Polysaccharides from Marine Bacteria and Their Anti-SARS-CoV-2 Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Structural Characterization of Marine Bacteria Polysaccharides
2.2. SARS-CoV-2 Strain
2.3. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR-RV)
2.4. The Cytotoxic Activity of the PSs Assay
2.5. The Study of Anti-SARS-CoV-2 Activity
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- PSs from marine bacteria A. nigrifaciens KMM 156 (PS1), C. amphilecti KMM 3890 (PS2), I. abyssalis KMM 227T (PS3), differing in chemical structure, exhibit anti-coronavirus activity, effectively inhibiting the stages of attachment and penetration of SARS-CoV-2 into the cells.
- This PSs can be considered a promising source of antiviral medicinal substances, including in the fight against the SARS-CoV-2 virus. However, further research is required to study the in-depth mechanisms of the antiviral activity of studied PSs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD50 | 50% Cytotoxic Dose |
| CI | Cytotoxicity Index |
| CPE | Cytopathogenic Effect |
| ED50 | 50% Effective Dose |
| MNCD | Maximum Non-Cytotoxic Dose |
| MTT | Methylthiazolyl Tetrazolium Bromide |
| PCRPI | Protection Index on the Results of RT-PCR-RV |
| PI | Protection Index |
| PSs | Polysaccharides |
| TCID50 | Tissue Culture Infectious Dose |
| RT-PCR-RV | Real-Time Reverse Transcription Polymerase Chain Reaction |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome-2 |
References
- Sharma, A.; Ahmad, F.I.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Shchelkanov, M.Y.; Popova, A.Y.; Dedkov, V.G.; Akimkin, V.G.; Maleev, V.V. Research history of coronaviruses. Russ. J. Infect. Immun. 2020, 10, 221–246. (In Russian) [Google Scholar] [CrossRef]
- Yoshimoto, F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020, 39, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.; Bhattacharya, M.; Sharma, G.; Lee, S.S. The 2019 novel coronavirus disease (COVID-19) pandemic: A zoonotic prospective. Asian Pac. J. Trop. Med. 2020, 13, 242–246. [Google Scholar] [CrossRef]
- Lorusso, A.; Calistri, P.; Petrini, A.; Savini, G.; Decaro, N. Novel coronavirus (SARS-CoV-2) epidemic: A veterinary perspective. Vet. Ital. 2020, 56, 5–10. [Google Scholar] [CrossRef]
- Munir, K.; Ashraf, S.; Munir, I.; Khalid, H.; Muneer, M.A.; Mukhtar, N.; Amin, S.; Ashraf, S.; Imran, M.A.; Chaudhry, U.; et al. Zoonotic and reverse zoonotic events of SARS-CoV-2 and their impact on global health. Emerg. Microbes Infect. 2020, 9, 2222–2235. [Google Scholar] [CrossRef]
- Benvenuto, D.; Giovanetti, M.; Ciccozzi, A.; Spoto, S.; Angeletti, S.; Ciccozzi, M. The 2019-new coronavirus epidemic: Evidence for virus evolution. J. Med. Virol. 2020, 92, 455–459. [Google Scholar] [CrossRef]
- Majumdar, A.; Malviya, N.; Alok, S. An overview on COVID-19 outbreak: Epidemic to pandemic. Int. J. Pharm. Sci. Res. 2020, 11, 1958–1968. [Google Scholar] [CrossRef]
- Wang, Y.T.; Landeras-Bueno, S.; Hsieh, L.E.; Terada, Y.; Kim, K.; Ley, K.; Shresta, S.; Saphire, E.O.; Regla-Nava, J.A. Spiking Pandemic Potential: Structural and Immunological Aspects of SARS-CoV-2. Trends Microbiol. 2020, 28, 605–618. [Google Scholar] [CrossRef]
- Lin, J.G.; Huang, G.J.; Su, Y.C. Efficacy analysis and research progress of complementary and alternative medicines in the adjuvant treatment of COVID-19. J. Biomed. Sci. 2023, 30, 30. [Google Scholar] [CrossRef]
- Mishra, N.; Gupta, E.; Walag, A.M.P.; Kharwar, R.N.; Singh, P.; Mishra, P. A Review of Marine Natural Product Resources with Potential Bioactivity Against SARS-COV-2. Trop. J. Nat. Prod. Res. 2023, 7, 2093–2103. [Google Scholar] [CrossRef]
- Wu, D.Y.; Hou, X.T.; Xia, Z.S.; Hao, E.W.; Xie, J.L.; Liang, J.Y.; Liang, Q.M.; Du, Z.C.; Deng, J.G. Analysis on oral medication rules of traditional Chinese medicine prescriptions for prevention of COVID-19. Chin. Herb. Med. 2021, 13, 502–517. [Google Scholar] [CrossRef]
- Chaisuwan, W.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Wangtueai, S.; Sommano, S.R.; You, S.; et al. The Antiviral Activity of Bacterial, Fungal, and Algal Polysaccharides as Bioactive Ingredients: Potential Uses for Enhancing Immune Systems and Preventing Viruses. Front. Nutr. 2021, 8, 772033. [Google Scholar] [CrossRef]
- Chhetri, B.K.; Tedbury, P.R.; Sweeney-Jones, A.M.; Mani, L.; Soapi, K.; Manfredi, C.; Sorscher, E.; Sarafianos, S.G.; Kubanek, J. Marine Natural Products as Leads against SARS-CoV-2 Infection. J. Nat. Prod. 2022, 85, 657–665. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.G.; Moon, K.-S.; Jung, S.-B.; Kwon, Y.M.; Kang, N.S.; Kim, J.-H.; Nam, S.-J.; Choi, G.; Baek, Y.-B. Identification and characterization of a marine bacterium extract from Mameliella sp. M20D2D8 with antiviral effects against influenza A and B viruses. Arch. Virol. 2024, 169, 41. [Google Scholar] [CrossRef] [PubMed]
- Kwon, P.S.; Oh, H.; Kwon, S.J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Osmanović, A.; Salihović, M.; Veljović, E.; Hindija, L.; Pazalja, M.; Malenica, M.; Selmanagić, A.; Špirtović-Halilović, S. Marine Origin vs. Synthesized Compounds: In Silico Screening for a Potential Drug Against SARS-CoV-2. Sci. Pharm. 2025, 93, 2. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Shah, M.; Shuvo, S.K.; Khan, H.; Chowdhury, M.A.R.; Bulbul, I.J.; Hossain, M.S.; et al. Multifaceted role of natural sources for COVID-19 pandemic as marine drugs. Environ. Sci. Pollut. Res. Int. 2022, 29, 46551. [Google Scholar] [CrossRef]
- Salimi, F.; Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 3. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Besednova, N.N.; Zaporozhets, T.S.; Kokoulin, M.S.; Khotimchenko, Y.S.; Shchelkanov, M.Y. Antiviral Potential of Marine Bacteria Polysaccharides. Russ. J. Mar. Biol. 2024, 50, 107–115. [Google Scholar] [CrossRef]
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral polymers: Past approaches and future possibilities. Macromolecules 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Eichberg, J.; Maiworm, E.; Oberpaul, M.; Czudai-Matwich, V.; Lüddecke, T.; Vilcinskas, A.; Hardes, K. Antiviral Potential of Natural Resources against Influenza Virus Infections. Viruses 2022, 14, 2452. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Pan, B. Exploring the structural, functional, and biocompatibility aspects of marine bacterial extracellular polysaccharides for biopharmaceutical applications. Theor. Nat. Sci. 2024, 37, 277–282. [Google Scholar] [CrossRef]
- Moon, K.-S.; Choi, G.; Jung, S.-B.; Kim, H.-J.; Park, J.-G.; Kwon, Y.M.; Cho, E.-S.; Shin, M.; Yu, J.-Y.; Choi, J.; et al. Influenza Virus-Based Antiviral Strategy: A Broad-Spectrum Potential of a Marine Bacterium Targeting Future Pandemics. Preprints 2024. [Google Scholar] [CrossRef]
- Gorshkova, R.P.; Nazarenko, E.L.; Zubkov, V.A.; Ivanova, E.P.; Ovodov, I.S.; Shashkov, A.S.; Knirel’, I.A. Structure of the repeating link of the acid polysaccharide of Alteromonas haloplanktis KMM 156. Bioorg Khim 1993, 19, 327–336. (In Russian) [Google Scholar] [PubMed]
- Kokoulin, M.S.; Kuzmich, A.S.; Kalinovsky, A.I.; Tomshich, S.V.; Romanenko, L.A.; Mikhailov, V.V.; Komandrova, N.A. Structure and anticancer activity of sulfated O-polysaccharide from marine bacterium Cobetia litoralis KMM 3880T. Carbohydr. Polym. 2016, 154, 55–61. [Google Scholar] [CrossRef]
- Kokoulin, M.S.; Komandrova, N.A.; Kalinovskiy, A.I.; Tomshich, S.V.; Romanenko, L.A.; Vaskovsky, V.E. Structure of the O-specific polysaccharide from the deep-sea marine bacterium Idiomarina abyssalis KMM 227T containing a 2-O-sulfate-3-N-(4-hydroxybutanoyl)-3,6-dide-oxy-D-glucose. Carbohydr. Res. 2015, 413, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Shchelkanov, M.Y.; Sakhuria, I.B.; Polyakova, E.B.; Baranova, V.V.; Pashkova, T.A.; Kornilaeva, G.V.; Karamov, E.V. Improving the quality of the MTT method using microdosing tips of a special design. Immunology 1998, 19, 57–59. (In Russian) [Google Scholar]
- Krylova, N.V.; Smolina, T.P.; Berlizova, M.V.; Leonova, G.N. Immunocorrective and Antiviral Activity of Polysaccharide from Marine Bacteria Against Tick-Borne Encephalitis Virus. Antibiot. Khimioter 2019, 64, 16–24. (In Russian) [Google Scholar]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Q.; Rückert, T.; Romagnani, C. Natural killer cell specificity for viral infections. Nat. Immunol. 2018, 19, 800–808. [Google Scholar] [CrossRef] [PubMed]
- van Erp, E.A.; van Kampen, M.R.; van Kasteren, P.B.; de Wit, J. Viral infection of human natural killer cells. Viruses 2019, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Letafati, A.; Ardekani, O.S.; Naderisemiromi, M.; Norouzi, M.; Shafiei, M.; Nik, S.; Mozhgani, S.H. Unraveling the dynamic mechanisms of natural killer cells in viral infections: Insights and implications. Virol. J. 2024, 21, 18. [Google Scholar] [CrossRef]
- Khan, M.T.; Ali, A.; Wang, Q.; Irfan, M.; Khan, A.; Zeb, M.T.; Zhang, Y.J.; Chinnasamy, S.; Wei, D.Q. Marine natural compounds as potents inhibitors against the main protease of SARS-CoV-2-a molecular dynamic study. J. Biomol. Struct. Dyn. 2021, 39, 3627–3637. [Google Scholar] [CrossRef]
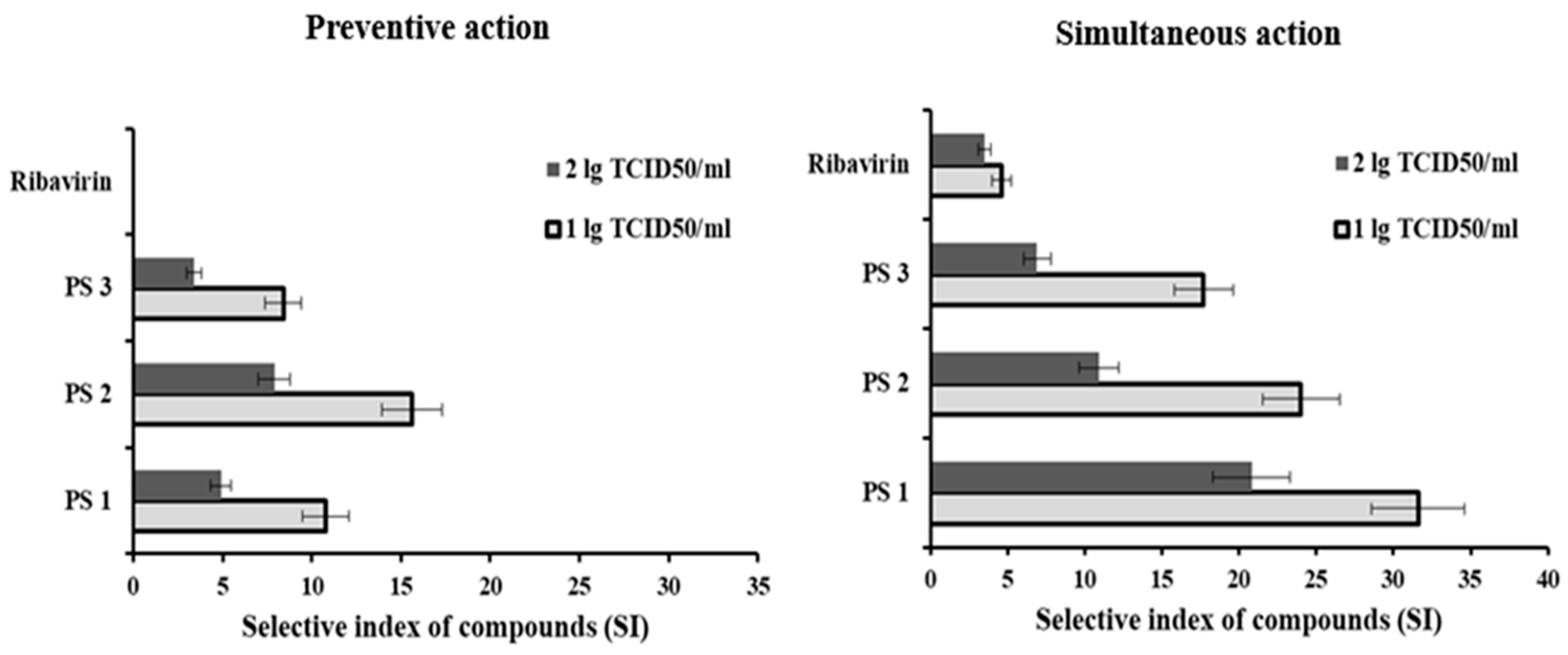
| PSs | Preventive Action | Simultaneous Action | ||||||
|---|---|---|---|---|---|---|---|---|
| 1.0 lg(TCID50/мл) | 2.0 lg(TCID50/мл) | 1.0 lg(TCID50/мл) | 2.0 lg(TCID50/мл) | |||||
| , мкг/мл | , мкг/мл | , мкг/мл | , мкг/мл | |||||
| PS1 | 185 ± 20 | 10.8 ± 1.3 | 410 ± 45 | 4.9 ± 0.6 | 63 ± 7 | 31.6 ± 3.0 | 96 ± 10 | 20.8 ± 2.5 |
| PS2 | 128 ± 14 | 15.6 ± 1.7 | 253 ± 28 | 7.9 ± 0.9 | 83 ± 9 | 24.0 ± 2.5 | 182 ± 20 | 10.9 ± 1.3 |
| PS3 | 238 ± 26 | 8.4 ± 0.9 | 581 ± 64 | 3.4 ± 0.4 | 113 ± 12 | 17.7 ± 1.9 | 289 ± 32 | 6.9 ± 0.9 |
| Ribavirin® | n/a | n/a | 160 ± 18 | 4.6 ± 0.6 | 207 ± 23 | 3.5 ± 0.4 | ||
| PSs | Preventive Action | Simultaneous Action | ||||
|---|---|---|---|---|---|---|
| Ctsi | Ctsi − Cti | Ctsi | Ctsi − Cti | |||
| PS1 | 18.8 ± 2.2 * | 2.4 ± 0.3 | 8.8 ± 1.1 | 26.3 ± 3.4 * | 9.9 ± 1.3 | 41.9 ± 5.0 |
| PS2 | 19.9 ± 2.4 * | 3.5 ± 0.4 | 13.1 ± 1.7 | 22.0 ± 2.6 * | 5.6 ± 0.7 | 21.9 ± 2.8 |
| PS3 | 17.9 ± 2.1 | 1.5 ± 0.2 | 5.4 ± 0.7 | 20.3 ± 2.4 | 3.9 ± 0.5 | 14.8 ± 1.8 |
| Ribavirin® | 16.9 ± 1.9 | 0.5 ± 0.1 | 1.7 ± 0.2 | 19.8 ± 2.2 * | 3.4 ± 0.4 | 12.7 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, T.A.; Krylova, N.V.; Kokoulin, M.S.; Persiyanova, E.V.; Maistrovskaya, O.S.; Milovankin, P.G.; Belov, Y.A.; Shchelkanov, M.Y. Polysaccharides from Marine Bacteria and Their Anti-SARS-CoV-2 Activity. Microbiol. Res. 2025, 16, 102. https://doi.org/10.3390/microbiolres16050102
Kuznetsova TA, Krylova NV, Kokoulin MS, Persiyanova EV, Maistrovskaya OS, Milovankin PG, Belov YA, Shchelkanov MY. Polysaccharides from Marine Bacteria and Their Anti-SARS-CoV-2 Activity. Microbiology Research. 2025; 16(5):102. https://doi.org/10.3390/microbiolres16050102
Chicago/Turabian StyleKuznetsova, Tatyana A., Natalia V. Krylova, Maksim S. Kokoulin, Elena V. Persiyanova, Olga S. Maistrovskaya, Pavel. G. Milovankin, Yurii A. Belov, and Mikhail Yu. Shchelkanov. 2025. "Polysaccharides from Marine Bacteria and Their Anti-SARS-CoV-2 Activity" Microbiology Research 16, no. 5: 102. https://doi.org/10.3390/microbiolres16050102
APA StyleKuznetsova, T. A., Krylova, N. V., Kokoulin, M. S., Persiyanova, E. V., Maistrovskaya, O. S., Milovankin, P. G., Belov, Y. A., & Shchelkanov, M. Y. (2025). Polysaccharides from Marine Bacteria and Their Anti-SARS-CoV-2 Activity. Microbiology Research, 16(5), 102. https://doi.org/10.3390/microbiolres16050102








