2. Microorganisms as Producers of Azo and Azoxy Compounds
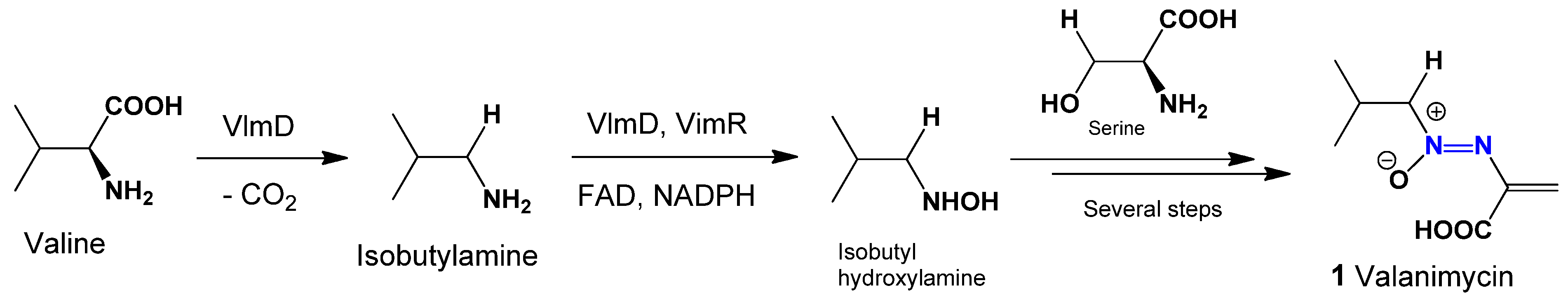
A well-known example of a natural azoxy antibiotic is valanimicin (
1), which is derived from the fermentation broth of
Streptomyces viridifaciens MG456-hF10. Recent research has identified the genes responsible for valanimicin biosynthesis. The VlmF protein, part of the major facilitator superfamily, provides resistance to valanimicin. Meanwhile, VlmD, VlmH, and VlmR convert L-valine into isobutyl-hydroxylamine, and VlmL facilitates the formation of L-seryl-tRNA from L-serine (see
Figure 1) [
17,
18].
Valanimicin is effective against both Gram-positive and Gram-negative bacteria, particularly
E. coli BE1121, a mutant strain of
E. coli K12 that is deficient in DNA repair. It has also shown toxicity against L1210, P388/S (doxorubicin-sensitive) and P388/ADR (doxorubicin-resistant) murine leukemia cells, with IC
50 values of 0.8, 2.7, and 1.4 pg/mL, respectively. In animal studies, valanimicin extended the survival of mice with Ehrlich carcinoma or L1210 leukemia cells [
19].
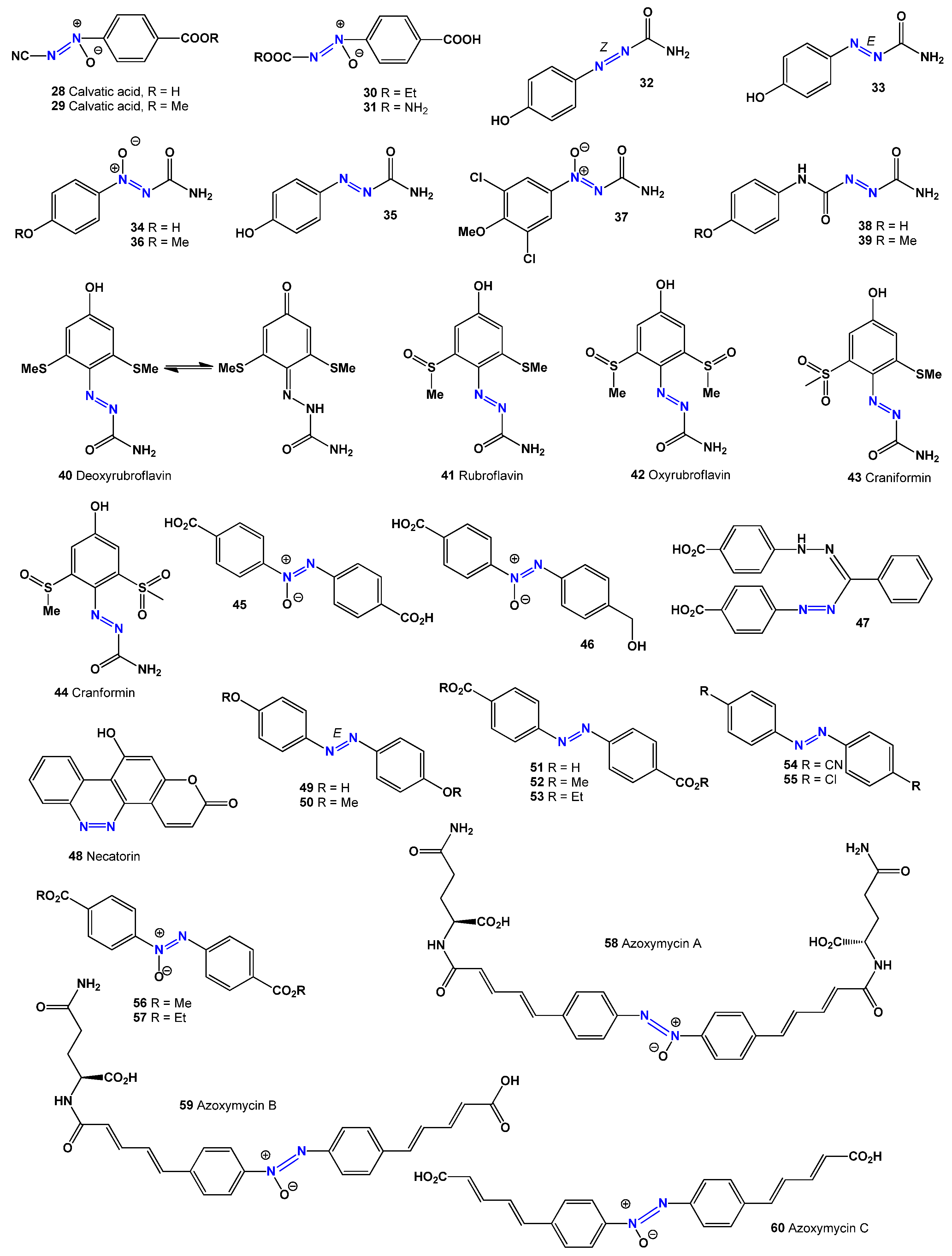
Several valinomycin derivatives have been discovered from different sources. A valanimicin derivative (
2, see structure in
Figure 2) was found in the culture broth of
Streptomyces viridifaciens MG456-hF10 during valanimicin biosynthesis [
20], with its structure confirmed using a more stable ammonia adduct (
3) [
21].
Table 1 summarizes the biological activities of compounds (
1–
27). The antibiotic LL-BH872α (4), which contains an α,β-unsaturated azoxy group, was isolated from
Streptomyces hinnulinis [
22]. More recently, LL-BH872a, 2z-OH (
5), produced by
Actinomadura sp., was found in apricot (
Prunus armeniaca) roots [
21], along with LL-BH872a, 2z-OH, 4′z-OH (
6), produced by
Streptomyces misionensis FERM 10459 [
23].
Several microorganisms produce antifungal antibiotics. For example, manivamycins A (
7) and B (
8), isolated from
Streptomyces prasinopilosus, showed broad antifungal and antibacterial activity against multiple
Candida and
Trichophyton species,
Cryptococcus neoformans, and
Staphylococcus aureus [
24]. Another antifungal agent, azoxybacillin (
9), was isolated from
Bacillus cereus NR2991 and was particularly effective against
Aspergillus fumigatus and
Trichophyton mentagrophytes [
25,
26]. Azoxyalkene (
10), an unstable azoxy compound, was discovered in
Actinomadura sp. (strain A7), which grows in apricot roots, though it only displayed weak antifungal activity against
Rhodotorula sp. [
21].
Elaiomycin (
11), an azoxy antibiotic isolated from
Streptomyces hepaticus, was found to strongly inhibit
Mycobacterium tuberculosis growth [
27,
28,
29,
30]. Elaomycins D–G (
12–
15), produced by
Streptomyces sp. HKI0708, exhibit antimicrobial, anti-mycobacterial, and cytotoxic activities [
31,
32]. Additionally, elaomycins K (
16), L (
17), and elaomycin K amide (
18), azoxy-type antibiotics found in
Streptomyces sp. Tü 6399, showed weak antibacterial effects against
Bacillus subtilis,
Staphylococcus lentus, and
Xanthomonas campestris, a plant pathogen [
33].
Antiparasitic antibiotics, particularly those targeting nematodes, are rare. Jietacins A (
19) and B (
20), isolated from
Streptomyces sp. [
34,
35], showed ten-times the activity of avermectin Bla, a veterinary nematicidal agent, against the pine wood nematode
Bursaphelenchus hylocomi [
36,
37]. Additionally, geralcin C (
21), D, and E, isolated from
Streptomyces sp. LMA-545, exhibited anticancer properties, with geralcin C showing an IC
50 of 0.8 μM against KB and HCT116 cell lines. It also inhibited
E. coli DnaG primase, a Gram-negative antimicrobial target, with an IC
50 of 0.7 μM [
38]. Lastly, propanosine (K-76,
22), identified in
Micromonospora chalcea 671-AV2 extracts, inhibited
Valsa ceratosperma growth [
39].
Lyophylline (
23) is an antibiotic obtained from the mushroom
Lyophyllum shimeji or its fungal endophytes. It has been found to prevent forebrain blister formation in the cranial mesenchyme at a concentration of 50 μg/mL [
40].
The antitumor antibiotics DC1881A (
24) and DC1881B (
25) are produced by
Streptomyces sp. DO-118 [
41]. Additionally, the azoxy compounds KA-7367A (
26) and KA-7367B (
27, activity see in
Table 2), which have antifungal properties, were discovered in
Streptomyces sp. (KC-7367, FERM BP-1277). KA-7367A (
26) showed activity against
Aspergillus fumigatus,
Candida albicans,
Cryptococcus neoformans,
Trichophyton mentagrophytes, and
T. rubrum [
42].
Calvacic acid (also called alvacic acid or calvatinic acid,
28) and its methyl derivatives (
29, see structure in
Figure 3, and
Table 3) belong to a group of antitumor antibiotics with a diazene N-oxide structure. These compounds are produced by the fungi
Calvatia craniformis (Agaricaceae) [
43],
Calvatia lilacina [
44], and
Lycoperdon pyriforme [
45]. Another antibiotic, clavulanic acid, inhibits the growth of Gram-positive and Gram-negative bacteria at concentrations of 3–6 μg/mL [
44]. It has cytotoxic effects, preventing the growth of Yoshida sarcoma cells [
43], and has shown carcinostatic activity against hepatoma and leukemia K562 cells [
46]. Clavulanic acid is also effective against
Helicobacter pylori, a Gram-negative bacterium associated with gastric infections [
47].
Amoxicillin–clavulanic acid is frequently used to treat many of the World Health Organization’s Priority Infectious Syndromes in adults and children. However, in some cases, a delayed antibiotic prescription or amoxicillin alone could be sufficient, reducing unnecessary use [
48,
49]. Additionally, clavulanic acid is being investigated for its potential in preventing and treating scopolamine-induced Alzheimer’s disease [
50]. Some of its analogues (
30 and
31) have been found to exhibit anti-microtubule activity [
51].
Azoformamide (
32), its (
E)-form (
33 and
35), and its azoxy derivatives (
34 and
36) have been isolated from the puffball fungus
Lycoperdon pyriforme [
52,
53] and have also been found in
Calvatia craniformis [
54]. A detection method for azoformamide in food additives has been developed [
55].
Table 1.
Biological activity of azo dyes produced by microorganisms (
1–
13) [
13,
24,
34].
Table 1.
Biological activity of azo dyes produced by microorganisms (
1–
13) [
13,
24,
34].
| No. | Dominated Activity | Rank | Additional Activity | Rank |
|---|
| 1 | Antineoplastic | Strong | Antibiotic | Strong |
| 2 | Antineoplastic | Strong | Hepatic disorders treatment | Strong |
| 3 | Antineoplastic | Strong | | |
| 4 | Antineoplastic | Moderate | | |
| 5 | Antiviral (Arbovirus) | Moderate | Antineoplastic | Moderate |
| 6 | Antineoplastic | Moderate | Antifungal | Weak |
| 7 | Antineoplastic | Moderate | Antifungal | Moderate |
| 8 | Mucositis treatment | Moderate | Antiviral (Arbovirus) | Moderate |
| 9 | Antifungal | Moderate | Antiviral (Arbovirus) | Moderate |
| 10 | Antiviral (Arbovirus) | Moderate | Antifungal | Moderate |
| 11 | Vasodilator | Moderate | Antifungal | Moderate |
| 12 | Antifungal | Moderate | Antiviral (Arbovirus) | Moderate |
| 13 | Antifungal | Moderate | Natural killer cell stimulant | Weak |
Extracts from the fungus
Lycoperdon pyriforme yielded two compounds, 4-methoxy-benzene-1-azoformamide (
33) and 4-methoxybenzene-1-OH N-azoxyformamide (
34), both of which were found to be effective against the parasitic nematode
Meloidogyne incognita. A related compound, 3,5-dichloro- 4-methoxybenzene-1-OH-azoxyformamide (
37), showed weaker nematicidal effects but higher cytotoxicity than compounds (
33) and (
34) [
56].
Azoxyformamides (
34 and
36) and two azoformamide derivatives (
38 and
39) were isolated from the fruiting bodies of
Calvatia craniiformis and
Lycoperdon hiemale, respectively. Compounds (
34) and (
39) inhibited rootlet growth in lettuce seedlings, suggesting that their azoxy structure contributes to this effect. Additionally, compounds (
34,
36, and
39) also inhibited the growth of pearl millet seedlings [
57,
58].
Fungi and their endophytes are known to produce colored pigments that contain azo groups. One such pigment, the red minor compound deoxyrubroflavin (
40), was isolated from the lump fungus
Calvatia rubro-flava [
59,
60]. Another pigment, the orange-colored rubroflavin (
41), was found in the dried fruiting bodies of
Calvatia rubro-flava and
Calvatia craniformis [
60,
61]. Additional pigments, including oxyrubroflavin (
42), craniformin (
43), and cranformin (
44, activity see in
Table 4), were also identified in
Calvatia rubro-flava [
60,
61,
62].
The insect-pathogenic fungus
Entomophthora virulenta produces a mixture of 4,4′-azoxybenzenedicarboxylic acid (
45) and 4,4′-hydroxymethylazoxybenzene carboxylic acid (
46), both of which have insecticidal properties [
63,
64]. A formazan derivative (
47) was isolated from
Agaricus silvicola [
64]. Another notable fungal compound, necatorin (
48), was discovered in
Lactarius necator. This mutagenic alkaloid contains an azo group within a polycyclic aromatic structure that includes a 1-benzopyran moiety and a ketone group at carbon C2 [
65,
66,
67,
68].
Table 2.
Biological activity of azo dyes produced by microorganisms (
14–
27) [
13,
36,
37,
41].
Table 2.
Biological activity of azo dyes produced by microorganisms (
14–
27) [
13,
36,
37,
41].
| No. | Dominated Activity | Rank | Additional Activity | Rank |
|---|
| 14 | Antifungal | Moderate | Natural killer cell stimulant | Weak |
| 15 | Vasodilator, peripheral | Weak | Antineoplastic | Weak |
| 16 | Antiviral (Arbovirus) | Strong | Mucositis treatment | Moderate |
| 17 | Pre-neoplastic | Strong | Mucositis treatment | Moderate |
| 18 | Mucositis treatment | Moderate | Natural killer cell stimulant | Weak |
| 19 | Hepatic disorders treatment | Strong | Antifungal | Weak |
| 20 | Hepatic disorders treatment | Strong | Antifungal | Weak |
| 21 | Antineoplastic | Strong | Antibiotic | Moderate |
| 22 | Antineoplastic | Strong | Antiviral (Picornavirus) | Moderate |
| 23 | Antineoplastic | Strong | Antiviral (Arbovirus) | Weak |
| 24 | Antineoplastic | Strong | Antibacterial | Moderate |
| 25 | Antineoplastic | Strong | Antibacterial | Moderate |
| 26 | Hepatic disorders treatment | Moderate | Antifungal | Moderate |
| 27 | Hepatic disorders treatment | Strong | Antibacterial | Moderate |
The azo dyes 4,4′-dihydroxyazobenzene (
49) and its methyl derivative (
50) were detected in fresh sporophores of
Agaricus xanthodermus and exhibited antimicrobial activity against
Staphylococcus species [
69,
70,
71]. Several fungi, including
Beauveria bassiana,
Beauveria brongniartii,
Metarhizium anisopliae, and
Verticillium lecanii, produce azo dye toxins (
51–
57) [
72,
73,
74,
75,
76].
Additionally, three aromatic azoxy compounds—azoxymycins A (
58), B (
59), and C (
60)—were discovered in
Streptomyces chattanoogensis L10 and
Streptomyces sp. strain P8-A2 [
77,
78,
79]. Azoxymycin B demonstrated cytotoxicity against human leukemia HL-60 cells, with an IC
50 value of 2.2 μM [
78].
Asterionellins A (
61), B (
62), and C (
63) are eight-membered compounds with an azoxy-like structure, discovered in the microalga
Asterionella sp. [
80,
81,
82]. In the fruiting bodies of
Agaricus xanthoderma, researchers identified an unstable agaritine derivative (
64) and a metabolite (
65) [
83]. Another compound, glutamylase phenol (
62), was also found in
Agaricus sp. [
84]. Among these, compound (
65) displayed strong antibiotic and anticancer properties [
84,
85].
Table 3.
Biological activity of azo dyes produced by microorganisms (
28–
41) [
13,
44,
47].
Table 3.
Biological activity of azo dyes produced by microorganisms (
28–
41) [
13,
44,
47].
| No. | Dominated Activity | Rank | Additional Activity | Rank |
|---|
| 28 | Anti-Helicobacter pylori | Strong | | |
| 29 | Anti-Helicobacter pylori | Strong | | |
| 30 | Anti-Helicobacter pylori | Strong | Fibrinolytic | Weak |
| 31 | Anti-Helicobacter pylori | Strong | Pre-neoplastic conditions treatment | Weak |
| 32 | Anti-inflammatory | Strong | Hemostatic | Weak |
| 33 | Anti-inflammatory | Strong | Hemostatic | Weak |
| 34 | Anti-Helicobacter pylori | Strong | | |
| 35 | Anti-Helicobacter pylori | Strong | Pre-neoplastic conditions treatment | Weak |
| 36 | Anti-Helicobacter pylori | Strong | | |
| 37 | Anti-Helicobacter pylori | Strong | Pre-neoplastic conditions treatment | Weak |
| 38 | Apoptosis agonist | Strong | Antineoplastic | Strong |
| 39 | Apoptosis agonist | Strong | Antineoplastic | Strong |
| 40 | Anti-inflammatory | Strong | Antineoplastic | Strong |
| 41 | Hemostatic | Strong | Anti-inflammatory | Strong |
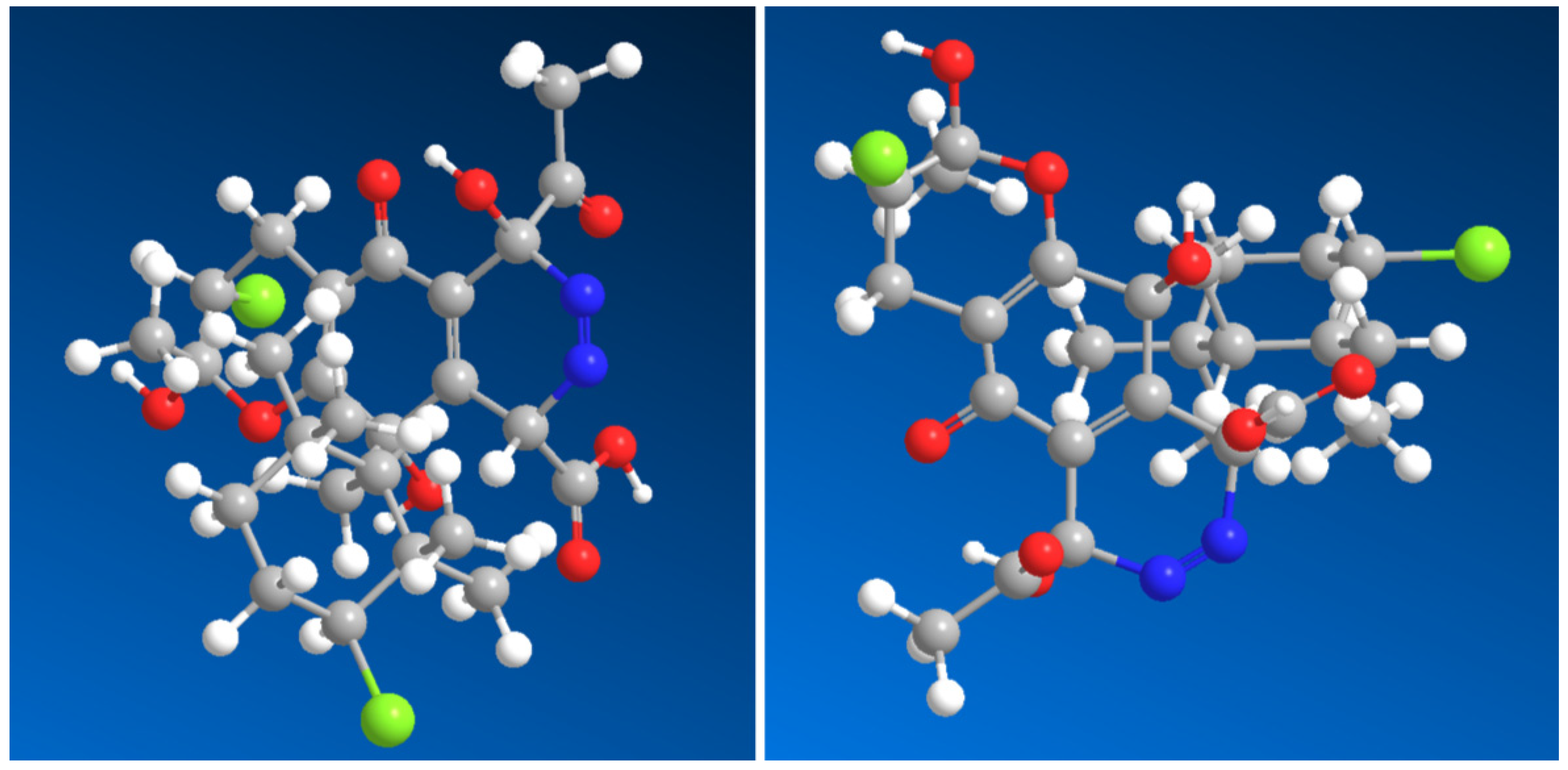
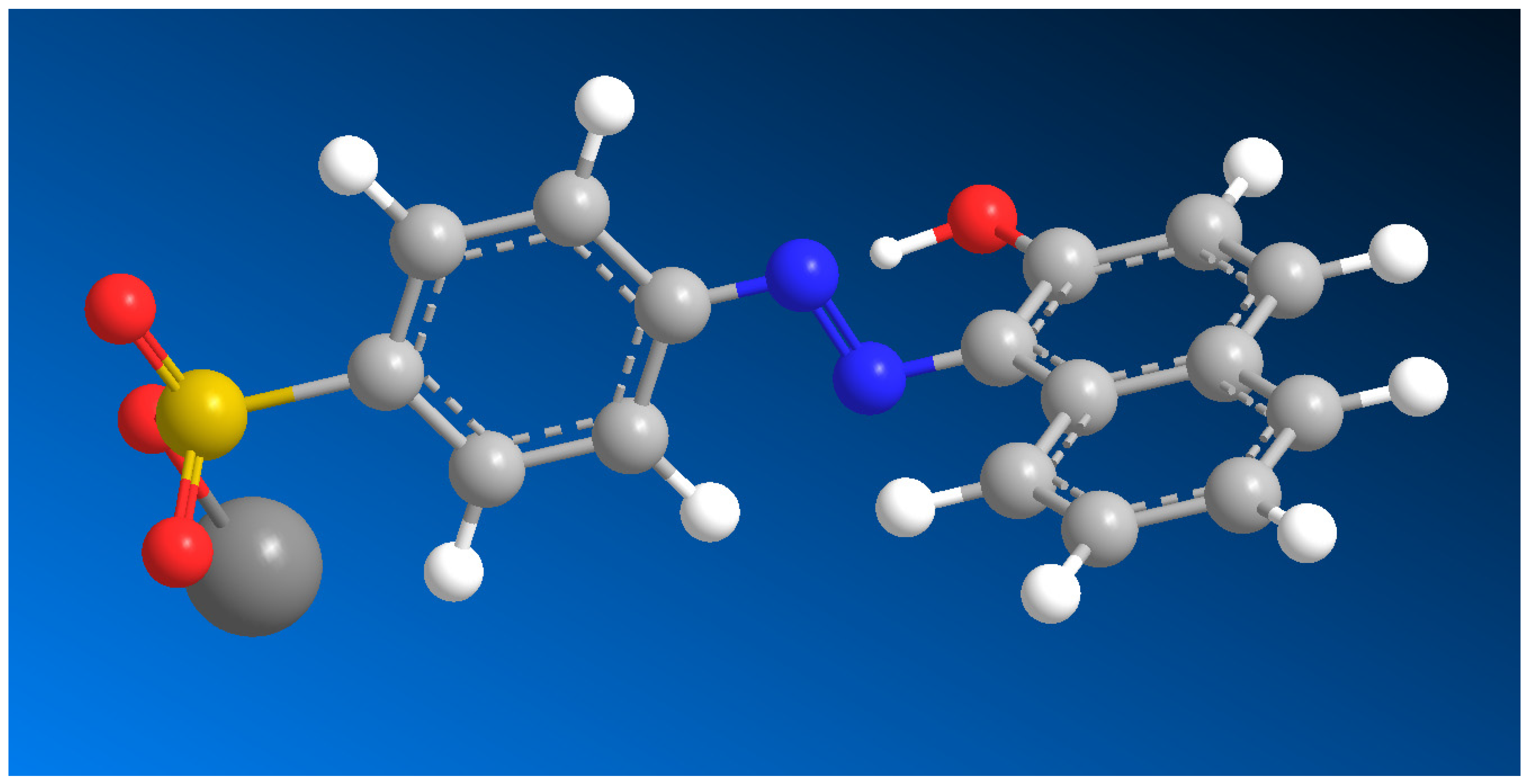
Azamerone, an anti-infective meroterpenoid obtained from marine
Streptomyces sp. grown in salt culture, features a unique chloropyranphthalazinone core with a distinctive side chain. It showed mild cytotoxic effects on mouse T-cell splenocytes and macrophages in laboratory tests. Additionally, the biosynthetic precursor of its azo-containing derivative (
66, 3D structure see in
Figure 4) was also detected in the same
Streptomyces sp. strain [
86,
87]. The Gram-positive bacterium
Streptomyces sp. Ank75 produces azoxy antibiotics
67 and
68, which have antifungal properties against
Candida albicans and
Mucor miehei [
88].
Researchers identified two vasodilators, WS-1228 A (triaxin C,
69) and WS-1228 B (triaxin D,
70), in the culture filtrate of
Streptomyces aureofaciens [
89,
90]. A few years later, Omura and colleagues [
91] discovered triaxins A (
69) and B (
70) in
Streptomyces sp. SK-1894 and found that they function as acyl-CoA synthetase inhibitors. These compounds, along with WS-1228 A and B, share a unique N-hydroxytriazene structure and are known for their vasodilatory effects. Additionally, they inhibit acyl-CoA synthetase, with IC
50 values of 5.5 μg/mL for triaxin A and 3.6 μg/mL for WS-1228 A. The triaxins (A, B, C, and D) differ in their inhibition levels, with triaxin C (
71) being the most potent, followed by triaxin A, triaxin D (
72), and triaxin B [
92,
93].
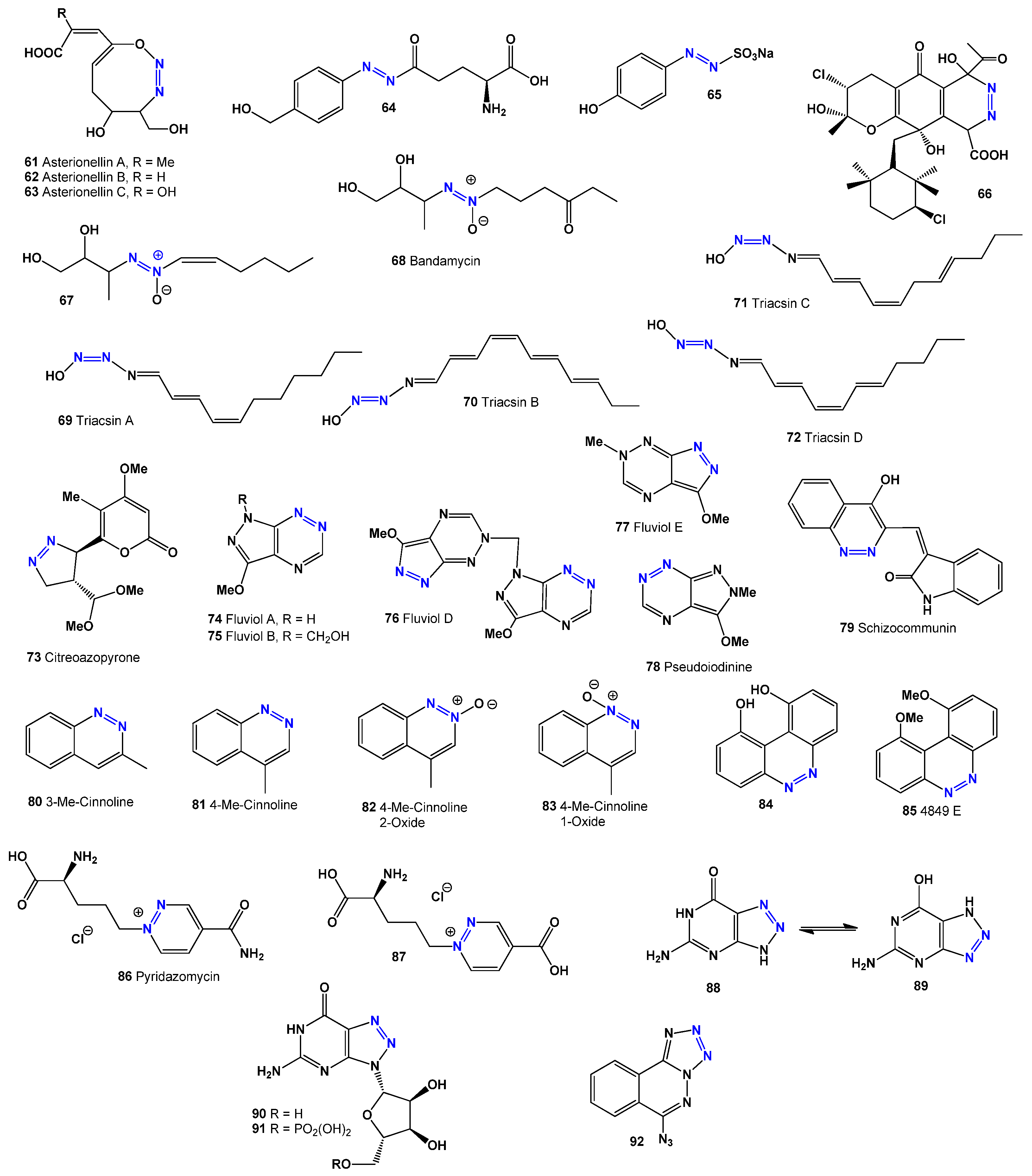
A rare compound, citreoazopyrone (
73), was isolated from a hybrid strain of
Penicillium citreo-viride and was found to inhibit lettuce seedling growth [
94]. The fluviol groups of antibiotics (
74–
77) and pseudoiodine (
78), produced by
Pseudomonas fluorescens and
Nostoc spongiaeforme, exhibit antimicrobial, antiviral, and antitumor effects [
95,
96,
97,
98]. The activities of compounds
78–
98 are detailed in
Table 5. Additionally, schizocommunin (
79), isolated from the fungus
Schizophyllum commune, showed strong cytotoxic effects against mouse lymphoma cells [
99].
Table 4.
Biological activity of azo dyes produced by microorganisms (
42–60) [
13,
72,
73,
74,
75,
76,
77,
78].
Table 4.
Biological activity of azo dyes produced by microorganisms (
42–60) [
13,
72,
73,
74,
75,
76,
77,
78].
| No. | Dominated Activity | Rank | Additional Activity | Rank |
|---|
| 42 | Anti-inflammatory | Strong | Hemostatic | Strong |
| 43 | Anti-inflammatory | Strong | Hemostatic | Strong |
| 44 | Anti-inflammatory | Strong | Hemostatic | Strong |
| 45 | Kidney function stimulant | Weak | Pre-neoplastic conditions | Weak |
| 46 | Immunosuppressant | Weak | Fibrinolytic | Weak |
| 47 | Pre-neoplastic conditions | Weak | Kidney function stimulant | Weak |
| 48 | Anti-mutagenic | Strong | Spasmolytic, urinary | Weak |
| 49 | Anti-seborrheic | Strong | | |
| 50 | Carminative | Strong | Anti-microbial | Moderate |
| 51 | Phobic disorders treatment | Strong | Kidney function stimulant (0.786) | Moderate |
| 52 | Fibrinolytic | Moderate | Pre-neoplastic conditions | Moderate |
| 53 | Acaricide | Strong | Anti-seborrheic | Strong |
| 54 | Alopecia treatment | Moderate | Anti-inflammatory, intestinal | Weak |
| 55 | Anti-seborrheic | Strong | Acaricide | Moderate |
| 56 | Pre-neoplastic conditions treatment | Weak | Immunosuppressant | Weak |
| 57 | Fibrinolytic | Moderate | Acaricide | Weak |
| 58 | Mucositis treatment | Strong | Immunosuppressant | Weak |
| 59 | Mucositis treatment | Moderate | Immunosuppressant | Weak |
| 60 | Antiviral(Arbovirus) | Weak | Immunosuppressant | Weak |
Researchers identified 3- and 4-methylcinnolines (
80 and
81) in the volatile components of
Hibiscus esculentus pods [
100], while azoxy compounds (
82 and
83) were found in yeast extract [
101]. Two cinnoline derivatives, compound
84 and 4849F (
85), were isolated from
Streptomyces sp. 4849.171. Compound
84 functions as an IL-4 receptor inhibitor, while 4849F (
85) shows antibacterial properties [
102].
Pyridazomycin (
86), an antifungal antibiotic from
Streptomyces violaceoniger sp.
griseofuscus, inhibits
Mucor hiemalis [
103,
104]. Both pyridazomycin (
86) and its chloride salt analogue (
87) exhibit antimicrobial activity [
105]. The compound 5-amino-1,4-dihydro-7H-1,2,3-triazolo [4,5-d]-pyrimidin-7-one (
88 or
89), also called 8-azaguanine, is produced from guanine by
Streptomyces albus [
106]. Its cytotoxic effects on various cancers, including carcinoma and sarcoma, were first reported over 70 years ago [
107].
Another form of 8-azaguanine, known as pathocidin (
89), is an antifungal antibiotic obtained from Actinomycetes [
108,
109,
110], which effectively inhibits fungal growth, including
Penicillium chrysogenum. Two related natural metabolites, 8-azaguanine-3N-β-D-ribofuranosyl (
90) and its 5′-phosphate version (
91, see structure in
Figure 5), have demonstrated anticancer effects against lymphoid leukemia and adenocarcinoma [
111].
The red dinoflagellate
Gymnodinium breve (syn.
Ptychodiscus brevis) produces the antibiotic 6-azidotetrazolo [5,1-a]phthalazine (
92) [
112]. Additionally, the pathogenic Gram-negative
Burkholderia species secretes extracellular enzymes involved in various biological processes, as well as toxins, antibiotics, and siderophores [
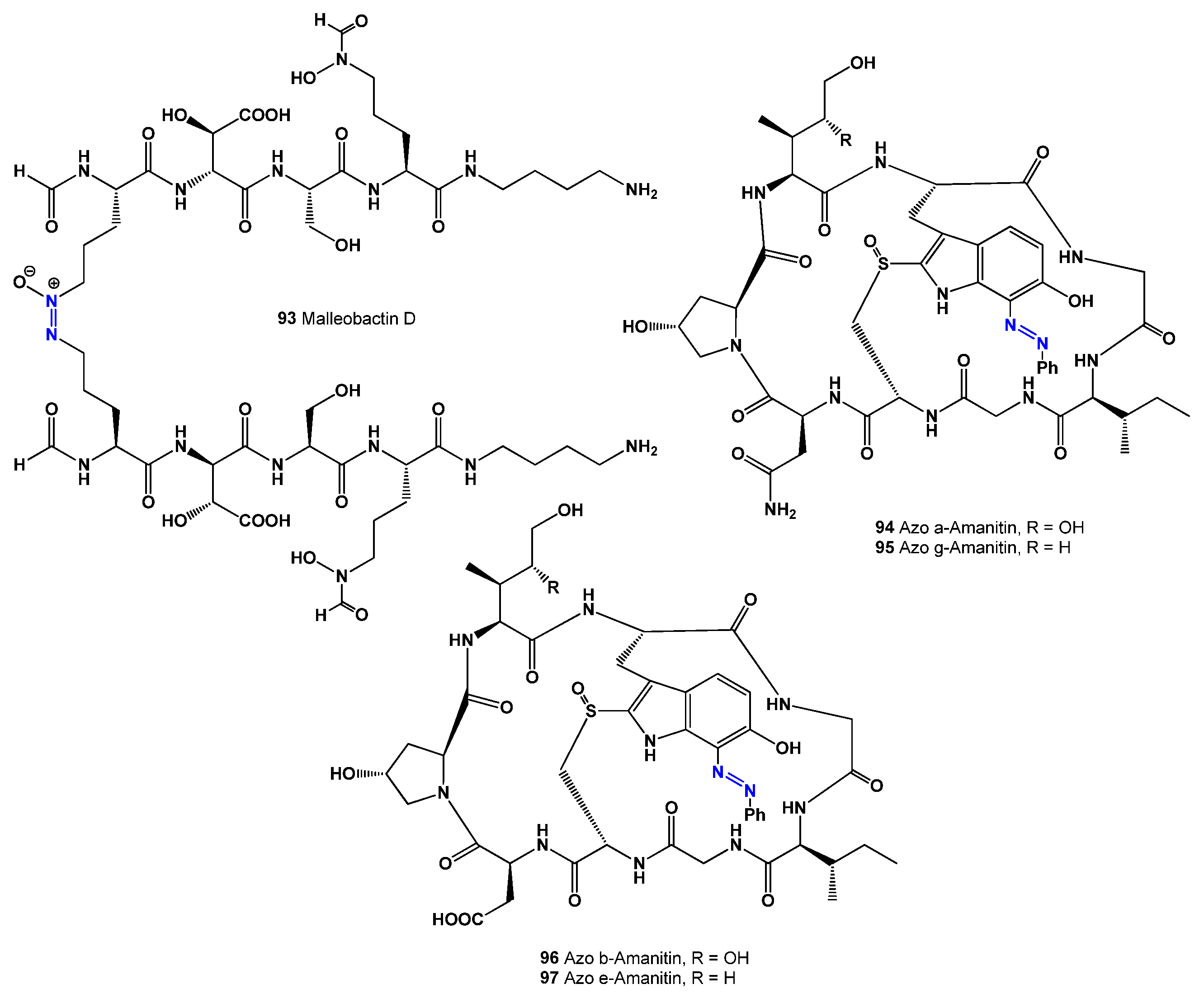
113]. A rare dimeric siderophore, malleobactin D (
93, see structure in
Figure 6, and activity shown in
Table 6), was identified in
Burkholderia pseudomallei (formerly
Pseudomonas pseudomallei) [
114].
Amatoxins are toxic bicyclic octapeptides found in several fungi from the Agaricales family, including
Amanita phalloides,
A. ocreata,
A. verna,
A. bisporigera,
Conocybe filaris, and
Galerina marginata (syn.
G. autumnalis), among others. These toxins are known for their harmful effects, including heat-induced toxicity, gastrointestinal issues, and the ability to inhibit RNA polymerase II [
115,
116,
117,
118]. Azoamanitins (
94–
97), which are semi-synthetic versions of amatoxins, have been studied for their potential in antiviral, antimicrobial, and anticancer treatments [
119,
120,
121].
3. Azo Dyes Derived from Terrestrial and Marine Sources
Azo dyes have been found in both terrestrial and marine environments, but it remains unclear whether bacteria or fungal endophytes played a role in their production, as these microorganisms often act as symbionts in plants and invertebrates [
1,
2,
3,
4,
8,
13,
79]. Some key compounds isolated from terrestrial and marine sources include the following:
Geleganidin D (
98, see structure in
Figure 7 and activity in
Table 7): A dimeric monoterpenoid indole alkaloid from
Gelsemium elegans with moderate cytotoxic activity against MCF-7 and PC-12 cells [
122].
G. elegans is used in clinical applications due to its indole alkaloids, which have antitumor, anti-inflammatory, analgesic, and immune-modulatory effects, though its therapeutic dose is close to toxic levels [
122].
Brachystemidin G (
99): An alkaloid from
Brachystemma calycinum with potent immunosuppressant properties, inhibiting murine T- and B-lymphocyte proliferation (IC50 = 5.6 µg/mL) [
123].
1,2,4-Triazine derivative (
100): Extracted from
Butea monosperma (family Caryophyllaceae) [
124]. This plant’s odor repels mosquitoes, its flowers are used as a dye, and its gum, known as kamarkas in Hindi, is used in traditional cooking [
125].
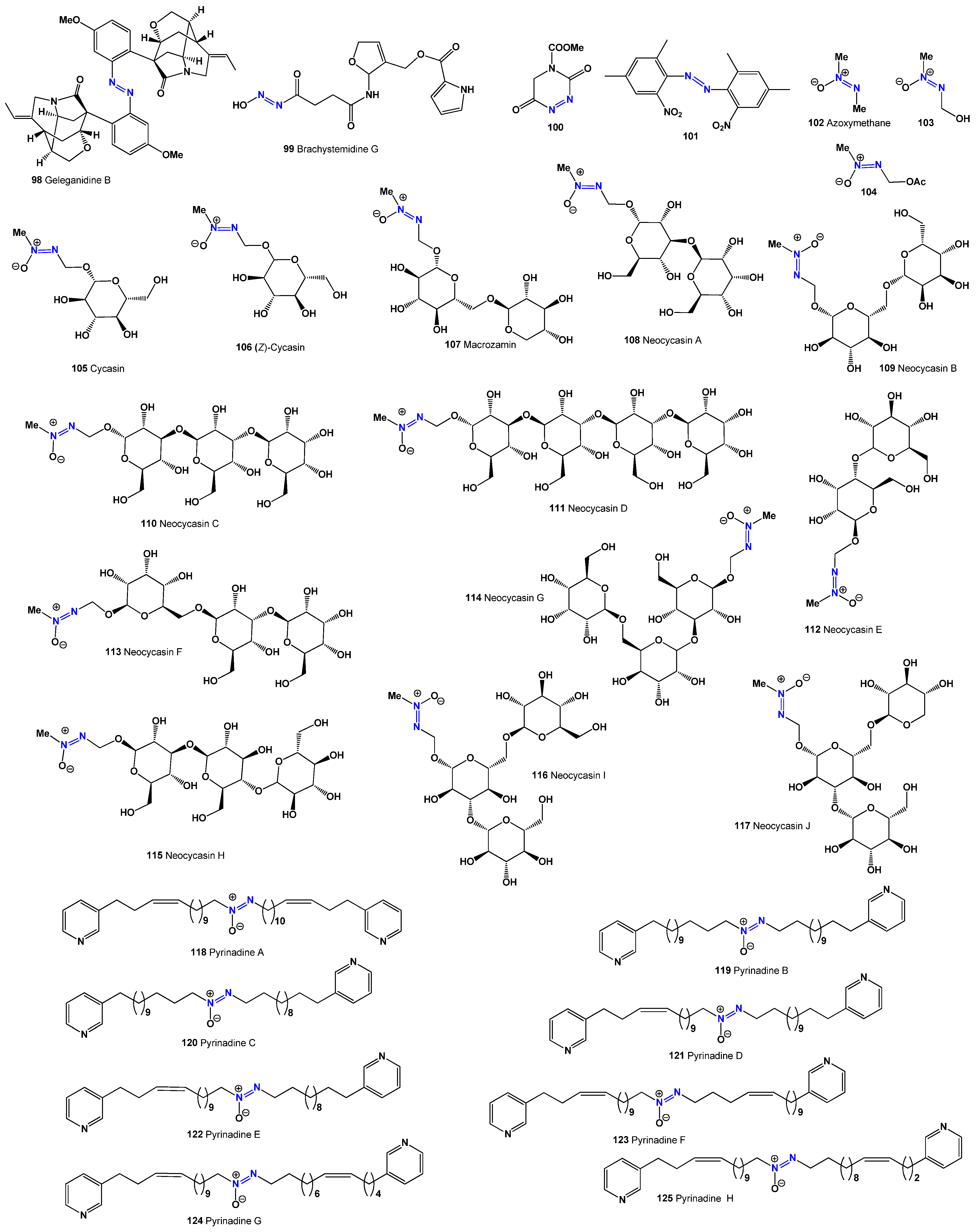
bis-(2,4-Dimethyl-6-nitrophenyl)-diazene (
101): Isolated from the leaves of
Aconitum sungpanense [
126]. Azoxyglycosides, which contain the aglycone methylazoxymethanol (MAM), have been found in Cycadaceae plants, with all known glycosides having β-glycosidic linkages [
127]. Metabolites, such as methylazoxymethane (
102), MAM (
103), methylazoxymethanol acetate (
104), and cycasins (
105,
106), have been extracted from Cycadaceae, Stangeriaceae, and Zamiaceae, which are coniferous trees found in tropical and subtropical regions [
127,
128,
129,
130,
131,
132]. MAM (
103) has been linked to liver and kidney carcinomas [
133], while cycasins (
105,
106) and macrosamine (
107) are highly toxic azoxyglycosides. Various insects, including caterpillars and weevils, feed on cycads’ roots, stems, leaves, and reproductive parts [
127,
132].
Azoxyglycosides are believed to function as natural herbivore deterrents [
134,
135,
136]. Cycasin, the primary azoxyglycoside found in cycads, has been shown to cause effects similar to those seen in carcinogenicity, mutagenicity, and neurotoxicity tests. The first isolation of neocycasin A (
108) was recorded in 1959 [
137], and, more recently, neocycasins B–J (
109–
117) have been identified from different plant species [
138,
139,
140,
141,
142,
143,
144,
145,
146].
The first cytotoxic
bis-3-alkylpyridine alkaloid with an azoxy moiety, pyrinadine A (
118), was discovered in the Okinawan marine sponge
Cribrochalina sp. [
147,
148]. Further cytotoxic
bis-3-alkylpyridine alkaloids, pyrinadines B–H (
118–
125), were later extracted from the same sponge, demonstrating cytotoxicity against P388 murine leukemia cells [
147,
148,
149]. The activities of these compounds (
118–
125) are summarized in
Table 7.
Table 7.
Biological activity of azo dyes produced by microorganisms (
98–
125) [
13,
122,
137,
144].
Table 7.
Biological activity of azo dyes produced by microorganisms (
98–
125) [
13,
122,
137,
144].
| No. | Dominated Activity | Rank | Additional Activity | Rank |
|---|
| 98 | Antineoplastic | Moderate | Antiprotozoal (Plasmodium) | Weak |
| 99 | β-1,3-Galactosyl-O-glycosyl-glycoprotein | Strong | β-1,6-N-acetylglucosaminyl
transferase inhibitor | Strong |
| 100 | Neurodegenerative diseases treatment | Strong | | |
| 101 | Antiparkinsonian | Strong | Anxiolytic | Strong |
| 102 | Acaricide | Moderate | Antiviral (Arbovirus) | Weak |
| 103 | Antineoplastic | Strong | | |
| 104 | Antineoplastic | Moderate | | |
| 105 | Antineoplastic | Moderate | | |
| 106 | Anti-infertility, female | Strong | Antineoplastic | Strong |
| 107 | Carcinogenic | Strong | Embryotoxic | Strong |
| 108 | Carcinogenic | Strong | Embryotoxic | Strong |
| 109 | Antineoplastic | Strong | | |
| 110 | Carcinogenic | Strong | Embryotoxic | Strong |
| 111 | Antineoplastic | Strong | | |
| 112 | Antineoplastic | Strong | Anti-infective | Strong |
| 113 | Antineoplastic | Strong | Anti-infective | Strong |
| 114 | Antineoplastic | Strong | Anti-infective | Strong |
| 115 | Antineoplastic | Strong | Anti-infective | Strong |
| 116 | Antineoplastic | Strong | Anti-infective | Strong |
| 117 | Antineoplastic | Strong | Anti-infective | Strong |
| 118 | Cytotoxic | Moderate | Anti-inflammatory | Weak |
| 119 | Cytotoxic | Moderate | Anti-inflammatory | Weak |
| 120 | Cytotoxic | Moderate | Anti-inflammatory | Weak |
| 121 | Cytotoxic | Moderate | Anti-inflammatory | Weak |
| 122 | Cytotoxic | Moderate | Anti-inflammatory | Weak |
| 123 | Cytotoxic | Moderate | Anti-inflammatory | Weak |
| 124 | Cytotoxic | Moderate | Anti-inflammatory | Weak |
| 125 | Cytotoxic | Moderate | Anti-inflammatory | Weak |
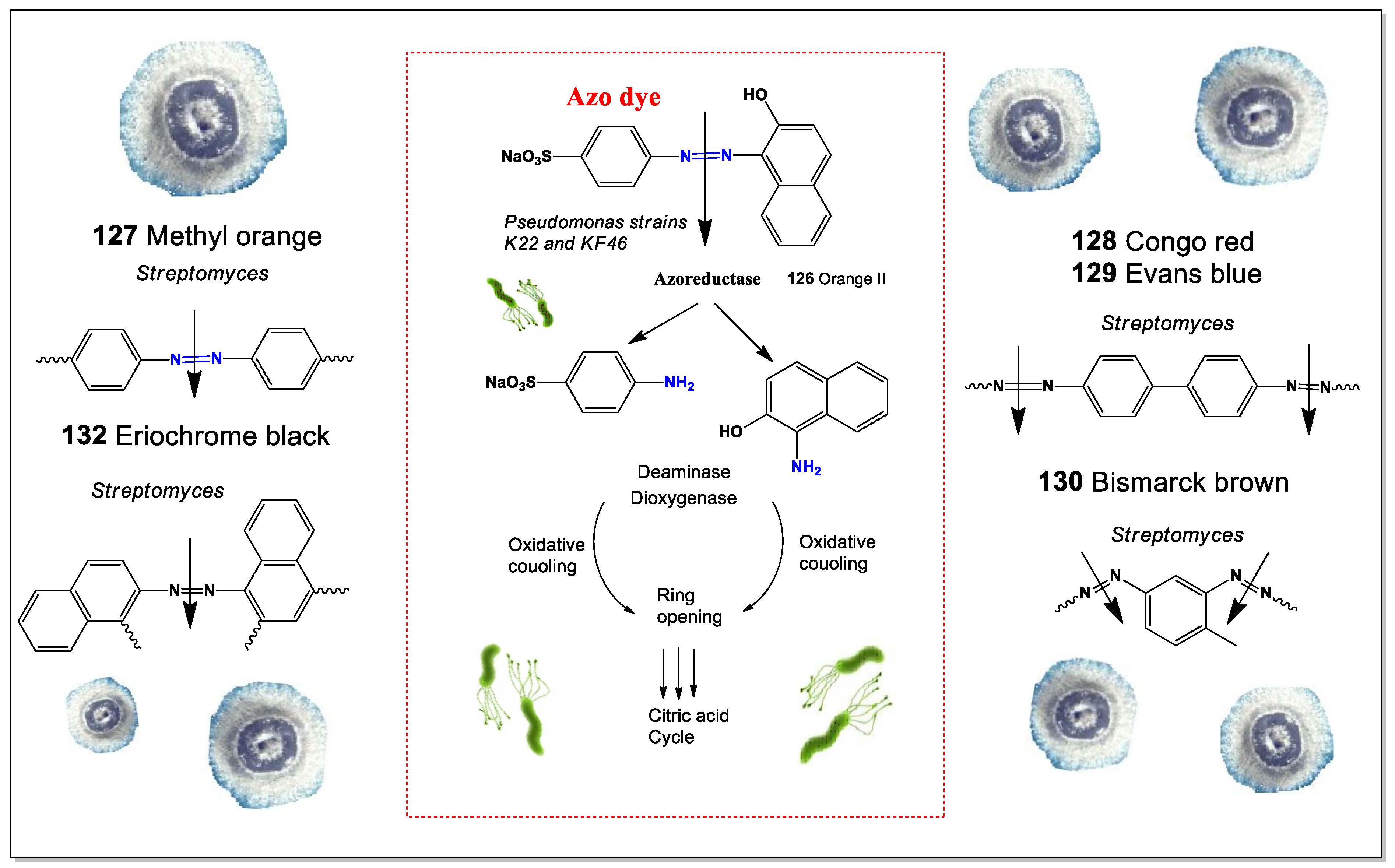
5. Microbial Degradation of Food Azo Dyes
Azo dyes are widely used in the food industry as color additives in products, such as soft drinks, jams, candies, and pickles (azo dye coloring in
Figure 11) [
170]. Their popularity comes from their ability to produce bright, vibrant colors while being inexpensive, stable, and free from unwanted tastes or odors.
However, while azo dyes make food visually appealing, they may also pose health risks. Consuming them within the acceptable daily intake is generally considered safe, but higher doses—especially in children—have been linked to various health issues. These include allergies, ADHD, asthma, anxiety, and even potential cytotoxic and genotoxic effects, which may contribute to cancer development. Additionally, azo dyes are highly stable and do not break down easily under aerobic conditions [
171,
172,
173].
Several synthetic azo dyes, such as Sunset Yellow (
133, see structure in
Figure 12, and activity see in
Table 9), Carmoisine (
134), Amaranth (
135), Ponceau 4R (
136), Brown HT (
137), and Allura Red (
138), are commonly added to food [
170]. They enhance color but offer no nutritional benefits, preservation properties, or health advantages. Because they are cost-effective, chemically stable, and provide intense coloration without altering taste, the food industry often prefers them over natural dyes.
Although food dyes are regularly tested for safety, their health effects remain controversial. Some studies suggest that the breakdown of azo dyes can produce harmful byproducts, raising concerns about their long-term safety [
174,
175].
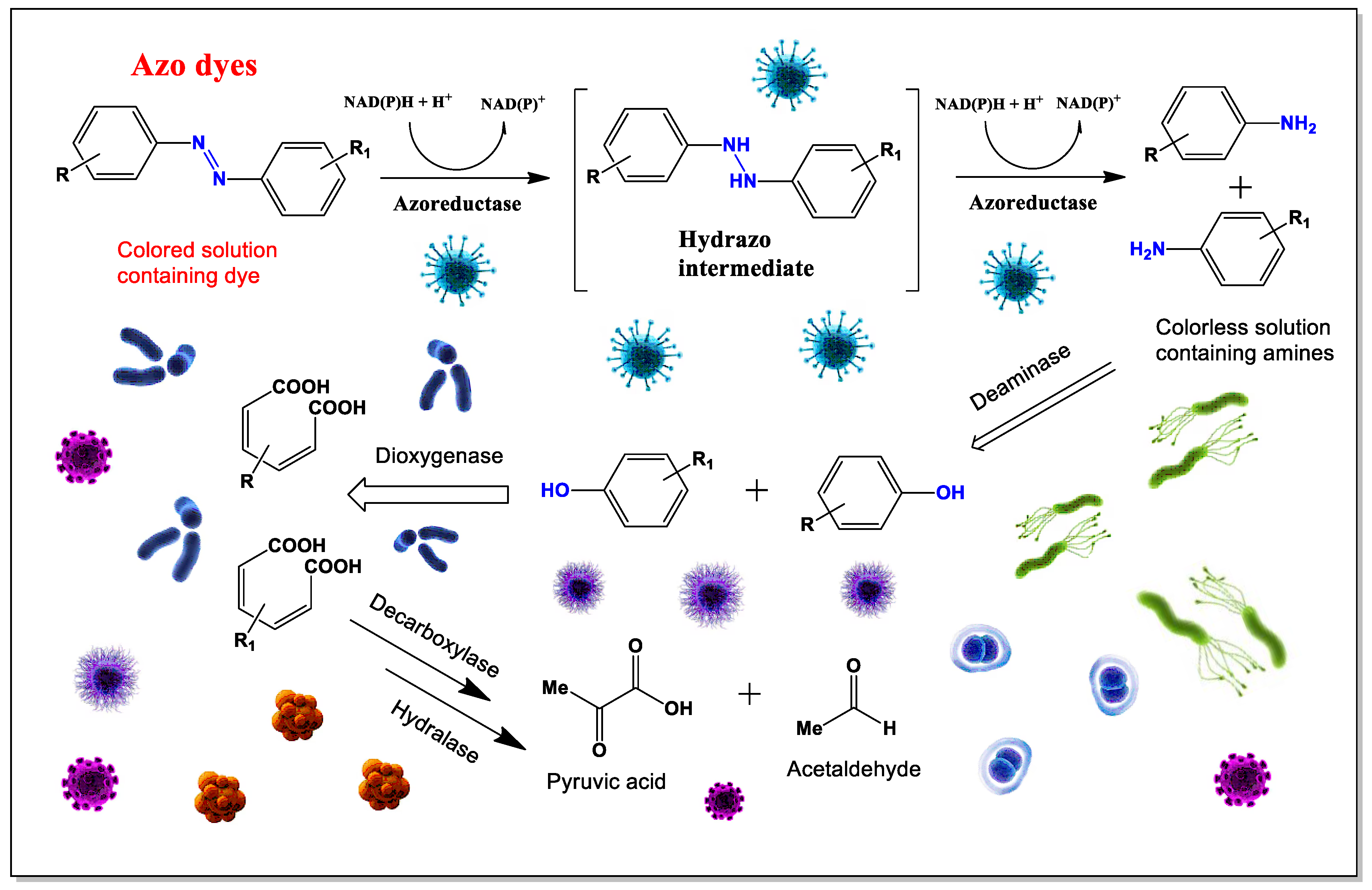
Food azo dyes may have potential health risks, primarily due to their breakdown by intestinal bacteria after ingestion. When these dyes are reduced by gut microflora, their azo bonds are cleaved, producing aromatic amines. These amines can then undergo further chemical modifications, such as N-hydroxylation or N-acetylation. In mammals, azo dye reduction mainly takes place in the anaerobic environment of the lower gastrointestinal tract, where bacterial azoreductase enzymes are responsible for the process. While mammalian enzymes in the liver and kidney also contribute to azo dye metabolism, their exact role is not yet fully understood [
176,
177].
Many anaerobic bacteria found in the cecal contents of both humans and experimental animals have been shown to break down azo dyes into aromatic amines. This reaction is facilitated by azoreductases, which require flavins for optimal activity and are highly sensitive to oxygen [
172,
173,
174,
175,
176,
177].
Aromatic amines and their derivatives are a major class of chemicals formed when azo dyes are broken down by azoreductases, which cleave the azo bond. Different microorganisms produce azoreductases with varying kinetic properties and substrate specificities. Research shows that flavin-containing azoreductases follow a Ping-Pong bi-bi reaction mechanism, where the enzyme’s activity depends on the substrate concentration. Many azoreductases show a higher affinity for NADPH over NADH, although they can utilize either as an electron source, with or without flavin [
178,
179,
180].
Not all bacteria produce azoreductases, but several known species do. These include strains from both animals and humans, such as
Aquiflexum sp.,
Bacillus sp.,
Clostridium perfringens,
Escherichia coli,
Enterococcus faecalis,
Pseudomonas aeruginosa,
Rhodobacter sphaeroides,
Shewanella oneidensis,
Staphylococcus aureus, and
Xenophilus azovorans KF46F [
178,
181]. Several azo dyes are commonly used in food production:
Sunset Yellow (
133) is an artificial dye used to enhance food appearance and shelf life. It has antioxidant properties, which increase with concentration, but also exhibits phytotoxicity [
182,
183,
184].
Carmoisine (
134) is a red azo dye used in foods that undergo heat treatment after fermentation. While azo dyes have been linked to carcinogenicity, especially bladder cancer, carmoisine itself has not been proven carcinogenic. Interestingly, its copper complex has demonstrated anticancer activity against colon cancer cells (HT-29), making it a potential candidate for future treatments [
185,
186,
187].
Amaranth (
135) is a red food dye used worldwide, although its use is banned in India due to concerns about its safety. Despite this, illegal production continues, with India contributing 2% of global output. Some studies suggest that amaranth may be carcinogenic under certain conditions [
188,
189].
Ponceau 4R (
136) is widely used in pharmaceuticals and food products. It is stable in light, heat, and acidic conditions but breaks down when exposed to ascorbic acid. While approved in Europe, Asia, and Australia, the US FDA has not approved it due to concerns about its potential to form non-sulfone aromatic amines, which may be carcinogenic [
190,
191].
Brown HT (
137), also called “food brown 3” or “chocolate brown HT”, is a pigment with antioxidant properties. It is found in small amounts in coal tar [
192,
193,
194].
Allura Red (
138) is a widely used food dye, commonly found in sweets, beverages, and processed foods. It dissolves in water but poorly in 50% ethanol. Long-term consumption may cause liver and kidney toxicity [
195,
196,
197]. Degradation of azo dyes and the cleavage of the (-N=N-) bond are shown in
Figure 13.