The Role of Microbial Dynamics, Sensorial Compounds, and Producing Regions in Cocoa Fermentation
Abstract
1. Introduction
2. Cocoa Fermentation Processes
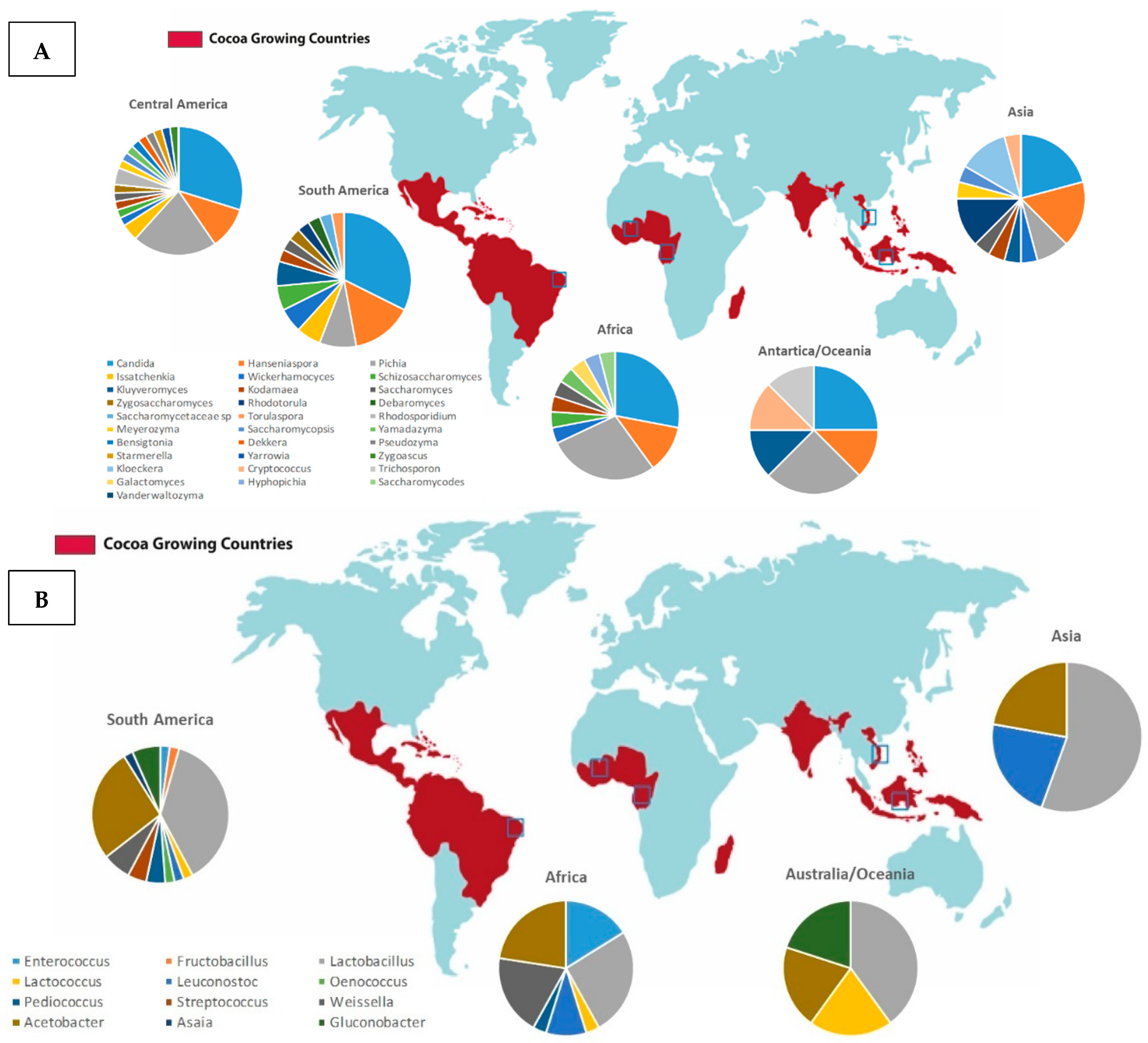
3. Microorganisms
3.1. Yeasts
3.2. Lactic Acid Bacteria
3.3. Acetic Acid Bacteria
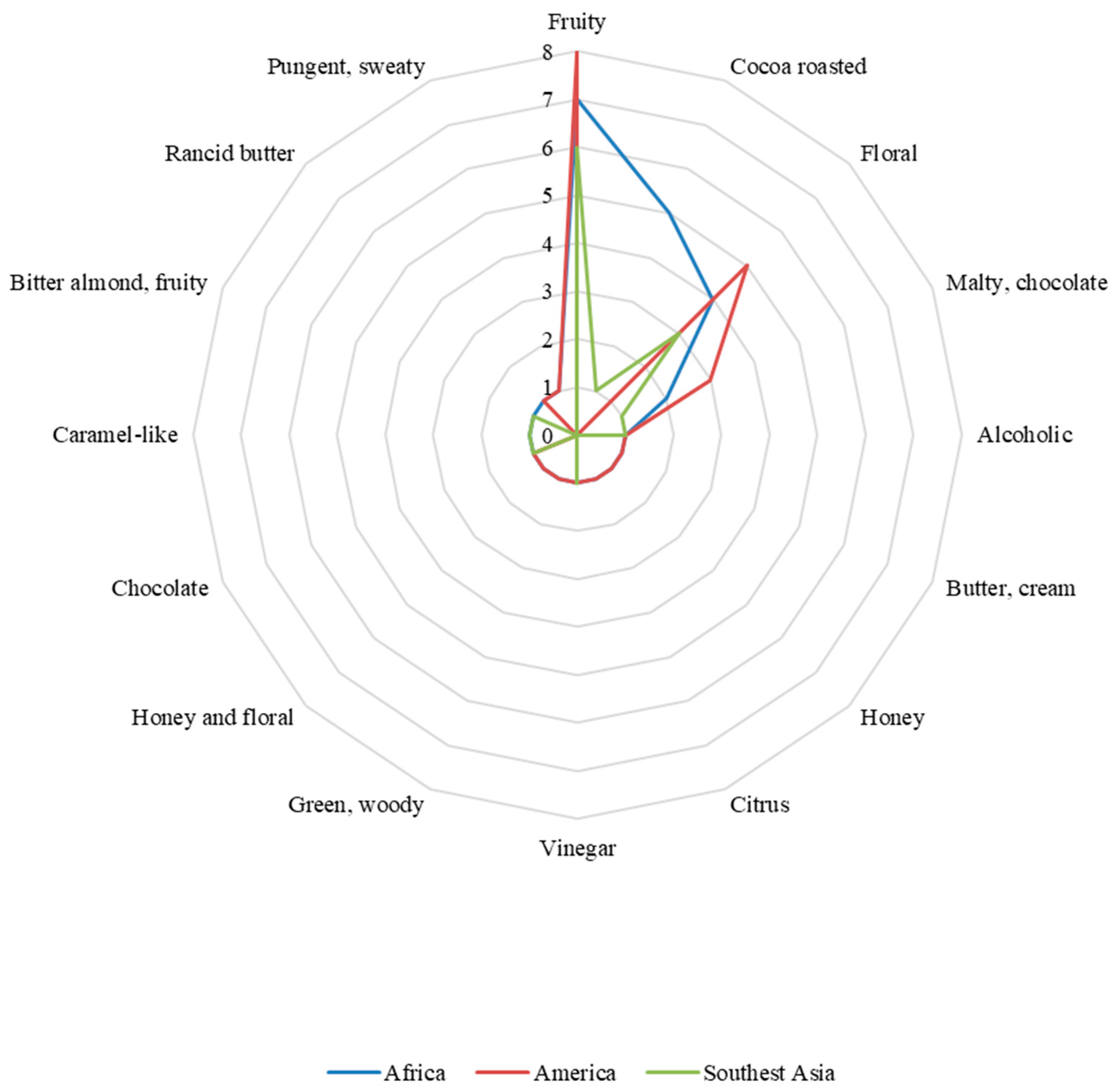
4. Regional Influence in the Sensorial Profile of Fermented Cocoa

5. Starter Cultures in CB Fermentation
| Starter Microorganisms | Study Objective | Key Findings | Unique Outcome | Ref. |
|---|---|---|---|---|
| Saccharomyces cerevisiae, Torulaspora delbrueckii | Effect of mixed cultures on Brazilian hybrid cocoa clones | T. delbrueckii dominated early; S. cerevisiae dominated later, enhancing flavor and aroma profiles. | Improved flavor | [133] |
| Pichia kudriavzevii, Hanseniaspora thailandica, Hanseniaspora opuntiae, Wickerhamomyces spp., S. cerevisiae | Impact of native yeast starters on antioxidant activity in Malaysian cocoa | Higher polyphenol and flavonoid content in H. thailandica and P. kudriavzevii compared to control. | Increased antioxidants | [134] |
| S. cerevisiae IMDO 050523, P. kudriavzevii IMDO 020508 | Monitoring starter cultures during fermentation and drying stages | The use of starter cultures extended fermentation by one additional day but increased production of desired metabolites, especially volatile organic compounds. | Enhanced aroma | [135] |
| Lactobacillus plantarum, Pediococcus acidilactici, Pichia fermentans | Co-culturing fructophilic lactic acid bacteria and yeast to enhance sugar metabolism and aroma formation during fermentation | Mixed cultures reduced residual sugars and suppressed wild microflora, suggesting these microbes optimize fermentation and aromatic profiles. | Enhanced color and flavor | [127] |
| S. cerevisiae, Pichia kudriavzevii | Effect of yeast strains on volatile compounds in Colombian cocoa | P. kudriavzevii increased desirable compounds in chocolate, suggesting mixed cultures could improve cocoa quality. | Unique regional identity | [136] |
| L. plantarum, S. cerevisiae, Acetobacter pasteurianus | Physiological characterization of starter cultures in cocoa fermentation | Microbial populations increased rapidly under controlled fermentation conditions, achieving higher concentrations by day 2–3 compared to natural fermentation. | Faster microbial growth | [137] |
| Torulaspora delbrueckii, Hanseniaspora uvarum, Limosilactobacillus plantarum, Acetobacter ghanensis | Inoculation of native microbial starter cultures to optimize fine-aroma cocoa bean fermentation | Starter cultures at various stages increased polyphenol content, improved efficiency by 24%, and decreased sugars and acids, allowing drying in 96 h. They also reduced filamentous fungi that could negatively affect flavor and aroma. | Decreased drying time and enhanced flavor | [14] |
| Hanseniaspora opuntiae, S. cerevisiae | Multiphase analysis of the chocolate production chain to assess the functionality of starter cultures | S. cerevisiae demonstrated superior functionality, resulting in better flavor profiles and acidic notes and outperforming Hanseniaspora opuntiae, which led to under-fermented beans. | Superior flavor profiles | [138] |
| S. cerevisiae, W. anomalus, C. tropicalis, P. kudriavzevii | Effect of solar predrying treatments and inoculation with yeast starter cultures on the chemical composition of cocoa beans from Colombia | Starter cultures and predrying treatments altered chemical composition, reducing polyphenol, caffeine, and lactic acid content. | Improved sensory attributes | [139] |
| S. cerevisiae | Amino acid profile during Criollo cocoa fermentation | Starter cultures increased essential amino acids (63.4% vs. 61.8% in spontaneous fermentation) and reduced fermentation time by 3–4 d. | Improved flavor and aroma profiles. | [140] |
| Lactiplantibacillus plantarum subsp. plantarum HL-15 | Alternative cocoa bean fermentation method using L. plantarum HL-15 as a starter culture and valorizing cocoa mucilage byproducts | Pulp removal and inoculation with L. plantarum HL-15 improved bean quality, suppressed fungal growth, and enhanced sensory qualities. | Reduced fungi and improved flavor | [141] |
| Issatchenkia orientalis, S. cerevisiae, W. anomalus, H. thailandica, P. kluyveri, Candida oleophila | Sensory and chemical selection of indigenous yeasts in cocoa fermentation | Different strains imparted unique sensory notes, with H. thailandica and P. kluyveri recognized by the panel for their outstanding flavor profiles. | Distinct flavor profiles | [142] |
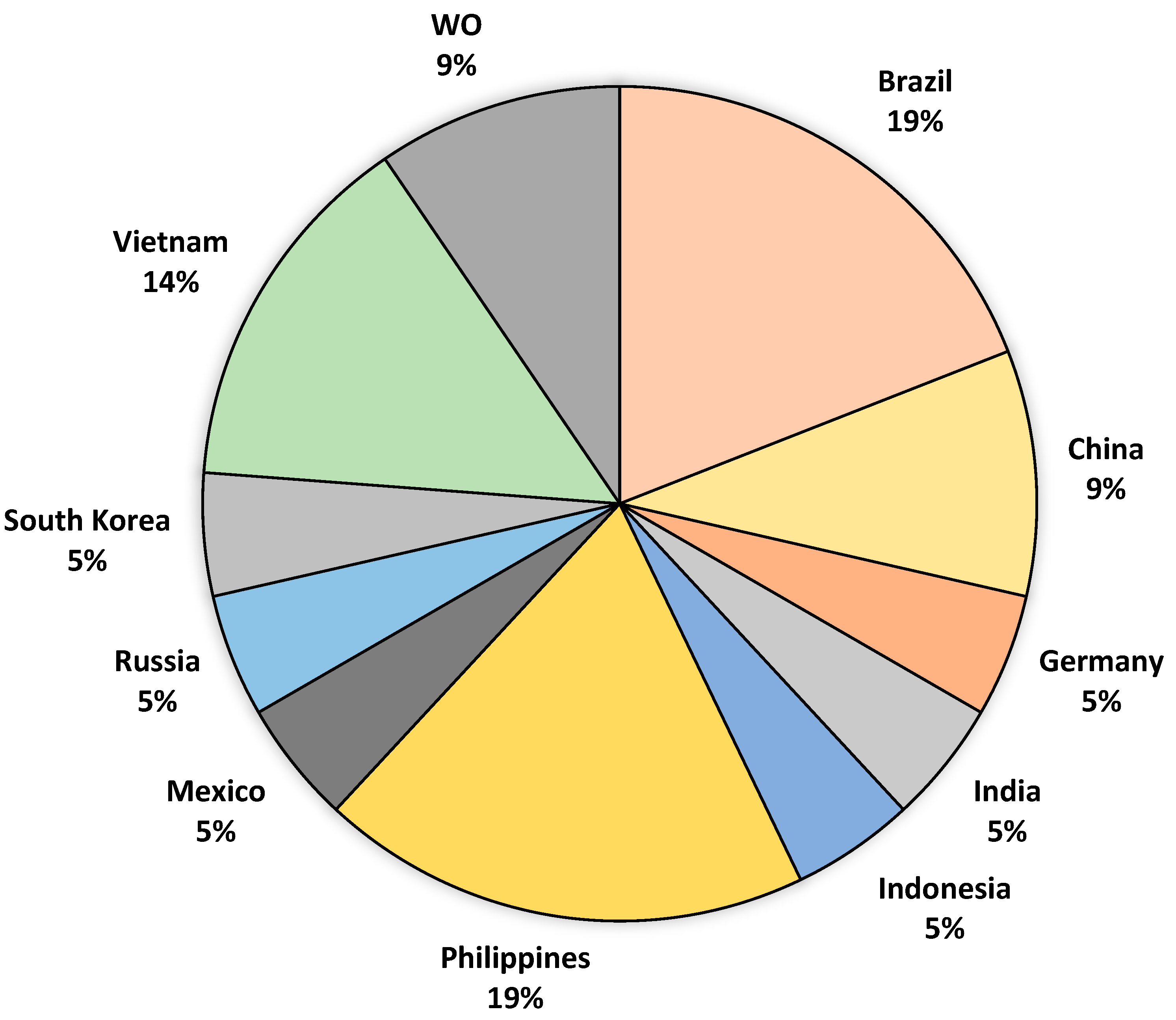
6. Patent Analysis on the Production of Organoleptic Compounds by the Fermentation of Cacao Beans
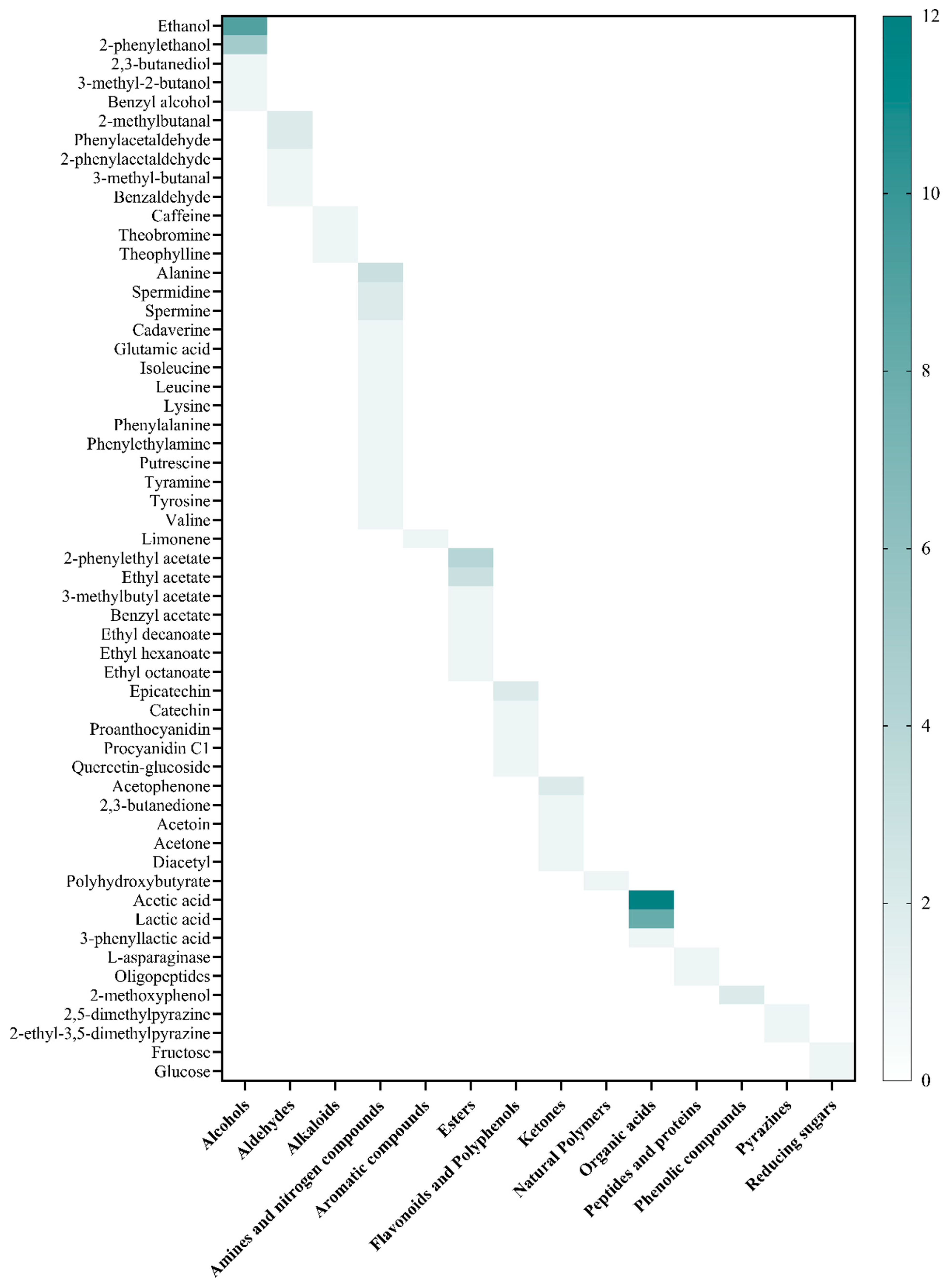
7. Bioprospection of Secondary Compounds from Cocoa Fermentation
8. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Future Market Insights Inc. Cocoa Market. Available online: https://www.futuremarketinsights.com/reports/cocoa-market (accessed on 1 October 2024).
- Skyquest Cocoa Market Size, Share, Growth Analysis, by Type (Fine Flavor Cocoa, Bulk/Ordinary Cocoa, and Others), by Processing Methods (Natural/Unfermented Cocoa, Fermented Cocoa, and Others), by Product Forms (Cocoa Beans, Cocoa Powder, Cocoa Butter, Cocoa Liquor), by Region—Industry Forecast 2024–2031. Available online: https://www.skyquestt.com/report/cocoa-market (accessed on 1 October 2024).
- Knowledge Based Value Research Global Cocoa Market Size, Share & Trends Analysis Report by Application, by Product Type (Cocoa Beans, Cocoa Powder & Cake, Cocoa Butter, Chocolate, and Others), by Regional Outlook and Forecast, 2023–2030. Available online: https://www.kbvresearch.com/cocoa-market/ (accessed on 1 October 2024).
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Volatile Organic Compounds and Sensory Profile of Dark Chocolates Made with Cocoa Beans Fermented with Pichia Kudriavzevii and Hanseniaspora Thailandica. J. Food Sci. Technol. 2022, 59, 2714–2723. [Google Scholar] [CrossRef]
- Tušek, K.; Valinger, D.; Jurina, T.; Sokač Cvetnić, T.; Gajdoš Kljusurić, J.; Benković, M. Bioactives in Cocoa: Novel Findings, Health Benefits, and Extraction Techniques. Separations 2024, 11, 128. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional Role of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Fermentation Processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Weckx, S. The Cocoa Bean Fermentation Process: From Ecosystem Analysis to Starter Culture Development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef]
- Sari, A.B.T.; Fahrurrozi, T.; Marwati, T.; Djaafar, T.F.; Hatmi, R.U.; Purwaningsih, P.; Wanita, Y.P.; Lisdiyanti, P.; Perwitasari, U.; Juanssilfero, A.B.; et al. Chemical Composition and Sensory Profiles of Fermented Cocoa Beans Obtained from Various Regions of Indonesia. Int. J. Food Sci. 2023, 2023, 5639081. [Google Scholar] [CrossRef]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef]
- Díaz-Muñoz, C.; De Vuyst, L. Functional Yeast Starter Cultures for Cocoa Fermentation. J. Appl. Microbiol. 2022, 133, 39–66. [Google Scholar] [CrossRef]
- Abijaude, J.; Sobreira, P.; Santiago, L.; Greve, F. Improving Data Security with Blockchain and Internet of Things in the Gourmet Cocoa Bean Fermentation Process. Sensors 2022, 22, 3029. [Google Scholar] [CrossRef]
- Coria-Hinojosa, L.M.; Velásquez-Reyes, D.; Alcázar-Valle, M.; Kirchmayr, M.R.; Calva-Estrada, S.; Gschaedler, A.; Mojica, L.; Lugo, E. Exploring Volatile Compounds and Microbial Dynamics: Kluyveromyces Marxianus and Hanseniaspora Opuntiae Reduce Forastero Cocoa Fermentation Time. Food Res. Int. 2024, 193, 114821. [Google Scholar] [CrossRef]
- Falconí, C.E.; Yánez-Mendizábal, V.; Haro, R.J.; Claudio, D.R. Inoculum of a Native Microbial Starter Cocktail to Optimize Fine-Aroma Cocoa (Theobroma cacao) Bean Fermentation. Agronomy 2023, 13, 2572. [Google Scholar] [CrossRef]
- Van de Voorde, D.; Díaz-Muñoz, C.; Hernandez, C.E.; Weckx, S.; De Vuyst, L. Yeast Strains Do Have an Impact on the Production of Cured Cocoa Beans, as Assessed with Costa Rican Trinitario Cocoa Fermentation Processes and Chocolates Thereof. Front. Microbiol. 2023, 14, 1232323. [Google Scholar] [CrossRef]
- Jethro Ekwala Misse Ngangue, R.; Minyaka, E.; Georges Bekwankoa Fofou, S.; Christophe Manz Koule, J.; Valery Nsoga, F.; Nchoutpouen Ngafon, M.; Tuem Somon, R.; Tibo Ambata Ambata, H.; Ndomou, M. Microbial Dynamics Associated with Spontaneous Fermentation of Cocoa (Theobroma cacao L.) in Cameroon and Evaluation of the Quality of Marketable Beans. Int. J. Nutr. Food Sci. 2022, 11, 38. [Google Scholar] [CrossRef]
- Llano, S.; Vaillant, F.; Santander, M.; Zorro-González, A.; González-Orozco, C.E.; Maraval, I.; Boulanger, R.; Escobar, S. Exploring the Impact of Fermentation Time and Climate on Quality of Cocoa Bean-Derived Chocolate: Sensorial Profile and Volatilome Analysis. Foods 2024, 13, 2614. [Google Scholar] [CrossRef]
- Deus, V.L.; Cerqueira E Silva, M.B.d.; Maciel, L.F.; Miranda, L.C.R.; Hirooka, E.Y.; Soares, S.E.; Ferreira, E.d.S.; Bispo, E.d.S. Influence of Drying Methods on Cocoa (Theobroma cacao L.): Antioxidant Activity and Presence of Ochratoxin A. Food Sci. Technol. 2018, 38, 278–285. [Google Scholar] [CrossRef]
- Dzelagha, B.F.; Ngwa, N.M.; Nde Bup, D. A Review of Cocoa Drying Technologies and the Effect on Bean Quality Parameters. Int. J. Food Sci. 2020, 2020, 8830127. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The Content of Polyphenolic Compounds in Cocoa Beans (Theobroma cacao L.), Depending on Variety, Growing Region, and Processing Operations: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef]
- Gutiérrez-Ríos, H.G.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Rayas-Duarte, P.; Cano-Sarmiento, C.; Figueroa-Hernández, C.Y.; González-Rios, O. Yeasts as Producers of Flavor Precursors during Cocoa Bean Fermentation and Their Relevance as Starter Cultures: A Review. Fermentation 2022, 8, 331. [Google Scholar] [CrossRef]
- Morales-Rodriguez, W.J.; Morante-Carriel, J.; Herrera-Feijoo, R.J.; Ayuso-Yuste, M.C.; Bernalte-García, M.J. Effect of Addition of Yeasts and Enzymes during Fermentation on Physicochemical Quality of Fine Aroma Cocoa Beans. J. Agric. Food Res. 2024, 16, 101126. [Google Scholar] [CrossRef]
- Collin, S.; Fisette, T.; Pinto, A.; Souza, J.; Rogez, H. Discriminating Aroma Compounds in Five Cocoa Bean Genotypes from Two Brazilian States: White Kerosene-like Catongo, Red Whisky-like FL89 (Bahia), Forasteros IMC68 PA121 and P7 (Pará). Molecules 2023, 28, 1548. [Google Scholar] [CrossRef]
- Pokharel, B. Cocoa Bean Fermentation: Impact on Chocolate Flavor and Quality. Int. J. Sci. Res. (IJSR) 2023, 12, 1668–1674. [Google Scholar] [CrossRef]
- Sandoval-Lozano, C.J.; Caballero-Torres, D.; López-Giraldo, L.J. Screening Wild Yeast Isolated from Cocoa Bean Fermentation Using Volatile Compounds Profile. Molecules 2022, 27, 902. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.P.; Chagas Junior, G.C.A.; de Aguiar Andrade, E.H.; Nascimento, L.D.d.; Chisté, R.C.; Ferreira, N.R.; Martins, L.H.d.S.; Lopes, A.S. How Climatic Seasons of the Amazon Biome Affect the Aromatic and Bioactive Profiles of Fermented and Dried Cocoa Beans? Molecules 2021, 26, 3759. [Google Scholar] [CrossRef]
- Chetschik, I.; Kneubühl, M.; Chatelain, K.; Schlüter, A.; Bernath, K.; Hühn, T. Investigations on the Aroma of Cocoa Pulp (Theobroma cacao L.) and Its Influence on the Odor of Fermented Cocoa Beans. J. Agric. Food Chem. 2018, 66, 2467–2472. [Google Scholar] [CrossRef]
- Ullrich, L.; Casty, B.; André, A.; Hühn, T.; Steinhaus, M.; Chetschik, I. Decoding the Fine Flavor Properties of Dark Chocolates. J. Agric. Food Chem. 2022, 70, 13730–13740. [Google Scholar] [CrossRef]
- Cherniienko, A.; Pawełczyk, A.; Zaprutko, L. Antimicrobial and Odour Qualities of Alkylpyrazines Occurring in Chocolate and Cocoa Products. Appl. Sci. 2022, 12, 11361. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Espírito-Santo, J.C.A.d.; Ferreira, N.R.; Marques-da-Silva, S.H.; Oliveira, G.; Vasconcelos, S.; Almeida, S.d.F.O.d.; Silva, L.R.C.; Gobira, R.M.; Figueiredo, H.M.d.; et al. Yeast Isolation and Identification during On-Farm Cocoa Natural Fermentation in a Highly Producer Region in Northern Brazil. Sci. Plena 2021, 16. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts Are Essential for Cocoa Bean Fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef]
- Almeida, S.d.F.O.d.; Silva, L.R.C.; Junior, G.C.A.C.; Oliveira, G.; Silva, S.H.M.d.; Vasconcelos, S.; Lopes, A.S. Diversity of Yeasts during Fermentation of Cocoa from Two Sites in the Brazilian Amazon. Acta Amaz. 2019, 49, 64–70. [Google Scholar] [CrossRef]
- Illeghems, K.; De Vuyst, L.; Weckx, S. Comparative Genome Analysis of the Candidate Functional Starter Culture Strains Lactobacillus Fermentum 222 and Lactobacillus Plantarum 80 for Controlled Cocoa Bean Fermentation Processes. BMC Genom. 2015, 16, 766. [Google Scholar] [CrossRef]
- Romanens, E.; Pedan, V.; Meile, L.; Schwenninger, S.M. Influence of Two Anti-Fungal Lactobacillus Fermentum-Saccharomyces Cerevisiae Co-Cultures on Cocoa Bean Fermentation and Final Bean Quality. PLoS ONE 2020, 15, e0239365. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Lefeber, T.; Bahrim, B.; Lee, O.S.; Daniel, H.M.; De Vuyst, L. Hanseniaspora Opuntiae, Saccharomyces Cerevisiae, Lactobacillus Fermentum, and Acetobacter Pasteurianus Predominate during Well-Performed Malaysian Cocoa Bean Box Fermentations, Underlining the Importance of These Microbial Species for a Successful Cocoa Bean Fermentation Process. Food Microbiol. 2013, 35, 73–85. [Google Scholar] [CrossRef]
- Peyer, L.C.; Axel, C.; Lynch, K.M.; Zannini, E.; Jacob, F.; Arendt, E.K. Inhibition of Fusarium Culmorum by Carboxylic Acids Released from Lactic Acid Bacteria in a Barley Malt Substrate. Food Control 2016, 69, 227–236. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Fleet, G.H.; Zhao, J. Unravelling the Contribution of Lactic Acid Bacteria and Acetic Acid Bacteria to Cocoa Fermentation Using Inoculated Organisms. Int. J. Food Microbiol. 2018, 279, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.T.T.; Zhao, J.; Fleet, G. The Effect of Lactic Acid Bacteria on Cocoa Bean Fermentation. Int. J. Food Microbiol. 2015, 205, 54–67. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and Its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Rincon-Delgadillo, M.I.; Lopez-Hernandez, A.; Wijaya, I.; Rankin, S.A. Diacetyl Levels and Volatile Profiles of Commercial Starter Distillates and Selected Dairy Foods. J. Dairy Sci. 2012, 95, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Tunick, M.H. Origins of Cheese Flavor; Drake, M., McGorrin, R.J., Cadwallader, K.R., Eds.; American Chemical Society: Washington, DC, USA, 2007; Volume 971, ISBN 9780841239685. [Google Scholar]
- Lefeber, T.; Janssens, M.; Camu, N.; De Vuyst, L. Kinetic Analysis of Strains of Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Pulp Simulation Media toward Development of a Starter Culture for Cocoa Bean Fermentation. Appl. Environ. Microbiol. 2010, 76, 7708–7716. [Google Scholar] [CrossRef]
- van Aalst, A.C.A.; Mans, R.; Pronk, J.T. An Engineered Non-Oxidative Glycolytic Bypass Based on Calvin-Cycle Enzymes Enables Anaerobic Co-Fermentation of Glucose and Sorbitol by Saccharomyces Cerevisiae. Biotechnol. Biofuels Bioprod. 2022, 15, 112. [Google Scholar] [CrossRef]
- Lima, C.O.d.C.; De Castro, G.M.; Solar, R.; Vaz, A.B.M.; Lobo, F.; Pereira, G.; Rodrigues, C.; Vandenberghe, L.; Martins Pinto, L.R.; da Costa, A.M.; et al. Unraveling Potential Enzymes and Their Functional Role in Fine Cocoa Beans Fermentation Using Temporal Shotgun Metagenomics. Front. Microbiol. 2022, 13, 994524. [Google Scholar] [CrossRef]
- Lopes, G.G.; Morgano, M.A.; Taniwaki, M.H. Advances in Bean-to-Bar Chocolate Production: Microbiology, Biochemistry, Processing, and Sensorial Aspects. Food Technol. 2024, 27, e2023133. [Google Scholar] [CrossRef]
- Hirko, B.; Mitiku, H.; Getu, A. Role of Fermentation and Microbes in Cacao Fermentation and Their Impact on Cacao Quality. Syst. Microbiol. Biomanuf. 2023, 3, 509–520. [Google Scholar] [CrossRef]
- González, A.F.R.; García, G.A.G.; Polanía-Hincapié, P.A.; López, L.J.; Suárez, J.C. Fermentation and Its Effect on the Physicochemical and Sensory Attributes of Cocoa Beans in the Colombian Amazon. PLoS ONE 2024, 19, e0306680. [Google Scholar] [CrossRef]
- Korcari, D.; Fanton, A.; Ricci, G.; Rabitti, N.S.; Laureati, M.; Hogenboom, J.; Pellegrino, L.; Emide, D.; Barbiroli, A.; Fortina, M.G. Fine Cocoa Fermentation with Selected Lactic Acid Bacteria: Fermentation Performance and Impact on Chocolate Composition and Sensory Properties. Foods 2023, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Kresnowati, M.T.A.P.; Suryani, L.; Affifah, M. Improvement of Cocoa Beans Fermentation by LAB Starter Addition. J. Med. Bioeng. 2013, 2, 274–278. [Google Scholar] [CrossRef]
- Sengun, I.Y. Acetic Acid Bacteria in Food Fermentations. Fermented Foods Part I Biochem. Biotechnol. 2016, 1, 76–96. [Google Scholar]
- Han, D.; Yang, Y.; Guo, Z.; Dai, S.; Jiang, M.; Zhu, Y.; Wang, Y.; Yu, Z.; Wang, K.; Rong, C.; et al. A Review on the Interaction of Acetic Acid Bacteria and Microbes in Food Fermentation: A Microbial Ecology Perspective. Foods 2024, 13, 2534. [Google Scholar] [CrossRef]
- Trcek, J.; Toyama, H.; Czuba, J.; Misiewicz, A.; Matsushita, K. Correlation between Acetic Acid Resistance and Characteristics of PQQ-Dependent ADH in Acetic Acid Bacteria. Appl. Microbiol. Biotechnol. 2006, 70, 366–373. [Google Scholar] [CrossRef]
- Matsushita, K.; Takaki, Y.; Shinagawa, E.; Ameyama, M.; Adachi, O. Ethanol Oxidase Respiratory Chain of Acetic Acid Bacteria. Reactivity with Ubiquinone of Pyrroloquinoline Quinone-Dependent Alcohol Dehydrogenases Purified from Acetobacter Aceti and Gluconohacter Suhoxydans. Biosci. Biotechnol. Biochem. 1992, 56, 304–310. [Google Scholar] [CrossRef]
- Camu, N.; De Winter, T.; Verbrugghe, K.; Cleenwerck, I.; Vandamme, P.; Takrama, J.S.; Vancanneyt, M.; De Vuyst, L. Dynamics and Biodiversity of Populations of Lactic Acid Bacteria and Acetic Acid Bacteria Involved in Spontaneous Heap Fermentation of Cocoa Beans in Ghana. Appl. Environ. Microbiol. 2007, 73, 1809–1824. [Google Scholar]
- Nielsen, D.S.; Teniola, O.D.; Ban-Koffi, L.; Owusu, M.; Andersson, T.S.; Holzapfel, W.H. The Microbiology of Ghanaian Cocoa Fermentations Analysed Using Culture-Dependent and Culture-Independent Methods. Int. J. Food Microbiol. 2007, 114, 168–186. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, J.; Singh, R.P. Biotransformation of Sweet Lime Pulp Waste into High-Quality Nanocellulose with an Excellent Productivity Using Komagataeibacter Europaeus SGP37 under Static Intermittent Fed-Batch Cultivation. Bioresour. Technol. 2018, 247, 73–80. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, Z.; Zhang, H.; Liebl, W.; Toyama, H.; Chen, F. Oxidative Fermentation of Acetic Acid Bacteria and Its Products. Front. Microbiol. 2022, 13, 879246. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chocolate Science and Technology, 2nd ed.; Wiley Blackwell; University of Ghana, Legon—Accra, Ghana; Formerly of Nestlé Product Technology Centre: York, UK, 2016; pp. 1–550. ISBN 978-1-1189-1378-9. [Google Scholar]
- Ghisolfi, R.; Bandini, F.; Vaccari, F.; Bellotti, G.; Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Bacterial and Fungal Communities Are Specifically Modulated by the Cocoa Bean Fermentation Method. Foods 2023, 12, 2024. [Google Scholar] [CrossRef]
- Lefeber, T.; Papalexandratou, Z.; Gobert, W.; Camu, N.; De Vuyst, L. On-Farm Implementation of a Starter Culture for Improved Cocoa Bean Fermentation and Its Influence on the Flavour of Chocolates Produced Thereof. Food Microbiol. 2012, 30, 379–392. [Google Scholar] [CrossRef] [PubMed]
- John, W.A.; Kumari, N.; Böttcher, N.L.; Koffi, K.J.; Grimbs, S.; Vrancken, G.; D’Souza, R.N.; Kuhnert, N.; Ullrich, M.S. Aseptic Artificial Fermentation of Cocoa Beans Can Be Fashioned to Replicate the Peptide Profile of Commercial Cocoa Bean Fermentations. Food Res. Int. 2016, 89, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, B.; Derewiaka, D.; Lenart, A.; Kowalska, J. Changes in the Composition and Content of Polyphenols in Chocolate Resulting from Pre-Treatment Method of Cocoa Beans and Technological Process. Eur. Food Res. Technol. 2019, 245, 2101–2112. [Google Scholar] [CrossRef]
- Chang, H.; Gu, C.; Wang, M.; Chang, Z.; Zhou, J.; Yue, M.; Chen, J.; Qin, X.; Feng, Z. Integrating Shotgun Metagenomics and Metabolomics to Elucidate the Dynamics of Microbial Communities and Metabolites in Fine Flavor Cocoa Fermentation in Hainan. Food Res. Int. 2024, 177, 113849. [Google Scholar] [CrossRef] [PubMed]
- Scalone, G.L.L.; Textoris-Taube, K.; De Meulenaer, B.; De Kimpe, N.; Wöstemeyer, J.; Voigt, J. Cocoa-Specific Flavor Components and Their Peptide Precursors. Food Res. Int. 2019, 123, 503–515. [Google Scholar] [CrossRef]
- Jamili, J.; Yanti, N.A.; Susilowati, P.E. Diversity and the Role of Yeast in Spontaneous Cocoa Bean Fermentation from Southeast Sulawesi, Indonesia. Biodiversitas 1970, 17, 90–95. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Comparison of Bioactive Components and Flavor Volatiles of Diverse Cocoa Genotypes of Theobroma Grandiflorum, Theobroma Bicolor, Theobroma Subincanum and Theobroma cacao. Food Res. Int. 2022, 161, 111764. [Google Scholar] [CrossRef]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of Predominant Yeasts to the Occurrence of Aroma Compounds during Cocoa Bean Fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]
- Koff, O.; Samagaci, L.; Goualie, B.; Niamke, S. Diversity of Yeasts Involved in Cocoa Fermentation of Six Major Cocoa-Producing Regions in Ivory Coast. Eur. Sci. J. ESJ 2017, 13, 496. [Google Scholar] [CrossRef][Green Version]
- Ouattara, H.G.; Niamké, S.L. Mapping the Functional and Strain Diversity of the Main Microbiota Involved in Cocoa Fermentation from Cote d’Ivoire. Food Microbiol. 2021, 98, 103767. [Google Scholar] [CrossRef]
- Mendoza Salazar, M.M.; Lizarazo-Medina, P.X. Assessment of the Fungal Community Associated with Cocoa Bean Fermentation from Two Regions in Colombia. Food Res. Int. 2021, 149, 110670. [Google Scholar] [CrossRef] [PubMed]
- Tigrero-Vaca, J.; Maridueña-Zavala, M.G.; Liao, H.-L.; Prado-Lince, M.; Zambrano-Vera, C.S.; Monserrate-Maggi, B.; Cevallos-Cevallos, J.M. Microbial Diversity and Contribution to the Formation of Volatile Compounds during Fine-Flavor Cacao Bean Fermentation. Foods 2022, 11, 915. [Google Scholar] [CrossRef]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing Cocoa Flavour Using Pichia Kluyveri and Kluyveromyces Marxianus in a Defined Mixed Starter Culture for Cocoa Fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Papalexandratou, Z.; Vuyst, L. Assessment of the Yeast Species Composition of Cocoa Bean Fermentations in Different Cocoa-Producing Regions Using Denaturing Gradient Gel Electrophoresis. FEMS Yeast Res. 2011, 11, 564–574. [Google Scholar] [CrossRef]
- Ziegleder, G. Composition of Flavor Extracts of Raw and Roasted Cocoas. Z. Lebensm. Unters. Forsch. 1991, 192, 521–525. [Google Scholar] [CrossRef]
- Ziegleder, G. Linalool Contents as Characteristic of Some Flavor Grade Cocoas. Z. Lebensm. Unters. Forsch. 1990, 191, 306–309. [Google Scholar] [CrossRef]
- Kadow, D.; Niemenak, N.; Rohn, S.; Lieberei, R. Fermentation-like Incubation of Cocoa Seeds (Theobroma cacao L.)—Reconstruction and Guidance of the Fermentation Process. LWT-Food Sci. Technol. 2015, 62, 357–361. [Google Scholar] [CrossRef]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of Cocoa Fermentation and Its Effect on Quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Bagnulo, E.; Scavarda, C.; Bortolini, C.; Cordero, C.; Bicchi, C.; Liberto, E. Cocoa Quality: Chemical Relationship of Cocoa Beans and Liquors in Origin Identitation. Food Res. Int. 2023, 172, 113199. [Google Scholar] [CrossRef]
- Ramos, C.L.; Dias, D.R.; Miguel, M.G.D.C.P.; Schwan, R.F. Impact of Different Cocoa Hybrids (Theobroma cacao L.) and S. Cerevisiae UFLA CA11 Inoculation on Microbial Communities and Volatile Compounds of Cocoa Fermentation. Food Res. Int. 2014, 64, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Batista, N.N.; Ramos, C.L.; Ribeiro, D.D.; Pinheiro, A.C.M.; Schwan, R.F. Dynamic Behavior of Saccharomyces Cerevisiae, Pichia Kluyveri and Hanseniaspora Uvarum during Spontaneous and Inoculated Cocoa Fermentations and Their Effect on Sensory Characteristics of Chocolate. LWT-Food Sci. Technol. 2015, 63, 221–227. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of Different Fruit Wines Made from Cacao, Cupuassu, Gabiroba, Jaboticaba and Umbu. LWT-Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Serra, J.L.; Mouchrek, A.N.; de Oliveira, A.C.; Correia, M.G.d.S.; Burgos, W.J.M.; Vandenberghe, L.P.d.S.; Neto, D.P.d.C.; Soccol, C.R.; Baeten, V.; Darnet, S.; et al. β-Glycosidase Activity Associated with the Formation of Aroma Compounds in Native Non-Saccharomyces Yeasts Isolated from Cocoa Bean Fermentation. BASE 2024, 28, 37–53. [Google Scholar] [CrossRef]
- Marseglia, A.; Musci, M.; Rinaldi, M.; Palla, G.; Caligiani, A. Volatile Fingerprint of Unroasted and Roasted Cocoa Beans (Theobroma cacao L.) from Different Geographical Origins. Food Res. Int. 2020, 132, 109101. [Google Scholar] [CrossRef]
- Hinneh, M.; Van de Walle, D.; Tzompa-Sosa, D.A.; De Winne, A.; Termote, S.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; De Cooman, L.; Dewettinck, K. Tuning the Aroma Profiles of FORASTERO Cocoa Liquors by Varying Pod Storage and Bean Roasting Temperature. Food Res. Int. 2019, 125, 108550. [Google Scholar] [CrossRef]
- Hashim, P.; Selamat, J.; Kharidah, S.; Ali, A. Changes in Free Amino Acid, Peptide-N, Sugar and P y Razine Concentration during Cocoa Fermentation. J. Sci. Food Agric. 1998, 78, 535–542. [Google Scholar] [CrossRef]
- Voigt, J.; Janek, K.; Textoris-Taube, K.; Niewienda, A.; Wöstemeyer, J. Partial Purification and Characterisation of the Peptide Precursors of the Cocoa-Specific Aroma Components. Food Chem. 2016, 192, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Fanning, E.; Eyres, G.; Frew, R.; Kebede, B. Linking Cocoa Quality Attributes to Its Origin Using Geographical Indications. Food Control 2023, 151, 109825. [Google Scholar]
- Quelal, O.M.; Hurtado, D.P.; Benavides, A.A.; Alanes, P.V.; Alanes, N.V. Key Aromatic Volatile Compounds from Roasted Cocoa Beans, Cocoa Liquor, and Chocolate. Fermentation 2023, 9, 166. [Google Scholar] [CrossRef]
- Crafack, M.; Keul, H.; Eskildsen, C.E.; Petersen, M.A.; Saerens, S.; Blennow, A.; Skovmand-Larsen, M.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Impact of Starter Cultures and Fermentation Techniques on the Volatile Aroma and Sensory Profile of Chocolate. Food Res. Int. 2014, 63, 306–316. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Alvarez, J.P.; Neto, D.P.D.C.; Thomaz, V.; Tanobe, V.O.A.; Rogez, H.; Góes-neto, A.; Ricardo, C. Great Intraspecies Diversity of Pichia Kudriavzevii in Cocoa Fermentation Highlights the Importance of Yeast Strain Selection for Flavor Modulation of Cocoa Beans. LWT-Food Sci. Technol. 2017, 84, 290–297. [Google Scholar] [CrossRef]
- Serra, J.L.; Moura, F.G.; Pereira, G.V.d.M.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the Microbial Community in Amazonian Cocoa Bean Fermentation by Illumina-Based Metagenomic Sequencing. LWT-Food Sci. Technol. 2019, 106, 229–239. [Google Scholar] [CrossRef]
- Taylor, A.J.; Cardenas-Torres, E.; Miller, M.J.; Zhao, S.D.; Engeseth, N.J. Microbes Associated with Spontaneous Cacao Fermentations—A Systematic Review and Meta-Analysis. Curr. Res. Food Sci. 2022, 5, 1452–1464. [Google Scholar] [CrossRef]
- Van Durme, J.; Ingels, I.; De Winne, A. Inline Roasting Hyphenated with Gas Chromatography-Mass Spectrometry as an Innovative Approach for Assessment of Cocoa Fermentation Quality and Aroma Formation Potential. Food Chem. 2016, 205, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Nielsen, D.S.; Hønholt, S.; Jakobsen, M. Occurrence and Diversity of Yeasts Involved in Fermentation of West African Cocoa Beans. FEMS Yeast Res. 2005, 5, 441–453. [Google Scholar] [CrossRef]
- Nielsen, D.S.; Hønholt, S.; Tano-Debrah, K.; Jespersen, L. Yeast Populations Associated with Ghanaian Cocoa Fermentations Analysed Using Denaturing Gradient Gel Electrophoresis (DGGE). Yeast 2005, 22, 271–284. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Camu, N.; Falony, G.; De Vuyst, L. Comparison of the Bacterial Species Diversity of Spontaneous Cocoa Bean Fermentations Carried out at Selected Farms in Ivory Coast and Brazil. Food Microbiol. 2011, 28, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Vrancken, G.; Takrama, J.F.; Camu, N.; Vos, P. De Yeast Diversity of Ghanaian Cocoa Bean Heap Fermentations. FEMS Yeast Res. 2009, 9, 774–783. [Google Scholar] [CrossRef]
- Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Detailed Analyses of the Bacterial Populations in Processed Cocoa Beans of Different Geographic Origin, Subject to Varied Fermentation Conditions. Int. J. Food Microbiol. 2016, 236, 98–106. [Google Scholar] [CrossRef]
- Visintin, S.; Alessandria, V.; Valente, A.; Dolci, P.; Cocolin, L.; Visintin, S.; Alessandria, V.; Valente, A.; Dolci, P.; Cocolin, L. Molecular Identification and Physiological Characterization of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria Isolated from Heap and Box Cocoa Bean Fermentations in West Africa. Int. J. Food Microbiol. 2016, 216, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, H.D.H.G.; Ouattara, H.D.H.G.; Droux, M.; Reverchon, S.; Nasser, W.; Niamke, S.L. Lactic Acid Bacteria Involved in Cocoa Beans Fermentation from Ivory Coast: Species Diversity and Citrate Lyase Production. Int. J. Food Microbiol. 2017, 256, 11–19. [Google Scholar] [CrossRef]
- Lagunes Gálvez, S.; Loiseau, G.; Paredes, J.L.; Barel, M.; Guiraud, J.P. Study on the Microflora and Biochemistry of Cocoa Fermentation in the Dominican Republic. Int. J. Food Microbiol. 2007, 114, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Papalexandratou, Z.; Vrancken, G.; De Bruyne, K.; Vandamme, P.; De Vuyst, L. Spontaneous Organic Cocoa Bean Box Fermentations in Brazil Are Characterized by a Restricted Species Diversity of Lactic Acid Bacteria and Acetic Acid Bacteria. Food Microbiol. 2011, 28, 1326–1338. [Google Scholar] [CrossRef]
- Illeghems, K.; De Vuyst, L.; Papalexandratou, Z.; Weckx, S. Phylogenetic Analysis of a Spontaneous Cocoa Bean Fermentation Metagenome Reveals New Insights into Its Bacterial and Fungal Community Diversity. PLOS ONE 2012, 7, e38040. [Google Scholar] [CrossRef]
- Vinícius, G.; Pereira, D.M.; Teixeira, K.; Gonzaga, E.; Almeida, D.; Coelho, S.; Freitas, R. Spontaneous Cocoa Bean Fermentation Carried out in a Novel-Design Stainless Steel Tank: In Fl Uence on the Dynamics of Microbial Populations and Physical—Chemical Properties. Int. J. Food Microbiol. 2013, 161, 121–133. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Segura-García, L.E.; Kirchmayr, M.; Orozco-Ávila, I.; Lugo-Cervantes, E.; Gschaedler-Mathis, A. Identification of Predominant Yeasts Associated with Artisan Mexican Cocoa Fermentations Using Culture-Dependent and Culture-Independent Approaches. World J. Microbiol. Biotechnol. 2015, 31, 359–369. [Google Scholar] [CrossRef]
- Fernandez Maura, Y.; Balzarini, T.; Clape Borges, P.; Evrard, P.; De Vuyst, L.; Daniel, H.M. The Environmental and Intrinsic Yeast Diversity of Cuban Cocoa Bean Heap Fermentations. Int. J. Food Microbiol. 2016, 233, 34–43. [Google Scholar] [CrossRef]
- Ardhana, M.; Fleet, G.H. The Microbial Ecology of Cocoa Bean Fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Meersman, E.; Steensels, J.; Mathawan, M.; Wittocx, P.J.; Saels, V.; Struyf, N.; Bernaert, H.; Vrancken, G.; Verstrepen, K.J. Detailed Analysis of the Microbial Population in Malaysian Spontaneous Cocoa Pulp Fermentations Reveals a Core and Variable Microbiota. PLoS ONE 2013, 8, e81559. [Google Scholar] [CrossRef]
- International Cocoa Organization Growing Cocoa. Available online: https://www.icco.org/growing-cocoa/ (accessed on 5 December 2024).
- Caligiani, A.; Marseglia, A.; Palla, G. Cocoa: Production, Chemistry, and Use, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780123849533. [Google Scholar]
- Diomande, D.; Antheaume, I.; Leroux, M.; Lalande, J.; Balayssac, S.; Remaud, G.S.G.S.; Tea, I. Multi-Element, Multi-Compound Isotope Profiling as a Means to Distinguish the Geographical and Varietal Origin of Fermented Cocoa (Theobroma cacao L.) Beans. Food Chem. 2015, 188, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.D.; Van de Walle, D.; De Clercq, N.; De Winne, A.; Kadow, D.; Lieberei, R.; Messens, K.; Tran, D.N.; Dewettinck, K.; Van Durme, J. Assessing Cocoa Aroma Quality by Multiple Analytical Approaches. Food Res. Int. 2015, 77, 657–669. [Google Scholar] [CrossRef]
- Besançon, L.; Lorn, D.; Kouamé, C.; Grabulos, J.; Lebrun, M.; Fontana, A.; Schorr-Galindo, S.; Boulanger, R.; Strub, C.; Colas de la Noue, A. Influence of Yeast Interactions on the Fermentation Process and Aroma Production in Synthetic Cocoa Pulp vs. Real Mucilage Media. Fermentation 2024, 10, 662. [Google Scholar] [CrossRef]
- Cambrai, A.; Marcic, C.; Morville, S.; Houer, P.S.; Bindler, F.; Marchioni, E. Differentiation of Chocolates According to the Cocoa’s Geographical Origin Using Chemometrics. J. Agric. Food Chem. 2010, 58, 1478–1483. [Google Scholar] [CrossRef]
- Cemin, P.; Reis Ribeiro, S.; de Candido de Oliveira, F.; Leal Leães, F.; Regina dos Santos Nunes, M.; Wagner, R.; Sant’Anna, V. Chocolates with Brazilian Cocoa: Tracking Volatile Compounds According to Consumers’ Preference. Food Res. Int. 2022, 159, 111618. [Google Scholar] [CrossRef]
- Putri, D.N.; De Steur, H.; Juvinal, J.G.; Gellynck, X.; Schouteten, J.J. Sensory Attributes of Fine Flavor Cocoa Beans and Chocolate: A Systematic Literature Review. J. Food Sci. 2024, 89, 1917–1943. [Google Scholar] [CrossRef]
- Lozano Tovar, M.D.; Tibasosa, G.; González, C.M.; Ballestas Alvarez, K.; Lopez Hernandez, M.D.P.; Rodríguez Villamizar, F. Isolation and Identification of Microbial Species Found in Cocoa Fermentation as Microbial Starter Culture Candidates for Cocoa Bean Fermentation in Colombia. Pelita Perkeb. 2020, 36, 236–248. [Google Scholar] [CrossRef]
- Verce, M.; Schoonejans, J.; Hernandez Aguirre, C.; Molina-Bravo, R.; De Vuyst, L.; Weckx, S. A Combined Metagenomics and Metatranscriptomics Approach to Unravel Costa Rican Cocoa Box Fermentation Processes Reveals Yet Unreported Microbial Species and Functionalities. Front. Microbiol. 2021, 12, 641185. [Google Scholar] [CrossRef]
- Nishimura, H.; Shiwa, Y.; Tomita, S.; Endo, A. Microbial Composition and Metabolic Profiles during Machine-Controlled Intra-Factory Fermentation of Cocoa Beans Harvested in Semitropical Area of Japan. Biosci. Microbiota Food Health 2024, 43, 2023–2036. [Google Scholar] [CrossRef]
- Daniel Fonseca Blanco, J.; Del Pilar López Hernandez, M.; Sabrina Ortiz Galeano, L.; Criollo Nuñez, J.; Denis Lozano Tovar, M. Effect of Addition of a Specific Mixture of Yeast, Lactic and Acetic Bacteria in the Fermentation Process to Improve the Quality and Flavor of Cocoa Beans in Colombia. Pelita Perkeb. 2020, 36, 154–172. [Google Scholar] [CrossRef]
- García-Ríos, E.; Lairón-Peris, M.; Muñiz-Calvo, S.; Heras, J.M.; Ortiz-Julien, A.; Poirot, P.; Rozès, N.; Querol, A.; Guillamón, J.M. Thermo-Adaptive Evolution to Generate Improved Saccharomyces Cerevisiae Strains for Cocoa Pulp Fermentations. Int. J. Food Microbiol. 2021, 342, 109077. [Google Scholar] [CrossRef] [PubMed]
- Djaafar, T.F.; Monika, D.C.; Marwati, T.; Triwitono, P.; Rahayu, E.S. Microbiology, Chemical, and Sensory Characteristics of Cocoa Powder: The Effect of Lactobacillus Plantarum HL-15 as Culture Starter and Fermentation Box Variation. Digit. Press Life Sci. 2020, 2, 00008. [Google Scholar] [CrossRef]
- Ferreira, O.d.S.; Chagas-Junior, G.C.A.; Chisté, R.C.; Martins, L.H.d.S.; Andrade, E.H.d.A.; do Nascimento, L.D.; Lopes, A.S. Saccharomyces Cerevisiae and Pichia Manshurica from Amazonian Biome Affect the Parameters of Quality and Aromatic Profile of Fermented and Dried Cocoa Beans. J. Food Sci. 2022, 87, 4148–4161. [Google Scholar] [CrossRef]
- Meersman, E.; Steensels, J.; Paulus, T.; Struyf, N.; Saels, V.; Mathawan, M.; Koffi, J.; Vrancken, G.; Verstrepen, K.J. Breeding Strategy To Generate Robust Yeast Starter Cultures for Cocoa Pulp Fermentations. Appl. Environ. Microbiol. 2015, 81, 6166–6176. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; Martins, L.H.d.S.; Lopes, A.S. Chemical Implications and Time Reduction of On-Farm Cocoa Fermentation by Saccharomyces Cerevisiae and Pichia Kudriavzevii. Food Chem. 2021, 338, 127834. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Kaasik, K.; Kauffmann, L.V.; Skorstengaard, A.; Bouillon, G.; Espensen, J.L.; Hansen, L.H.; Jakobsen, R.R.; Blennow, A.; Krych, L.; et al. Linking Cocoa Varietals and Microbial Diversity of Nicaraguan Fine Cocoa Bean Fermentations and Their Impact on Final Cocoa Quality Appreciation. Int. J. Food Microbiol. 2019, 304, 106–118. [Google Scholar] [CrossRef]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rogez, H.; Góes-Neto, A.; Azevedo, V.; Brenig, B.; Aburjaile, F.; Soccol, C.R. Co-Culturing Fructophilic Lactic Acid Bacteria and Yeast Enhanced Sugar Metabolism and Aroma Formation during Cocoa Beans Fermentation. Int. J. Food Microbiol. 2021, 339, 109015. [Google Scholar] [CrossRef]
- Haruna, L.; Abano, E.E.; Teye, E.; Tukwarlba, I.; Adu, S.; Agyei, K.J.; Kuma, E.; Yeboah, W.; Lukeman, M. Effect of Partial Pulp Removal and Fermentation Duration on Drying Behavior, Nib Acidification, Fermentation Quality, and Flavor Attributes of Ghanaian Cocoa Beans. J. Agric. Food Res. 2024, 17, 101211. [Google Scholar] [CrossRef]
- Kresnowati, M.T.A.P.; Febriami, H. Mapping the effects of starter culture addition on cocoa bean fermentation. ASEAN Eng. J. 2015, 5, 25–37. [Google Scholar] [CrossRef]
- Tsaaqifah, H.; Fahrurrozi, F.; Meryandini, A. Selection Of Lactic Acid Bacteria as Starter Culture for Cocoa Fermentation (Theobroma cacao L.). J. Penelit. Pendidik. IPA 2023, 9, 825–831. [Google Scholar] [CrossRef]
- Lewis Dopgima, L.; Jerome, F.-C.; Fossi Bertrand, T.; Lawrence Tatanah, N.; Rauwitta Omabit, A.; Yannick, T.; Ekwa Yawa, M.; Erasmus Nchuaji, T.; Lohr Lewis Fombang, E.; Vincent Pryde Kehdinga, T.; et al. Comparison of Cocoa Bean Quality Produced with Different Starter Cultures and Fermentation Methods. Int. J. Microbiol. Biotechnol. 2023, 8, 10–18. [Google Scholar] [CrossRef]
- García Gonzalez, E.; Mendez Orejuela, J.; Sierra Banguera, J.; Chamorro Moreno, D.; Ordoñez Narváez, G.; Ochoa Muñoz, A.; Montalvo Rodriguez, C. Ecology and Population Dynamics of Yeast Starter Cultures in Cocoa Beans Fermentation. BioTechnologia 2022, 103, 343–353. [Google Scholar] [CrossRef]
- Visintin, S.; Ramos, L.; Batista, N.; Dolci, P.; Schwan, F.; Cocolin, L. Impact of Saccharomyces Cerevisiae and Torulaspora Delbrueckii Starter Cultures on Cocoa Beans Fermentation. Int. J. Food Microbiol. 2017, 257, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Influence of Selected Native Yeast Starter Cultures on the Antioxidant Activities, Fermentation Index and Total Soluble Solids of Malaysia Cocoa Beans: A Simulation Study. LWT 2020, 122, 108977. [Google Scholar] [CrossRef]
- Díaz-Muñoz, C.; Van de Voorde, D.; Comasio, A.; Verce, M.; Hernandez, C.E.; Weckx, S.; De Vuyst, L. Curing of Cocoa Beans: Fine-Scale Monitoring of the Starter Cultures Applied and Metabolomics of the Fermentation and Drying Steps. Front. Microbiol. 2021, 11, 616875. [Google Scholar] [CrossRef]
- Junior, G.C.A.C.; Ferreira, N.R.; Andrade, E.H.d.A.; Do Nascimento, L.D.; de Siqueira, F.C.; Lopes, A.S. Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces Cerevisiae KY794742 and Pichia Kudriavzevii KY794725. Molecules 2021, 26, 344. [Google Scholar] [CrossRef]
- Cocoa Fermentation: Starter Addition Effect. Agrobiol. Rec. 2022, 9, 37–44. [CrossRef]
- Díaz-Muñoz, C.; Van de Voorde, D.; Tuenter, E.; Lemarcq, V.; Van de Walle, D.; Soares Maio, J.P.; Mencía, A.; Hernandez, C.E.; Comasio, A.; Sioriki, E.; et al. An In-Depth Multiphasic Analysis of the Chocolate Production Chain, from Bean to Bar, Demonstrates the Superiority of Saccharomyces Cerevisiae over Hanseniaspora Opuntiae as Functional Starter Culture during Cocoa Fermentation. Food Microbiol. 2023, 109, 104115. [Google Scholar] [CrossRef]
- Tejeda, J.F.; Arango-Angarita, J.; Cuervo, J.L. Effect of Solar Pre-Drying and Yeast Starter Inoculation Treatments on the Chemical Composition of Cocoa (Theobroma cacao L.) Beans from Southwestern Colombia. Foods 2023, 12, 4455. [Google Scholar] [CrossRef] [PubMed]
- Balcázar-Zumaeta, C.R.; Fernández-Romero, E.; Lopes, A.S.; Ferreira, N.R.; Chagas-Júnior, G.C.A.; Yoplac, I.; López-Trigoso, H.A.; Tuesta-Occ, M.L.; Maldonado-Ramirez, I.; Maicelo-Quintana, J.L.; et al. Amino Acid Profile Behavior during the Fermentation of Criollo Cocoa Beans. Food Chem. X 2024, 22, 101486. [Google Scholar] [CrossRef]
- Marwati, T.; Djaafar, T.F.; Hatmi, R.U.; Kobarsih, M.; Indrasari, S.D.; Fitrotin, U.; Fajariyah, A.; Wibowo, N.A.; Anantama, M.S.; Wikandari, R.; et al. Alternative Fermentation Method of Cocoa Beans: The Use of Lactiplantibacillus Plantarum Subsp. Plantarum HL-15 as Starter Culture and Valorization of Cocoa Pulp by-Product. J. Agric. Food Res. 2024, 18, 101398. [Google Scholar] [CrossRef]
- Mendoza Salazar, M.M.; Martínez Álvarez, O.L.; Ardila Castañeda, M.P.; Lizarazo Medina, P.X. Bioprospecting of Indigenous Yeasts Involved in Cocoa Fermentation Using Sensory and Chemical Strategies for Selecting a Starter Inoculum. Food Microbiol. 2022, 101, 103896. [Google Scholar] [CrossRef]
- Aziz, N.; Nur, Y.S.; Djulardi, A. Preparing Fermented Cocoa Pods Useful for Producing Quail Eggs with Low Cholesterol and Omega-3 Contents, by Mixing Cocoa Pod Substrate and Tofu Residues, Sterilizing, Adding Pleurotus Ostreatus Inoculum, Fermenting and Heating Product. ID202000442-U1. Patent number: S00201909258, 2019. [Google Scholar]
- He, J.; Li, M.; Yang, N.; Shuai, X.; Jiang, T.; He, Y.; Zhang, R. Nutritional Composition Useful for e.g. Reducing Blood Sugar, Contains Yeast Glucan, Barley, Chitosan Oligosaccharide, Inulin, Citrus Fruit Fiber, Seaweed, Momordica Charantia Polypeptide Powder, Kudzu Vine Root and Buckwheat. CN110800994-A. Patent number: 201911020687.4, 2019. [Google Scholar]
- Shi, M. Preparing High-Temperature-Resistant Chocolate Involves Heating and Melting Cocoa Butter to Obtain Cocoa Butter Liquid, Subjecting Skim Milk Powder and Stachyose to One Refining and Refining to Obtain One Refining Material. CN110810602-A. Patent number: 201911283924.6, 2019. [Google Scholar]
- Vieira, A.M.J. Producing Brandy Involves Harvesting Ripe Fruits, Collecting Manually or Mechanically with Aid of Collection Tools into Storage Containers, and Then Transporting for Reception Area and Heavy Goods. BR102019020655-A2. Patent number: 102019020655-1, 2019. [Google Scholar]
- Langan, J.P.; Nadal, M.; Clark, A.J. Producing Myceliated Cacao Product, Comprises Fermenting Harvested Cacao Comprising Beans, Pulp and/or Pods from Fruit Pods of Species Theobroma cacao, and Providing Fungal Inoculum Comprising Filamentous Fungus. WO2022046913-A1. Patent number: 2021/047560, 2022. [Google Scholar]
- Dharamkar, R.R.; Kapse, A.; Pradeep, H.; Joshi, M.R.; Basri, S.J.T.; Upadhyay, M.; Pradhan, P. Producing Cocoa Polyphenols-Enriched Cocoa Component for Use in Food Composition, Involves Reacting Fat from Cocoa Beans and/or Processing Cocoa Beans to Produce Ingredient That Retains Polyphenol Levels, e.g. Procyanidins. IN202221007331-A. Patent number: 202221007331, 2022. [Google Scholar]
- Rossel, F. Producing Cocoa Drink, Comprises Fermenting Cocoa Beans, Fermenting, Drying Cocoa Beans, Drying in Aqueous Soaking Liquid, Soaking Cocoa Beans, and Removing Cocoa Beans from Soaking Liquid. DE102019123661-A1. Patent number: 10 2019 123 661.4, 2019. [Google Scholar]
- Mota-Gutierrez, J.; Barbosa-Pereira, L.; Ferrocino, I.; Cocolin, L. Traceability of Functional Volatile Compounds Generated on Inoculated Cocoa Fermentation and Its Potential Health Benefits. Nutrients 2019, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, H.; Tzompa Sosa, D.A.; De Winne, A.; Ruales, J.; De Clippeleer, J.; De Leersnyder, I.; De Wever, J.; Everaert, H.; Messens, K.; Dewettinck, K. Dynamics of Volatile Compounds and Flavor Precursors during Spontaneous Fermentation of Fine Flavor Trinitario Cocoa Beans. Eur. Food Res. Technol. 2019, 245, 1917–1937. [Google Scholar] [CrossRef]
- Lee, A.H.; Neilson, A.P.; O’Keefe, S.F.; Ogejo, J.A.; Huang, H.; Ponder, M.; Chu, H.S.S.; Jin, Q.; Pilot, G.; Stewart, A.C. A Laboratory-Scale Model Cocoa Fermentation Using Dried, Unfermented Beans and Artificial Pulp Can Simulate the Microbial and Chemical Changes of on-Farm Cocoa Fermentation. Eur. Food Res. Technol. 2019, 245, 511–519. [Google Scholar] [CrossRef]
- Velásquez-Reyes, D.; Rodríguez-Campos, J.; Avendaño-Arrazate, C.; Gschaedler, A.; Alcázar-Valle, M.; Lugo-Cervantes, E. Forastero and Criollo Cocoa Beans, Differences on the Profile of Volatile and Non-Volatile Compounds in the Process from Fermentation to Liquor. Heliyon 2023, 9, e15129. [Google Scholar] [CrossRef]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of Turning, Pod Storage and Fermentation Time on Microbial Ecology and Volatile Composition of Cocoa Beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of Volatile and Non-Volatile Compounds in Cocoa (Theobroma cacao L.) during Fermentation and Drying Processes Using Principal Components Analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Agyirifo, D.S.; Wamalwa, M.; Otwe, E.P.; Galyuon, I.; Runo, S.; Takrama, J.; Ngeranwa, J. Metagenomics Analysis of Cocoa Bean Fermentation Microbiome Identifying Species Diversity and Putative Functional Capabilities. Heliyon 2019, 5, e02170. [Google Scholar]
- Silva, G.S.; Dala-Paula, B.M.; Bispo, E.S.; Gloria, M.B.A. Bioaccessibility of Bioactive Amines in Dark Chocolates Made with Different Proportions of Under-Fermented and Fermented Cocoa Beans. Food Chem. 2023, 404, 134725. [Google Scholar] [CrossRef] [PubMed]
- Novita Sari, I.; Setiawan, T.; Seock Kim, K.; Toni Wijaya, Y.; Won Cho, K.; Young Kwon, H. Metabolism and Function of Polyamines in Cancer Progression. Cancer Lett. 2021, 519, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Tabur, S.; Ozmen, S.; Oney-Birol, S. Promoter Role of Putrescine for Molecular and Biochemical Processes under Drought Stress in Barley. Sci. Rep. 2024, 14, 19202. [Google Scholar] [CrossRef]
- Nascimento, L.L.; Pereira, M.S.; de Almeida, L.S.; da Silveira Ferreira, L.; de Moura Pita, B.L.; de Souza, C.O.; Ribeiro, C.D.F.; Fricks, A.T. Innovation in Cocoa Fermentation: Evidence from Patent Documents and Scientific Articles. Fermentation 2024, 10, 251. [Google Scholar] [CrossRef]
- Bowser, S.M.; Moore, W.T.; McMillan, R.P.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Hulver, M.W.; Neilson, A.P. High-Molecular-Weight Cocoa Procyanidins Possess Enhanced Insulin-Enhancing and Insulin Mimetic Activities in Human Primary Skeletal Muscle Cells Compared to Smaller Procyanidins. J. Nutr. Biochem. 2017, 39, 48–58. [Google Scholar] [CrossRef]
- Gil, M.; Uribe, D.; Gallego, V.; Bedoya, C.; Arango-Varela, S. Traceability of Polyphenols in Cocoa during the Postharvest and Industrialization Processes and Their Biological Antioxidant Potential. Heliyon 2021, 7, e07738. [Google Scholar] [CrossRef]
- Vieira, A.M.J. Producing Brandy Involves Harvesting Ripe Fruits, Collecting Manually or Mechanically with Aid of Collection Tools into Storage Containers, and Then Transporting for Reception Area and Heavy Goods 2021. BR102019020655-1A2, 23 February 2021. [Google Scholar]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa Has More Phenolic Phytochemicals and a Higher Antioxidant Capacity than Teas and Red Wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Wickramasuriya, A.M.; Dunwell, J.M. Cacao Biotechnology: Current Status and Future Prospects. Plant Biotechnol. J. 2018, 16, 4–17. [Google Scholar]

| Patent | Category | Objective | Ingredients/Organisms | Technological Approach | Key Benefits | Ref. |
|---|---|---|---|---|---|---|
| ID202000442-U1 | Mevastatin | Produce Mevastatin-rich quail feed | Cocoa husks, tofu pulp, Pleurotus ostreatus | Fermentation using agricultural byproducts | Low cholesterol, omega-3 enriched feed; promotes sustainability by reducing food waste | [143] |
| CN110800994-A | Polysaccharides | Produce selenium-enriched oligosaccharide tablets | Aspergillus niger, mushroom powder, barley grass, deer antler | Low-temperature mixing, ethanol granulation, TCM principles | High selenium, natural health benefits for heart, liver, immunity | [144] |
| CN110810602-A | Exopolysaccharide | Produce heat-resistant chocolate | Cocoa butter, lard, palm oil, Bifidobacterium, Lactobacillus strains | Emulsifiers, thickening agents (fenugreek gum), non-traditional grinding | Increased melting point and shelf life, suitable for variable storage conditions | [145] |
| BR102019020655-A2 | Alcohols | Produce cacao-based aguardente | Cacao fruit, Saccharomyces cerevisiae, Saccharomyces bayanus | Enzyme-yeast sequencing, optimized must fermentation | Improved fermentation efficiency; quality alcoholic beverage derived from cacao | [146] |
| WO2022046913-A1 | Enzymatic Digestion | Produce myceliated cacao with improved flavor | Cacao beans, pulp, pods, filamentous fungi | Natural fermentation with biotechnological enhancement | Flavorful cacao without artificial sweeteners, appealing to natural food markets | [147] |
| IN202221007331-A | Polyphenols and Antioxidants | Produce cacao with high polyphenol content | Cacao beans (fat removal, preserved polyphenols) | Efficient extraction and preservation methods | Nutrient-dense products with high polyphenol content for improved health benefits | [148] |
| DE102019123661-A1 | Vitamins and Tannins | Develop fermented cacao drink with optimized flavor | Cacao seeds, natural microorganisms | Controlled fermentation, seed drying and soaking | Enhanced flavor profile in cacao drink, potential for new beverage blends like coffee infusions | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, S.d.M.; Martínez-Burgos, W.J.; dos Reis, G.A.; Ocán-Torres, D.Y.; dos Santos Costa, G.; Rosas Vega, F.; Alvarez Badel, B.; Sotelo Coronado, L.; Lima Serra, J.; Soccol, C.R. The Role of Microbial Dynamics, Sensorial Compounds, and Producing Regions in Cocoa Fermentation. Microbiol. Res. 2025, 16, 75. https://doi.org/10.3390/microbiolres16040075
Campos SdM, Martínez-Burgos WJ, dos Reis GA, Ocán-Torres DY, dos Santos Costa G, Rosas Vega F, Alvarez Badel B, Sotelo Coronado L, Lima Serra J, Soccol CR. The Role of Microbial Dynamics, Sensorial Compounds, and Producing Regions in Cocoa Fermentation. Microbiology Research. 2025; 16(4):75. https://doi.org/10.3390/microbiolres16040075
Chicago/Turabian StyleCampos, Sofia de M., Walter J. Martínez-Burgos, Guilherme Anacleto dos Reis, Diego Yamir Ocán-Torres, Gabriela dos Santos Costa, Fernando Rosas Vega, Beatriz Alvarez Badel, Liliana Sotelo Coronado, Josilene Lima Serra, and Carlos Ricardo Soccol. 2025. "The Role of Microbial Dynamics, Sensorial Compounds, and Producing Regions in Cocoa Fermentation" Microbiology Research 16, no. 4: 75. https://doi.org/10.3390/microbiolres16040075
APA StyleCampos, S. d. M., Martínez-Burgos, W. J., dos Reis, G. A., Ocán-Torres, D. Y., dos Santos Costa, G., Rosas Vega, F., Alvarez Badel, B., Sotelo Coronado, L., Lima Serra, J., & Soccol, C. R. (2025). The Role of Microbial Dynamics, Sensorial Compounds, and Producing Regions in Cocoa Fermentation. Microbiology Research, 16(4), 75. https://doi.org/10.3390/microbiolres16040075








