may lead to significant health risks, including liver damage and carcinogenicity [
22,
23]. Due to these health risks, many dental professionals have searched for safer alternatives, such as essential oil-based solvents. Essential oils are composed of volatile compounds and are biosynthesized by plants. In recent years, essential oils have been widely used in the field of medicine and dentistry due to their effectiveness, non-toxicity, biocompatibility, antimicrobial properties, and non-carcinogenic nature. Several of these oils have been used as effective gutta–percha solvents and alternatives to chloroform in root canal treatments [
3,
20,
24,
25].
According to the previous literature, the following essential oils were reported as effective GP solvents: eucalyptus oil [
26], orange oil [
27], clove oil [
28], rosemary oil [
29], grapefruit oil [
30], and castor oil [
31]. However, the antibacterial properties of most of these essential oils, GP solvents, which help to disinfect surfaces while dissolving GP, were not evaluated. This study aims to evaluate the antimicrobial properties of the aforementioned six essential oils and chloroform, specifically against the persistent endodontic pathogens
E. faecalis and
C. albicans, and identify those that are most effective in reducing the microbial count while facilitating gutta–percha removal.
4. Discussion
Solvents are solutions used in endodontic retreatment to soften the GP root-filling material. Various types of GP solvents have been used, including chloroform, xylene, halothane, trichloroethane, and tetrachloroethylene [
28,
31,
33]. However, none of these solvents fulfills all the criteria of an ideal solvent, which should be nontoxic and non-carcinogenic to surrounding tissues, the patient, and clinicians; should effectively soften GP; and should exhibit antibacterial properties [
3,
18,
34,
35].
As a promising alternative to synthetic GP solvents, several essential oils have been reported as effective GP solvents, including eucalyptus oil, orange oil, clove oil, rosemary oil, grapefruit oil, and castor oil [
3,
28,
33]. A few studies [
3,
36] have explored the antibacterial properties of GP solvents against
E. faecalis, while only one study [
14] compared their antifungal activity against
C. albicans. However, none of these studies assessed the antimicrobial activities of various GP solvents or examined their effectiveness over different time intervals.
This study compared the antimicrobial activities of seven GP solvents: chloroform, eucalyptus oil, orange oil, clove oil, rosemary oil, grapefruit oil, and castor oil. For the first time, the antimicrobial properties of four of these essential oils—GP solvents, clove oil, rosemary oil, grapefruit oil, and castor oil—were compared with the more efficient and commonly used solvent chloroform [
28,
29,
36]. Unlike previous studies [
3,
14,
36,
37], which measured the activities after 24 h, this study took into account the actual clinical scenario by assessing their antimicrobial effects at time intervals of 3 min, 10 min, and 24 h based on the data collected, which indicated that the time needed for retreatment ranged from 1.5 to 10.8 min [
38]. GP solvents typically remain in the canals for 3 to 10 min to soften the obturating GP before removal [
18,
31,
39]. In this study, the effectiveness of a 24 h interval of GP solvents was also assessed for comparison to previous studies, particularly in terms of its impact when contact is sustained over a long period.
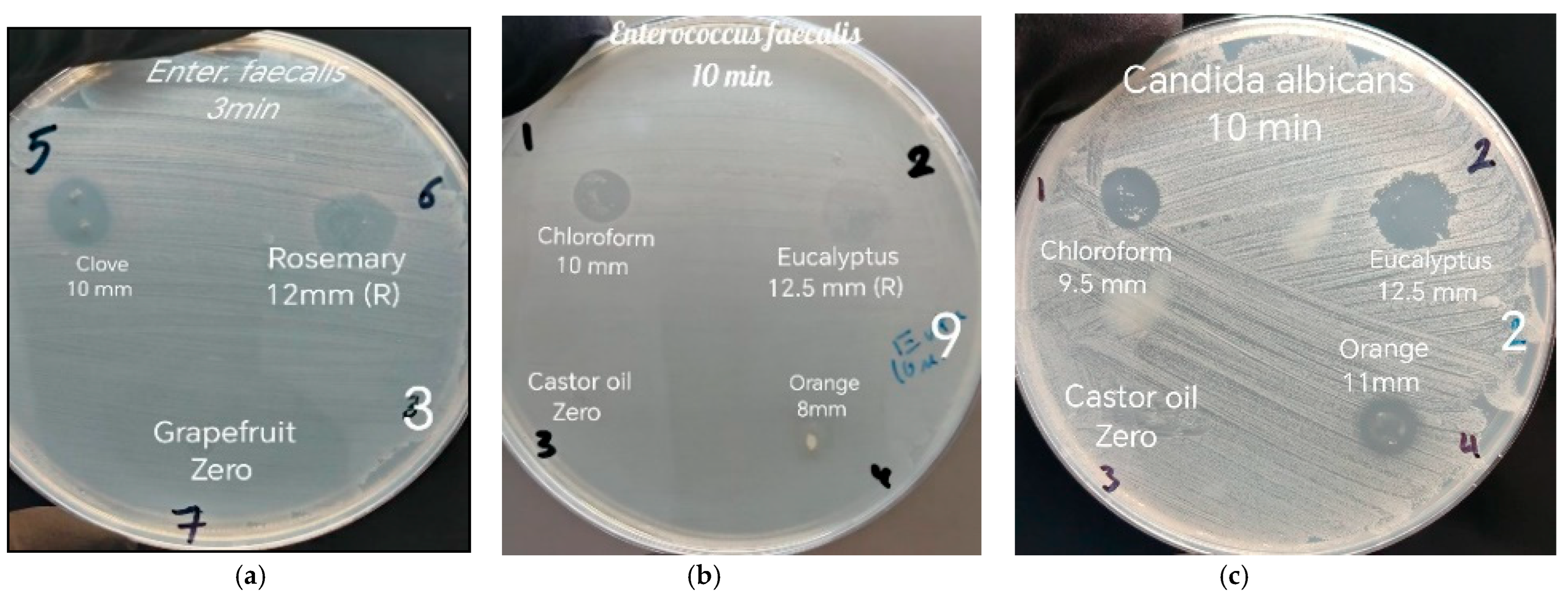
The results obtained were classified into four groups. Group 1 (ZOI with no persistence) included chloroform, orange oil, and clove oil for
E. faecalis and chloroform, orange oil, clove oil, and rosemary oil for
C. albicans. The results align with the findings of Maria et al. (2021), who observed that orange oil had a significantly greater ZOI than chloroform at the 24 h mark [
3]. These results suggest that each solvent produced the largest ZOI at a specific time interval. This is consistent with Chouhan et al. (2017), who emphasized that the antibacterial effectiveness of essential oils is influenced by several factors, such as their chemical composition, concentration, and interaction with bacterial cell membranes over time [
40].
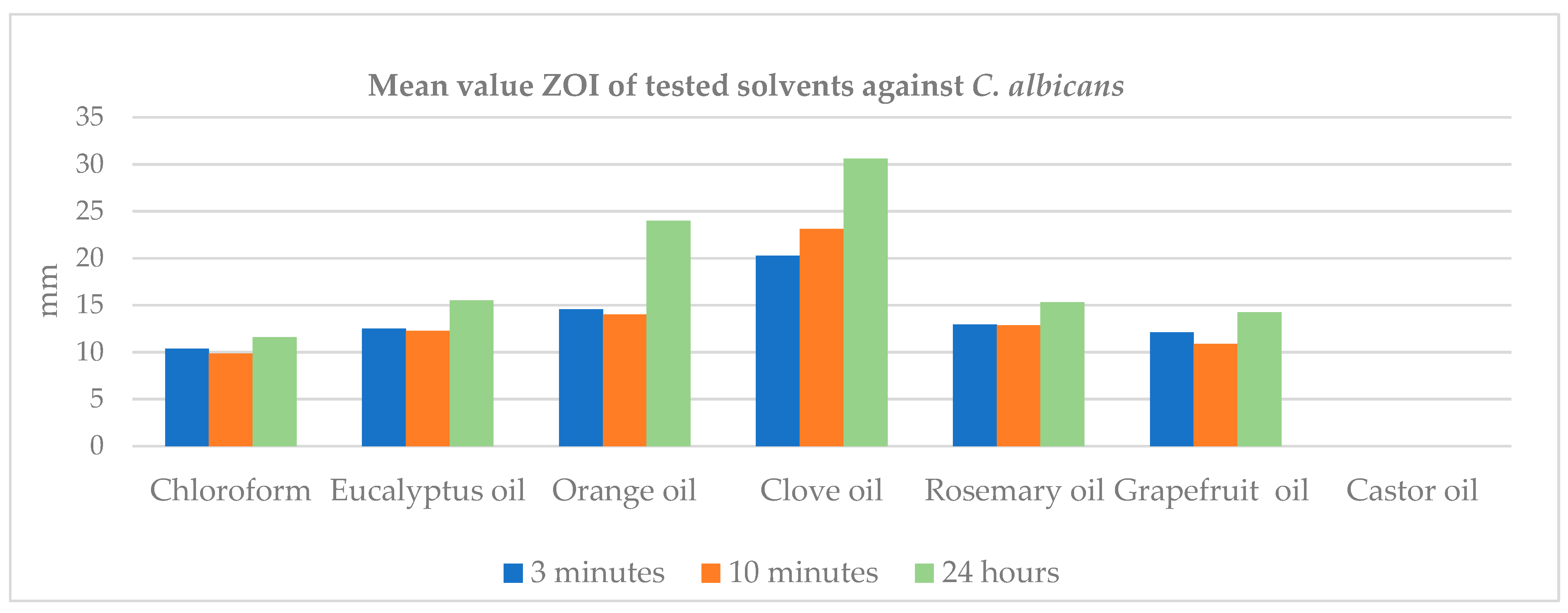
In contrast, when evaluating the GP solvents against
C. albicans, the results showed greater consistency. The ZOI ranked from highest to lowest as follows: clove oil, orange oil, rosemary oil, and chloroform at all three time intervals. Clove oil was significantly more effective against
C. albicans. This could be attributed to eugenol, the most abundant ingredient in clove oil, which is recognized as one of the key active ingredients in essential oils effective against
C. albicans, while chloroform was considerably less effective among the other three GP solvents at all three time intervals. These results are somewhat consistent with Dutta et al. (2023), who demonstrated that chloroform exhibited lower antifungal activity compared to eucalyptus oil, xylene, and turpentine oil and showed similar antifungal activity to orange oil [
14] while increasing the ZOI for all GP solvents against
C. albicans over time, most probably related to stability and sustained release of active components, and their antifungal mechanisms of action include different cellular targets. For example, the chemical stability of essential oil components, such as eugenol in clove oil and terpene derivatives in rosemary oil, enables them to continue releasing their antifungal effects even after initial exposure and inhibits the hyphae formation of
Candida species. This sustained release contributes to prolonged antifungal activity against
C. albicans [
41,
42].
Group 2 (ZOI with persistence) included oil and rosemary oil for
E. faecalis and eucalyptus oil and grapefruit oil for
C. albicans. Rosemary oil demonstrated persistence at the 3 min time point only against
E. faecalis, while eucalyptus oil exhibited ZOI with persistence at both 3 min and 10 min time intervals against
E. faecalis and only at 3 min against
C. albicans. The persistence of
E. faecalis and
C. albicans to some essential oils at short contact times (such as 3 min or 10 min) can be attributed to several factors related to the chemical composition and mode of action of essential oils, as well as the inherent properties of the microorganisms [
43]. For example, the major constituent of eucalyptus oil is oxygenated monoterpenes (1,8-cineole) (87.32%) and monoterpenes hydrocarbons (12.45%). Most of its strong antimicrobial activity is due to the presence of 1,8-cineole [
3,
44,
45]. Rosemary essential oil is primarily composed of monoterpenes and their derivatives, making up 95–98% of its content, with the remaining 2–5% consisting of sesquiterpenes [
46,
47,
48]. Essential oils with higher levels of monoterpenes may act more slowly, whereas those with phenolic compounds may have faster action [
40]. Although eucalyptus oil and rosemary oil possess potent antimicrobial properties [
3,
45,
47,
48], their antimicrobial activities may take more time to become highly effective. The efficacy of essential oils can be dependent on concentration and exposure time. At shorter contact times, the concentration of these active compounds may not reach levels high enough to induce cell membrane disruption, inhibition of enzyme activity, or other cellular damage, allowing the bacteria to adapt and resist the effects. It has been reported that compounds with slow action require 30–60 min to exhibit effective antimicrobial activity [
49]. In addition, both
E. faecalis and
C. albicans have relatively robust cell walls and membranes that can limit the uptake of essential oils.
E. faecalis, a Gram-positive bacterium, has a thick peptidoglycan layer that can act as a barrier to many antimicrobial agents [
50,
51]. In addition,
E. faecalis is known to harbor various resistance mechanisms, such as efflux pumps and changes in membrane permeability [
52,
53], which can decrease susceptibility to essential oils. These resistance mechanisms may be activated or more pronounced at shorter contact times. Similarly,
C. albicans has a complex cell wall that includes polysaccharides, like glucan [
54], which can reduce the effectiveness of antimicrobial agents during brief exposure. Moreover, brief contact may allow these organisms to repair any damage or activate resistance mechanisms [
55].
Group 3 (ZOI only at specific time intervals) included grapefruit oil at the 24 h mark for
E. faecalis. Grapefruit oil did not produce any ZOI against
E. faecalis at the 3 min and 10 min intervals, while ZOI of 7.6 ± 0.31 was observed against
E. faecalis at the 24 h interval, which was significantly lower than all the other tested GP solvents, except for castor oil, which showed no ZOI. Grapefruit oil primarily consists of limonene (88.6–91.5%), β-pinene (0.8–1.2%), linalool (1.1–0.7%), and α-terpinene (0.7–1.0%) [
56]. This means that over 96% of grapefruit oil is made up of monoterpenes [
57], which tend to act more slowly and require longer contact times to exhibit noticeable effects, such as a ZOI [
40].
Group 4 (no ZOI) included castor oil for both
E. faecalis and
C. albicans. Castor oil did not produce ZOI at any time intervals tested for both
E. faecalis and
C. albicans. Castor oil is a type of vegetable oil extracted from the seeds of the castor plant (Ricinus communis Linn). The primary fatty acid components in castor oil include ricinoleic acid (92%), oleic acid (3.53%), linoleic acid (2.90%), stearic acid (1.02%), and myristic acid (0.55%). The castor oil derivatives, K-soap, free fatty acids, and methyl esters are responsible for their antibacterial activity, especially against
Escherichia coli and
Staphylococcus aureus bacteria [
58]. Some studies [
59,
60,
61] have shown that castor oil exhibits antimicrobial properties against various bacterial and fungal species. However, its activity against
E. faecalis and
C. albicans specifically is not always significant. Salles et al. (2015) concluded that castor oil had a non-significant action on
E. faecalis but could reduce colony counts for other microorganisms like
C. albicans and
Staphylococcus aureus [
60]. Momoh et al. (2012) showed that fungi were less susceptible to the castor oil extract than bacteria [
59]. Alezzi et al. (2022) indicated in their review that castor oil could reduce but not eliminate the colony count of
E. faecalis and
C. albicans in comparison to the significant antimicrobial activity of NaOCl and chlorhexidine [
61]. These might indicate that although castor oil may have a mild inhibitory effect on certain microorganisms, it may fail to produce ZOI in some laboratory studies. The antimicrobial properties of castor oil can vary based on factors such as concentration, the study design, the methodology used, and specific strains of microorganisms tested.
The current study also compared the impact of the duration of application of the tested GP solvents on
E. faecalis and
C. albicans. For orange oil, clove oil, and rosemary oil, the ZOI increased as the duration of application lengthened, with the highest ZOI observed at 24 h, followed by 10 min and 3 min, respectively. In contrast, the order was different for chloroform and eucalyptus oil. Chloroform exhibited the highest ZOI at 24 h (10.85), followed by 3 min (10.45) and 10 min (10.1). For eucalyptus oil, ZOI was the highest at 10 min (10.5), followed by 3 min (8.95) and 24 h (8.05). However, both the 10 min and 3 min applications showed a higher ZOI than the 24 h application, which was associated with persistence. These results, in agreement with Chouhan et al. (2017), indicated that the antibacterial activity of essential oils can change according to the duration of their application [
40]. The time factor is crucial because the longer the essential oil is in contact with the bacteria, the more likely it is that its bioactive compounds (such as terpenes, aldehydes, limonene, phenols, and eugenol) will penetrate the bacterial cell membranes and exert their antimicrobial effects. With short contact times, there may not be enough time for these compounds to have a significant effect [
40,
43,
62,
63] and be associated with persistence. The duration of application can influence the bacteria’s growth phase. Essential oils may be more effective during specific phases of bacterial growth (such as the exponential growth phase) and less effective if the bacteria are in a dormant state [
64].