Abstract
Multidrug-resistant (MDR) bacterial pathogens pose a serious threat to global health, underscoring the urgent need for innovative therapeutic strategies. In this work, we designed and characterized thiol-modified antisense oligonucleotide-capped gold nanoparticles (ASO-AuNPs) to resensitize antibiotic-resistant bacteria. Transmission electron microscopy and UV–Vis spectroscopy confirmed the morphology, size, and optical properties of AuNPs and ASO-AuNPs. Minimum inhibitory concentrations (MIC) of ampicillin were determined for non-resistant Escherichia coli DH5α (16 ppm) and an ampicillin-resistant E. coli DH5α strain (PSK, 32,768 ppm). When co-administered with ampicillin, ASO-AuNPs (0.1 and 0.2 nM) significantly reduced bacterial growth compared to the antibiotic-alone control (p < 0.05), demonstrating the capacity of ASO-AuNPs to restore antibiotic efficacy. These findings provide a proof of concept that antisense oligonucleotide-functionalized nanomaterials can be harnessed to overcome beta-lactam resistance, setting the stage for further optimization and translation into clinical applications.
1. Introduction
Multidrug-resistant (MDR) bacterial pathogens pose one of the most critical challenges to modern healthcare, as infections caused by these organisms lead to prolonged hospital stays, increased morbidity, and a heightened risk of mortality. Several convergent factors exacerbate this crisis. On one hand, the overuse and misuse of antibiotics in both clinical and agricultural settings accelerate the natural selection of resistant strains. On the other hand, inadequate sanitation and hygiene practices, particularly in areas with limited access to healthcare infrastructure, facilitate the rapid dissemination of resistant pathogens within communities [1]. Compounding these issues is the stagnation in the development of novel antibiotics over the past few decades, largely attributable to diminishing economic incentives for pharmaceutical companies and the complex regulatory pathways for bringing new antibiotics to market. Consequently, infections caused by bacteria carrying multiple resistance mechanisms—rendering them impervious to many conventional therapies—have become alarmingly prevalent [2]. These factors collectively contribute to the escalating burden of MDR pathogens, emphasizing the urgent need for innovative strategies to combat antibiotic resistance.
An array of resistance mechanisms can make pathogens exceedingly difficult to eradicate. For instance, some bacteria develop efflux pumps that actively expel antibiotics before they can reach their intracellular targets [3], whereas others acquire enzymes that degrade or modify antibiotic molecules [4]. In parallel, structural alterations in bacterial cell envelopes or target sites can further compromise the efficacy of multiple drug classes [5]. As a result, infections that were once manageable with first-line antibiotics now demand broader-spectrum agents, increasing both treatment costs and the likelihood of adverse side effects. The continual emergence of new resistant strains, including carbapenem-resistant Enterobacteriaceae and extended-spectrum beta-lactamase (ESBL) producers, underscores the vast genetic adaptability of bacteria. Heightening the urgency, the World Health Organization has consistently warned that we are approaching a “post-antibiotic era”, wherein a range of critical infections could become untreatable [6].
Given this challenge, antisense oligonucleotides (ASOs) are garnering considerable attention as a promising alternative or adjunct to conventional antibiotics. ASOs are short, single-stranded nucleic acid sequences (commonly 13–30 nucleotides) [7] designed to bind a specific target mRNA through Watson–Crick base pairing, thereby inhibiting protein translation or triggering RNase H-mediated degradation [8]. By selectively knocking down genes responsible for antibiotic resistance, ASOs may restore the potency of existing antibiotics, offering a targeted approach that could reduce collateral damage to the normal microbiota. Moreover, ASOs can be synthesized and re-designed with relative speed compared to the protracted process of discovering and developing new antibiotic molecules. This flexibility not only facilitates rapid iterations to combat emerging resistance mutations but also lowers the probability of generating resistance to the ASO itself, given its highly specific mode of action [9,10].
Despite these advantages, the practical implementation of ASO-based therapies against bacterial pathogens faces multiple hurdles. Among these is the challenge of delivering highly charged, relatively large ASOs across bacterial membranes, which often feature sophisticated permeability barriers and active efflux systems [11]. Bacterial cells, especially Gram-negative species like Escherichia coli or Pseudomonas aeruginosa, possess outer membranes containing lipopolysaccharides that significantly hinder the uptake of macromolecules. As such, innovative delivery vehicles or facilitating strategies are needed to overcome these physiological barriers [9,12,13]. Various approaches, including liposomal encapsulation, conjugation with cell-penetrating peptides, and chemical modification of ASOs (e.g., phosphorothioate backbones), are currently under investigation to enhance intracellular targeting. Nevertheless, each method introduces its own complexities, such as potential cytotoxicity or stability issues, emphasizing that optimizing ASO design and delivery requires a delicate balance [14,15,16].
One particularly promising delivery platform is based on gold nanoparticles (AuNPs), which stand out for their unique physical and chemical properties, as well as their established track record in nanomedicine [17,18,19]. AuNPs can be synthesized in various sizes and shapes, affording precise control over their surface chemistry and biodistribution. Moreover, AuNP surfaces can be readily functionalized with biomolecules (e.g., peptides, antibodies, or nucleic acids) via electrostatic interactions or the formation of robust Au-S bonds with thiol groups [20]. This covalent-type conjugation confers stability to the resulting complexes under physiological conditions, a critical requirement for potential therapeutic applications [21]. In addition, AuNPs are often reported to have relatively low cytotoxicity when prepared and administered at appropriate concentrations, making them suitable candidates for drug delivery in vivo [22,23]. Even though gold is more expensive than other potential nanoparticle materials (e.g., silica or iron oxide), its well-characterized surface chemistry, robust Au–S bonding, and favorable colloidal stability make it an ideal platform for proof-of-concept studies. This foundational work can be extended in future studies to more cost-effective carriers, provided they offer comparable biocompatibility and conjugation efficiency.
Importantly, the conjugation of ASOs to AuNPs via a terminal thiol group can improve cellular uptake, protect ASOs from nucleases, and potentially enable controlled release at the target site. Recent studies highlight that this approach may facilitate higher local concentrations of ASOs at bacterial surfaces, thereby enhancing inhibition of resistance genes [24]. Moreover, AuNP-based carriers are amenable to further functional modifications—such as targeting ligands or additional antimicrobial peptides—which could synergistically improve efficacy [13]. Given these advantages, AuNPs represent a compelling platform to systematically overcome the intrinsic and acquired barriers that limit the effectiveness of conventional ASOs in antimicrobial settings [25].
Table 1 provides a brief comparison of recent ASO-based or nanocarrier-based strategies to contextualize our work within the broader field of antimicrobial research.

Table 1.
Comparison of selected recent approaches for antisense-based or nanocarrier-based strategies aimed at combating antibiotic resistance.
Based on these considerations, the present study explores a novel strategy that employs thiol-modified ASOs conjugated to AuNPs to resensitize antibiotic-resistant bacteria. By targeting specific resistance genes, this platform aims to silence the production of resistance-associated proteins, thereby restoring the bactericidal or bacteriostatic properties of existing antibiotics. We hypothesize that the dual benefits of ASOs’ target specificity and AuNPs’ robust delivery features will enable more efficient inhibition of resistance pathways, ultimately contributing to the development of next-generation antimicrobial therapies. This work thus lays the foundation for broader applications of ASO-AuNP conjugates in managing both emerging and established MDR pathogens, offering renewed hope in the global battle against antibiotic resistance.
2. Materials and Methods
2.1. Chemicals
All chemicals used in this study were of at least analytical grade to ensure minimal impurities and consistent experimental performance. The main suppliers were CTR Scientific (Monterrey, NL, Mexico) for gold(III) chloride, sodium citrate, dithiothreitol (DTT), and phosphate-buffered saline (PBS). A 100–5000 bp DNA Marker Plus (Ready-to-use) was obtained from BioBasic (Markham, ON, Canada) for molecular weight analysis of nucleic acids. Luria–Bertani (LB) broth and other microbiological media (BD Difco™, Becton Dickinson, Ciudad de Mexico, Mexico) were sterilized by autoclaving at 121 °C for 20 min before use. Deionized (DI) water (18.2 MΩ·cm) was used for all solution preparations, and filtration through a 0.22 µm membrane filter was performed where necessary to ensure sterility. Unless otherwise noted, all reagents were used as received without further purification.
2.2. Bacterial Strain and Culture Conditions
The Escherichia coli (E. coli) DH5α strain, purchased from Invitrogen (Carlsbad, CA, USA), served as the primary host for plasmid propagation and baseline antibiotic susceptibility assessment. LB broth (10 g/L tryptone, 5 g/L yeast extract, 10 g/L sodium chloride, pH 7.0 ± 0.2) was used as the standard growth medium. For routine cultivation, a single colony of E. coli DH5α was inoculated into 5 mL of LB broth and incubated at 37 °C with shaking at 150 rpm in a Lab Companion incubator shaker (BioFree Co., Seoul, Republic of Korea) for 16–18 h. This overnight culture was then subcultured at a 1:100 dilution into fresh LB broth and grown under identical conditions until the optical density at 600 nm (OD600) reached approximately 0.2 (exponential phase).
Bacterial growth was monitored by measuring OD600 using a UV–Vis spectrophotometer (Optizen 2120UV PLUS, Mecasys Co., Seoul, Republic of Korea) calibrated with sterile LB broth as the blank. To ensure experimental reproducibility and purity, cultures were periodically streaked onto LB agar plates (supplemented with 15 g/L agar) and checked for colony morphology. All culture manipulations were carried out in a Class II biological safety cabinet (Esco Lifesciences, Singapore) under aseptic conditions to prevent contamination. Following each experiment, all bacterial cultures, consumables, and media were autoclaved or disinfected using an appropriate chemical agent (e.g., 10% bleach solution) before disposal.
2.3. Antisense Oligonucleotides
Antisense oligonucleotides (ASOs) targeting the ampR gene were designed using a multi-software, stepwise approach. First, in silico sequence analysis and preliminary design were performed in SnapGene Viewer 1.0 (http://www.snapgene.com/ (accessed on 1 February 2025)) to identify the open reading frames (ORFs) of the ampR gene of interest. Subsequently, IDT OligoAnalyzer 1.0 (http://www.idtdna.com/calc/analyzer (accessed on 11 November 2024)) and NUPACK 4.0 (http://www.nupack.org/ (accessed on 15 January 2024)) were employed to predict secondary structures, melting temperatures, and potential off-target interactions. The primary goal was to ensure that the designed ASOs exhibited strong, stable base pairing with the target mRNA while avoiding self-dimerization or formation of significant secondary structures that could impair their binding efficiency.
To validate target specificity, we consulted the National Center for Biotechnology Information (NCBI) “genbank” databases (https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 2 November 2023)) for any overlapping sequences in E. coli genomes. We further employed BLAST (Basic Local Alignment Search Tool, https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 2 November 2023)) searches to confirm that each candidate ASO exhibited fewer than 85% homology to off-target regions in both the chromosomal DNA and plasmid sequences of E. coli, thus reducing the risk of unintended gene silencing.
Following the algorithm described by Hoynes-O’Connor and Moon, attention was focused on the first eight codifying codons of the beta-lactamase enzyme AmpR to maximize the impact of mRNA hybridization and degradation [31]. By targeting the 5′ region of the mRNA, the ASOs were designed to interfere at an early stage of protein translation, thereby enhancing the likelihood of inhibiting beta-lactamase expression. After iterative optimization of sequence length (typically 20–25 nucleotides), GC content, and secondary structure constraints, a final antisense sequence of 5′-GACACGGAAATGTTGAATACTCAT-3′ was selected.
The antisense oligonucleotide (ASO) selected was designed according to Hoynes-O’Connor and Moon [31] and their protocol highlights three critical factors—ASO length, thermodynamic stability (∆G interaction), and mismatch percentage—that substantially influence the efficacy of gene silencing. For instance, extending the ASO beyond 15 nucleotides correlates with enhanced repression efficiency, while lower ∆G values (<−40 kcal/mol) reflect increased thermodynamic stability of the ASO–mRNA duplex. Up to 15% mismatch can still permit meaningful repression, but higher mismatch percentages risk losing specificity.
Based on these guidelines, our ASO was predicted to bind strongly and specifically to ampR mRNA, thus effectively inhibiting beta-lactamase production. To confirm off-target risk, we performed BLAST analyses against both random E. coli annotations (Supplementary Table S1) and the E. coli K12 genome (Supplementary Table S2). For Supplementary Table S1, the alignments fell into three categories: (1) 100% identity at 24 nt, representing a precise match with the ampR gene; (2) 95.83% identity at 24 nt, indicating point mutations of ampR; and (3) 100% identity for segments shorter than 24 nt, corresponding to random, ampR-unrelated genes. Meanwhile, Supplementary Table S2 lists all the possible alignments within the E. coli K12 genome, although many fail to meet the strict requirements set by Hoynes-O’Connor and Moon (>15 nt overlap, <15% mismatch, and ∆G < −40 kcal/mol) to drive significant gene repression. Consequently, we expect minimal off-target inhibition under our experimental conditions.
Nevertheless, previous reports demonstrate that partial mismatches can still yield minor unintended effects [32,33,34]. Therefore, future work will include in vivo validation to confirm that this ASO does not substantially silence non-ampR genes. Such thorough bioinformatic screening and empirical testing are critical to reduce potential toxicity linked to off-target RNA suppression and to ensure the safety and specificity of ASO-based therapeutics.
To facilitate covalent attachment to gold nanoparticles (AuNPs), the ASOs were synthesized (Biobasic, Markham, ON, Canada) with a 5′ thiol group and a poly-A spacer. The thiol group (–SH) permits robust Au–S bond formation, enhancing conjugate stability and ensuring efficient delivery of ASOs to the bacterial cell surface. The poly-A spacer further reduces steric hindrance by distancing the core antisense region from the nanoparticle surface, thus preserving optimal binding orientation and accessibility to the ampR mRNA target. All ASOs were received lyophilized, dissolved in nuclease-free water to 100 µM stocks, and stored at −20 °C until subsequent use in nanoparticle functionalization and in vitro tests.
2.4. Transformation of E. coli DH5α into an Ampicillin-Resistant Strain
To investigate a robust, proof-of-concept approach, we employed Escherichia coli (E. coli) DH5α as our initial model. Although this strain does not naturally produce beta-lactamase, clinical isolates of E. coli frequently acquire plasmids carrying resistance genes, underscoring its relevance as a platform to study horizontally transferred resistance. To generate an ampicillin-resistant derivative of E. coli DH5α, we introduced plasmid pSK9065 (Addgene, Watertown, MA, USA) into the parental host via electroporation. Before electroporation, electrocompetent cells were prepared following a protocol modified from Li et al.; specifically, 1.4 mL of LB broth was inoculated with 100 μL of a freshly grown overnight culture of E. coli DH5α and incubated at 37 °C with vigorous shaking (900 rpm) until the optical density at 600 nm reached 0.6, indicative of a mid-exponential phase [35].
Cells were pelleted by centrifugation (9000 rpm, 2 min, 24 °C), and the supernatant was discarded. The cell pellet was resuspended in 1 mL of pre-chilled, sterile 10% glycerol or an equivalent non-ionic wash solution to remove residual salts. This washing step was repeated twice to ensure minimal ionic content, which is crucial for preventing arcing during electroporation. After the final wash, the pellet was gently resuspended in 30 μL of ultrapure water and maintained at room temperature until the electroporation step.
Separately, ~300 ng of the pSK9065 plasmid was diluted in 10 μL of ultrapure water (or 1× TE buffer) and placed into a pre-chilled 1 mm gap electroporation cuvette (Eppendorf, Hamburg, Germany). The 30 μL of electrocompetent cells were then carefully mixed with the plasmid DNA solution in the cuvette, ensuring minimal bubble formation. Electroporation was performed using an Eppendorf Electroporator set to deliver a 2.5 kV pulse (2500 V), with a typical time constant (τ) in the range of 4.5–5.0 ms.
Immediately after electroporation, the cuvette contents were gently transferred to a microcentrifuge tube containing 1 mL of sterile LB broth to facilitate cell recovery. The mixture was incubated at 37 °C for 1 h with shaking (150–200 rpm) to allow phenotypic expression of the newly introduced resistance marker. Subsequently, 100 µL of the recovery culture was spread onto LB agar plates supplemented with 100 ppm ampicillin (in accordance with the manufacturer’s guidelines). The plates were incubated at 37 °C for approximately 24 h.
Following incubation, discrete colonies appearing on the selective agar were enumerated, restreaked for purity if needed, and designated as the ampicillin-resistant (PSK) strain. To confirm plasmid retention, individual colonies were grown in LB containing 100 ppm ampicillin and subjected to plasmid isolation (see below). Stock cultures of confirmed PSK strains were prepared in 20% glycerol and stored at −80 °C for long-term preservation.
2.5. Plasmid DNA Extraction
Plasmid DNA was isolated from E. coli cultures using the PureLink™ Quick Plasmid Miniprep Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, with minor modifications for optimal yield. Briefly, 3–5 mL of overnight LB culture (OD600 ≈ 2.0–3.0) was harvested by centrifugation at 10,000× g for 2–3 min at room temperature. The supernatant was carefully discarded, and the bacterial pellet was resuspended in 250 µL of the resuspension buffer (supplemented with RNase A, if provided in the kit). The resuspended cells were thoroughly mixed by vortexing or gentle pipetting to ensure a homogeneous suspension.
Following resuspension, 250 µL of lysis buffer was added, and the tube was inverted 4–6 times to lyse the cells. It was critical to avoid vigorous shaking to minimize shearing of chromosomal DNA. After a 1–2 min incubation at room temperature, 350 µL of neutralization buffer was introduced to precipitate cellular debris and chromosomal DNA. The solution was inverted several times and centrifuged at 12,000–14,000× g for 5 min to clear the lysate. The supernatant, containing the plasmid DNA, was transferred carefully into a PureLink™ spin column placed in a 2 mL collection tube.
The spin column was then centrifuged at 12,000–14,000× g for 1 min, allowing the plasmid DNA to bind the silica membrane. The flow-through was discarded, and 500 µL of the wash buffer (diluted with ethanol, as per the kit guidelines) was added to the column. Another 1 min spin at 12,000–14,000× g removed the remaining impurities. After discarding the wash solution, an additional spin for 1 min was performed to remove residual ethanol, preventing carryover into the elution step.
Finally, the plasmid DNA was eluted from the column by adding 50 µL of the elution buffer (preheated to 60 °C for improved recovery) directly onto the membrane, waiting 1 min, and then centrifuging for 1 min at 12,000–14,000× g. Eluted DNA was quantified using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) or an equivalent method, and samples were stored at −20 °C until further analysis.
2.6. Restriction Digestion and Gel Electrophoresis
To verify plasmid integrity and confirm the presence of the ampR gene insert, 5 µL of the extracted plasmid DNA (approximately 500–1000 ng) was digested with 5–10 units of BamHI restriction enzyme (Thermo Scientific, Waltham, MA, USA) in a 20 µL reaction volume. The reaction mixture included 2 µL of the recommended 10× reaction buffer, and digestion proceeded for 1–2 h at 37 °C in a thermocycler or heating block.
Subsequently, 0.7% agarose gels were prepared by dissolving agarose in 1× TAE or 1× TBE buffer and adding GelRed (Biotium, Fremont, CA, USA) according to the manufacturer’s guidelines for DNA visualization. Digested plasmid samples, along with an undigested control and a 100–5000 bp DNA Marker Plus (BioBasic, Markham, ON, Canada), were loaded into the gel wells. Electrophoresis was performed at 48 V for 20 min using the MiniPCR™ system (miniPCR Bio, Cambridge, MA, USA) or an equivalent apparatus. After electrophoresis, bands were visualized under a UV transilluminator or blue light imager, and images were recorded. The observed fragment patterns were compared to the theoretical band sizes predicted from the plasmid map, confirming the successful presence and proper configuration of the desired insert.
2.7. Synthesis of AuNPs
Gold nanoparticles were prepared using a sodium citrate reduction method adapted from Ghaffari, with minor modifications to optimize yield and nanoparticle size distribution [36]. To begin, 10 mL of a 0.01% (w/v) aqueous gold (III) chloride (AuCl3) solution was placed in a borosilicate glass flask and heated to a gentle boil under constant stirring (approximately 500–800 rpm) using a magnetic stirrer–hotplate. The use of high-quality, sterile-filtered water (18.2 MΩ·cm) was crucial to minimize contamination and ensure the consistent nucleation and growth of the nanoparticles.
Once the solution reached boiling point, 100 µL of a 10% (w/v) sodium citrate solution was introduced dropwise. Sodium citrate acts both as a reducing agent—converting Au (III) to elemental gold (Au0)—and as a stabilizer by adsorbing onto the nanoparticle surface to prevent aggregation. Immediately after the addition of sodium citrate, the color of the reaction mixture began to transition from pale yellow to colorless, then into grayish or purple hues, and ultimately evolved into a characteristic ruby-red or purplish color. This visual color change signaled the formation of colloidal gold nanoparticles.
The reaction was then removed from the heat and allowed to cool to room temperature with continued stirring for an additional 10–20 min, ensuring complete reduction and consistent nanoparticle size. Throughout the procedure, the flask was covered with aluminum foil or kept under dim light conditions to minimize photoreduction or potential light-induced aggregation.
Once the reaction mixture had cooled, the resultant AuNP suspension was transferred into an amber or foil-wrapped container to protect it from prolonged exposure to light. Dynamic light scattering (DLS) or UV–Vis spectroscopy can be performed at this stage to confirm the presence of a surface plasmon resonance (SPR) peak at approximately 520–530 nm, which is indicative of spherical gold nanoparticles in the 10–50 nm size range.
Finally, the AuNP solution was stored at 4 °C in the dark until needed. Under these storage conditions, the colloid remained stable for several weeks without noticeable precipitation. However, periodic checks by UV–Vis spectroscopy were conducted to assess any potential shifts in the SPR peak that might indicate aggregation or instability. These gold nanoparticles served as the foundation for all subsequent conjugation steps with antisense oligonucleotides.
2.8. ASO Thiol Activation and AuNP Conjugation
2.8.1. Thiol Activation of ASOs
To facilitate the formation of robust Au-S bonds, the 5′-terminal alkanethiol group on the antisense oligonucleotides (ASOs) was chemically reduced to expose the active thiol moiety. This step involved breaking the internal disulfide linkage originally protecting the thiol group. A modified protocol based on that of Hurst et al. was employed for this purpose [24]. Briefly, in a 0.5 mL microcentrifuge tube, 52 µL of ultrapure water, 10 µL of ASO solution (100 µM), 20 µL of Dithiothreitol (DTT, 0.5 M), and 18 µL of phosphate-buffered saline (PBS, 1 M, pH 8) were combined. The resulting mixture was incubated at room temperature for 1 h to facilitate the disulfide reduction by DTT. After the reaction, a NAPTM-5 purification column was utilized to isolate the reduced ASOs from the reaction components. The purified ASO solution was concentrated (Savant SPD 2010 SpeedVac, Thermo Scientific) then stored at −20 °C for future use.
2.8.2. Conjugation of ASOs to Gold Nanoparticles
Surface functionalization of AuNPs with ASOs was achieved through a two-step incubation process followed by overnight stabilization [24]. In the first step, 60 μL of ASO solution (yielding a final concentration of 2.15 μM) was combined with 7 mL of AuNP suspension (resulting in a final concentration of 2.15 nM). This mixture was incubated for 16 h at room temperature to facilitate ASO adsorption onto the AuNP surface via Au-S bond formation. The second step aimed to stabilize the ASO-AuNP conjugates by gradually increasing the ionic strength of the solution. PBS was added dropwise until a final concentration of 10 mM (pH 7.4) was reached, followed by the addition of NaCl to achieve a final concentration of 50 mM. This initial low-salt incubation lasted for 1 h, allowing for controlled electrolyte introduction and minimizing aggregation tendencies. Subsequently, the salt concentration was further increased to 100 mM and the mixture incubated for an additional 24 h to promote stable conjugate formation. Finally, the resulting ASO-AuNP conjugates were stored at 4 °C for future applications. The morphology and the average diameter of AuNPs and ASO-AuNPs were verified by transmission electron microscopy, while the optical properties were characterized by UV–Vis spectroscopy [37].
2.9. TEM Characterization of AuNPs and ASO-AuNPs
Both AuNPs and ASO-AuNPs were deposited onto a carbon-coated copper grid and characterized using high-resolution transmission electron microscopy (HR-TEM) with a JEOL JEM-ARM200F microscope operating in scanning transmission electron microscopy (STEM) mode at 60 kV, utilizing high-angle annular dark field (HAADF) detectors. All images of the Au NPs were acquired in both bright field (BF) and dark field (DF) modes.
2.10. Bacterial Susceptibility Testing
The minimum inhibitory concentrations (MICs) of ampicillin for E. coli DH5α and PSK strain were determined by the microtiter plate dilution method [38] according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI). Afterwards, we evaluate the potential antimicrobial effect of the ASO-AuNPs/ampicillin combination against the ampicillin-resistant PSK strain after 24 h treatment. Based on the MICs results, the PSK strain was treated with 1/3 MIC ampicillin combined with ASO-AuNPs (0.1 and 0.2 nM). MIC and 1/3 MIC ampicillin, and ASO-AuNPs alone were included as controls.
2.11. Statistical Analysis
The results were expressed as the mean ± standard deviation. The data were analyzed by using a one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc analysis.
3. Results and Discussion
3.1. Characterization of AuNPs and ASO-AuNPs
Gold nanoparticles were synthesized by the chemical reduction of AuCl3 using sodium citrate. The reddish-purple color at the end of the reaction is commonly associated with the formation of AuNPs, a finding subsequently confirmed by UV–Vis spectroscopy. First, we characterized the optical properties of AuNPs and ASO-AuNPs by UV–Vis spectroscopy (Figure 1). Spectral scans over the range of 300–800 nm revealed a distinct surface plasmon resonance (SPR) peak at approximately 523 nm for the unmodified AuNPs, consistent with literature values that characterize spherical gold nanoparticles [39,40]. Following functionalization with thiol-modified antisense oligonucleotides (ASOs), the SPR maximum exhibited a slight red shift to ~525 nm, accompanied by a discernible decrease in absorbance peak intensity. This reduction in peak intensity has been reported previously and is generally attributed to the increased local refractive index around the nanoparticle surface caused by oligonucleotide capping. Additionally, partial nanoparticle aggregation or changes in the AuNP dielectric environment can also contribute to alterations in the SPR profile. The data here suggest successful conjugation of ASOs onto the nanoparticle surfaces, forming a stable bio-nanoconjugate (ASO-AuNPs) [41,42,43].

Figure 1.
Representative UV–Vis absorption spectra comparing uncapped gold nanoparticles (AuNPs) and antisense oligonucleotide-capped gold nanoparticles (ASO-AuNPs). AuNPs were synthesized by sodium citrate reduction of AuCl3, producing a characteristic surface plasmon resonance (SPR) peak near 523 nm. For the ASO-AuNPs, 2.15 μM of thiol-modified antisense oligonucleotides (ASOs) was incubated with 2.15 nM AuNPs at room temperature for 16 h. To enhance the colloidal stability, NaCl was incrementally added until a final concentration of 100 mM was reached. The slight red shift in the SPR peak (to ~525 nm) and the reduced peak intensity confirm successful ASO conjugation onto the nanoparticle surfaces.
3.2. Transmission Electron Microscopy and Size Distribution
Transmission electron microscopy images (Figure 2A–D) revealed that gold nanoparticles (AuNPs) produced by sodium citrate reduction had a predominantly spherical shape with a mean particle diameter of 19.42 ± 2.57 nm. By contrast, the antisense oligonucleotide-functionalized AuNPs (ASO-AuNPs; Figure 2E–H) exhibited a slightly larger mean diameter of 21 ± 8.24 nm. Notably, histogram analyses confirmed that unmodified AuNPs spanned 11.54–25.69 nm in diameter, with a principal size peak near 19.41 nm (Figure 2D). In comparison, ASO-AuNPs measured 1.66–54.22 nm, albeit with a main size distribution peak of 21.00 nm (Figure 2H). The broader range detected in ASO-AuNPs can reflect slight aggregation or variations in capping density, which are not uncommon in nanoparticle conjugation processes.
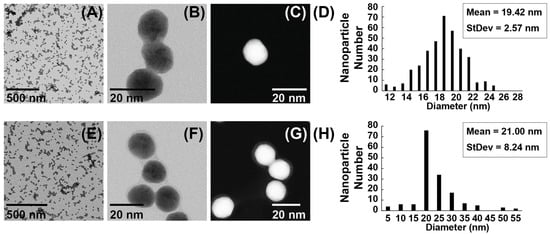
Figure 2.
TEM images and size distribution histogram of AuNPs and ASO-AuNPs. (A–C) TEM micrographs of gold nanoparticles (AuNPs) synthesized by sodium citrate reduction, highlighting their spherical shape. (D) A corresponding size distribution histogram, revealing a principal diameter peak near 19.42 nm. (E–G) TEM micrographs of antisense oligonucleotide-functionalized AuNPs (ASO-AuNPs). A faint “halo” around the nanoparticle periphery is indicative of ASO adsorption. (H) A size distribution histogram of ASO-AuNPs, which still centers near ~21.00 nm despite an extended tail.
An intriguing feature observed in the ASO-AuNP micrographs was the presence of a faint “halo” surrounding individual nanoparticles (Figure 2F,G). This visual phenomenon is typically attributed to the adsorbed oligonucleotide layer on the AuNP surface, corroborating the successful chemisorption of thiolated ASOs. The formation of a distinct corona around nanoparticles has been noted previously as an indicator of biomolecule conjugation and can be used to infer coverage density or conformational arrangement of the adsorbed ligands [44].
The density of ASO molecules on the gold nanoparticle surface plays a pivotal role in determining the overall antibacterial efficacy of ASO-AuNPs. While a higher loading of ASOs can increase local oligonucleotide concentration and potentially enhance mRNA binding, excessively dense packing may introduce steric hindrance that reduces hybridization efficiency. Moreover, an overly crowded surface could inhibit nanoparticle uptake by bacterial cells, further diminishing gene silencing and antibacterial effects [45]. Indeed, it has been demonstrated that oligonucleotide loading strongly influences cellular uptake and gene regulation efficiency for DNA-modified gold nanoparticles [46]. In this study, we selected the initial ASO/AuNP ratio based on established protocols for thiol–Au conjugation, yielding stable conjugates with minimal aggregation [46]. However, future work will systematically optimize and vary the ASO-to-AuNP ratio to balance these competing factors—ensuring both robust oligonucleotide density and efficient bacterial uptake—to further enhance the therapeutic potential of ASO-AuNPs.
3.3. Colloidal Stability of ASO-AuNPs
The ASO-AuNP conjugate demonstrates stability in saline solutions up to 0.1 M NaCl, primarily attributed to the robust chemisorption of the ASO onto the gold nanoparticle surface. Colloidal stability in AuNPs is dependent on the type and strength of their capping agent. While residual citrate ions from the synthesis process initially stabilize the nanoparticles, their weak binding affinity renders them susceptible to displacement by more strongly interacting ligands [47,48]. The ASO molecule, featuring a terminal thiol group with a pronounced affinity for gold, effectively displaces the labile citrate ions, forming a robust Au-S bond and consequently assuming the role of the primary stabilizing agent [45]. Consistent with our results, Li et al. described a similar binding system where thiol-modified oligonucleotides conjugated onto AuNPs, resulting in enhanced nanoparticle stability. Their findings lend further support to the hypothesis that the robust chemisorption of oligonucleotides, mediated by thiolate–gold interactions, significantly contributes to the overall stability of AuNP conjugates [49].
Such stability is essential for biomedical applications, including antisense strategies aimed at overcoming bacterial antibiotic resistance, as it ensures that nanoparticles remain well-dispersed in physiological media.
3.4. Generation and Confirmation of Ampicillin-Resistant E. coli
To establish an ampicillin-resistant E. coli strain, plasmid pSK9065 (Addgene; Watertown, MA, USA) was introduced into E. coli DH5α via electroporation (see Materials and Methods). The pSK9065 plasmid encodes a broad-spectrum beta-lactamase enzyme capable of hydrolyzing a wide range of beta-lactam antibiotics, including those enzymes typically inhibited by conventional beta-lactamase inhibitors such as clavulanic acid, sulbactam, and tazobactam [50]. This type of beta-lactamase, particularly the serine-type, is naturally present in various enterobacteria and non-fermenting gram-negative bacilli [51,52]. This characteristic, combined with their resistance profile, contributes to their association with high mortality rates [53].
Following transformation, colonies that grew on LB agar supplemented with 100 ppm ampicillin were designated as the PSK strain, reflecting successful uptake of pSK9065. To further confirm plasmid presence and integrity, plasmid DNA was isolated from overnight cultures of these colonies and subjected to restriction enzyme digestion. As shown in Figure 3, lane 1 represents the plasmid DNA extracted from the parental E. coli DH5α (no resistance plasmid), while lane 2 shows the undigested plasmid DNA from the PSK strain. BamHI digestion of the PSK plasmid in lane 3 yielded two clear fragments corresponding to approximately 6102 bp and 773 bp, matching the predicted restriction pattern for pSK9065.

Figure 3.
Agarose gel electrophoresis of plasmid DNA extracted from E. coli DH5α and the ampicillin-resistant PSK strain. Lane 1: Plasmid DNA from parental E. coli DH5α. Lane 2: Undigested plasmid DNA from the PSK strain. Lane 3: PSK plasmid DNA digested with BamHI, yielding two bands of ~6102 bp and ~773 bp. MW: Molecular weight marker.
These findings verify that the ampicillin-resistant colonies harbored the intact pSK9065 plasmid, encoding the AmpR beta-lactamase that confers the broad-spectrum resistance phenotype. The transformed PSK strain thus provided a robust model for downstream experiments evaluating the efficacy of antisense oligonucleotide-capped gold nanoparticles (ASO-AuNPs) in reversing antibiotic resistance.
Although E. coli DH5α does not inherently produce beta-lactamase, the introduction of pSK9065 simulates the clinically relevant scenario where plasmids bearing resistance genes can be transferred to otherwise susceptible strains. This design allowed for a controlled assessment of the ASO-AuNPs’ gene-silencing potential prior to exploring other bacteria that endogenously carry such resistance genes (e.g., certain Pseudomonas aeruginosa strains or MRSA).
3.5. Antimicrobial Susceptibility Testing
3.5.1. Minimum Inhibitory Concentration
To ascertain the impact of plasmid-encoded resistance on E. coli DH5α, we measured the minimum inhibitory concentration (MIC) of ampicillin against both the parental (susceptible) DH5α strain and the transformed, ampicillin-resistant (PSK) strain. MIC determinations followed a microdilution approach consistent with CLSI guidelines (see Materials and Methods).
In line with expected susceptibility profiles for laboratory E. coli strains, the MIC of ampicillin for DH5α was 16 ppm (Figure 4A). This value falls within the commonly reported range of 8–32 ppm, thereby validating the strain’s susceptibility [54,55]. Notably, the MIC for the PSK strain increased to 32,768 ppm (Figure 4B), which is approximately 2000-fold higher than that of the parental strain, demonstrating the effective incorporation of the ampR gene and its associated beta-lactamase activity. According to published guidelines, an ampicillin MIC of ≥32 ppm is indicative of resistance; thus, PSK aligns with the multidrug resistance phenotype it was designed to exhibit [56,57]. These striking differences in the ampicillin MIC confirm that PSK constitutes a robust model for investigating potential therapeutic interventions against beta-lactam resistance. Consequently, subsequent experiments utilized this transformed strain to evaluate the capacity of antisense oligonucleotide-capped gold nanoparticles (ASO-AuNPs) to reverse antibiotic resistance mechanisms.
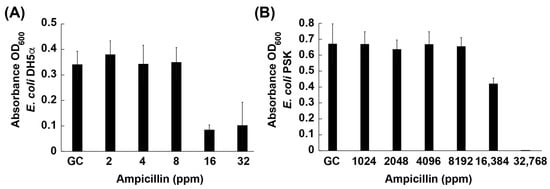
Figure 4.
Minimum inhibitory concentration (MIC) of ampicillin in (A) E. coli DH5α (ampicillin-susceptible) and (B) PSK (ampR plasmid-harboring, ampicillin-resistant). GC: growth control; ppm: parts per million of ampicillin.
3.5.2. Combination Therapy for Ampicillin-Resistant Bacteria
The antisense oligonucleotides (ASOs) conjugated onto gold nanoparticles (AuNPs) are hypothesized to inhibit ampR expression by either physically blocking ribosome binding to ampR mRNA or by inducing RNase H-mediated degradation of the mRNA transcript [58]. In general, antisense molecules that hybridize to a target mRNA can prevent translation machinery from accessing the ribosome binding site (RBS), effectively silencing gene expression [58]. Additionally, ASO–mRNA duplexes may recruit RNase H enzymes, leading to mRNA cleavage and further reducing beta-lactamase production [58].
To explore whether antisense oligonucleotide-capped gold nanoparticles (ASO-AuNPs) could restore ampicillin efficacy in the ampicillin-resistant PSK strain, we employed a sub-minimum inhibitory concentration (sub-MIC) approach. Ampicillin was used at one-third of its MIC (1/3 MIC), a level intended to impose selective pressure on the bacteria without fully inhibiting growth. ASO-AuNPs were tested at two final concentrations (0.1 and 0.2 nM) in combination with sub-MIC ampicillin, and bacterial growth was monitored via OD600 at 24 h. The following control groups were included: Untreated Control (no antibiotic, no ASO-AuNPs), MIC Control (ampicillin at 32,768 ppm, achieving complete inhibition), Sub-MIC Control (1/3 MIC of ampicillin alone), and ASO-AuNP Controls (0.1 or 0.2 nM ASO-AuNPs without ampicillin).
Figure 5 illustrates that the full MIC of ampicillin (32,768 ppm) completely suppressed bacterial growth, whereas sub-MIC ampicillin (1/3 MIC) partially reduced growth relative to the untreated control. Importantly, neither 0.1 nor 0.2 nM of ASO-AuNPs alone produced a statistically significant reduction in bacterial growth, suggesting that the nanoparticle–oligonucleotide conjugates do not exhibit intrinsic bactericidal or bacteriostatic activity at these concentrations.
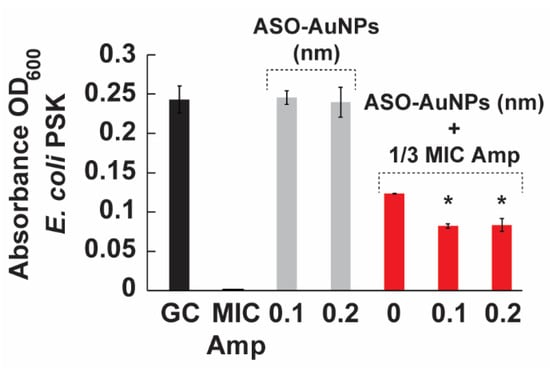
Figure 5.
The effect of ASO-AuNPs on the growth of the PSK strain. Bacteria was cultured in LB broth for 24 h at 37 °C. Growth of the strain was monitored at OD600. The cells were treated with the MIC of ampicillin (32,768 ppm), 1/3 MIC of ampicillin, ASO-AuNPs (0.1 and 0.2 nM), and the combination of ASO-AuNPs at either 0.1 or 0.2 nM and 1/3 MIC ampicillin. Data are shown as the mean ± standard deviation of three samples. (*) indicates values significantly different (p < 0.05) from the corresponding control, as determined by one-way ANOVA followed by Tukey’s post-hoc test. Amp: ampicillin, and ASO: antisense oligonucleotides.
However, when co-administered with 1/3 MIC ampicillin, ASO-AuNPs at 0.1 and 0.2 nM exhibited an additional growth reduction of ~30% compared to 1/3 MIC ampicillin alone. The observed trend indicates that ASO-AuNPs are capable of enhancing ampicillin activity. Several factors—such as dose optimization, timing of treatment, or specific gene expression levels in the PSK strain—could influence the magnitude and consistency of this effect. Further refinement of the ASO design or nanoparticle loading may enhance the synergy between antisense-mediated gene silencing and sub-MIC antibiotic stress.
Several previous investigations have leveraged antisense oligonucleotides (ASOs) or nanocarrier-based approaches to combat antimicrobial resistance. Table 1 summarizes selected recent strategies, including liposome- and nanoparticle-based delivery of ASOs targeting key resistance genes in Pseudomonas aeruginosa, Staphylococcus aureus, and other organisms. These studies highlight how blocking genes such as oprM or mecA can significantly lower the minimum inhibitory concentration (MIC) of antibiotics in multidrug-resistant pathogens, demonstrating that antisense approaches can resensitize bacteria to otherwise ineffective therapies. In the context of our current work, thiol-modified ASOs targeting ampR were conjugated to gold nanoparticles (AuNPs), resulting in a ~30% reduction in bacterial growth when used alongside sub-MIC ampicillin. Although this partial restoration of susceptibility is more modest than in some previous liposome-based examples, our platform offers certain advantages. First, the robust Au-S bond confers notable colloidal stability and may protect ASOs from nucleases. Second, AuNPs permit the multivalent display of oligonucleotides and are readily adaptable for further functionalization (e.g., attaching targeting ligands or antimicrobial peptides). Third, the modular nature of ASOs enables rapid re-design to address evolving resistance mutations or alternative genes.
Overall, these results underscore the feasibility of ASO-AuNP-mediated approaches to attenuate antibiotic resistance. We anticipate that insights gained from this E. coli-based model, in which resistance genes are plasmid-encoded, can be translated to more complex clinical isolates, including MRSA or carbapenem-resistant Pseudomonas aeruginosa. Although the bacterial envelope architecture and specific resistance pathways differ among species, the fundamental mechanism of ASO-mediated gene silencing remains broadly adaptable. Although additional optimization is warranted, the partial restoration of ampicillin efficacy highlights the potential of combining oligonucleotide-based therapeutics with conventional antibiotics as a strategy to combat multidrug-resistant pathogens.
Several investigations have highlighted the promise of antisense oligonucleotides (ASOs) in neutralizing critical resistance determinants in clinically relevant pathogens. For instance, it has been shown that liposome-encapsulated ASOs, targeting the oprM gene in Pseudomonas aeruginosa, drastically reduced the minimum inhibitory concentrations (MICs) of five frontline antibiotics, including piperacillin, ciprofloxacin, and levofloxacin. This enhancement of antibiotic efficacy—ranging from 2- to 16-fold—was attributed to the suppression of an efflux pump subunit encoded by oprM, underscoring the importance of efflux mechanisms in multidrug resistance (MDR) among P. aeruginosa strains. Notably, efflux-based resistance mechanisms are common in a wide spectrum of Gram-negative bacteria, suggesting that similar ASO-mediated gene silencing approaches could be applied beyond P. aeruginosa [13].
In a parallel demonstration, Meng et al. (2015) developed a liposome-based ASO delivery system aimed at the mecA gene in methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus epidermidis [9]. By targeting the gene responsible for conferring resistance to nearly all beta-lactam antibiotics (via altered penicillin-binding proteins), they reported a 2- to 16-fold improvement in susceptibility to multiple beta-lactams, including oxacillin and cefoxitin. Given the global burden of MRSA in both community and hospital settings, these findings highlight how specific gene silencing can potentially restore the therapeutic value of widely used antimicrobials.
Building on these foundational reports, our investigation sought to explore a proof of concept for using thiol-modified ASOs in conjugation with gold nanoparticles (AuNPs) to overcome ampicillin resistance in Escherichia coli. Unlike liposomal delivery systems, AuNPs can offer distinct advantages—such as enhanced stability in biological fluids, the capacity for multivalent presentation of oligonucleotides, and potential for co-functionalization with other targeting moieties or adjuvants. Indeed, prior research has shown that AuNPs, when capped with DNA or RNA sequences, can remain stable under physiologically relevant ionic strengths, an essential feature for clinical translation [59].
Our results demonstrated a positive trend toward resensitization with an approximately 30% reduction in bacterial growth under sub-MIC ampicillin conditions. This outcome can be enhanced by several factors, such as dose and delivery optimization—ASO concentration, nanoparticle loading capacity, and the ratio of ASOs to AuNP surfaces are pivotal for achieving effective intracellular uptake and robust mRNA knockdown; bacterial strain specificity—while E. coli is a common model organism, different MDR pathogens (e.g., Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii) might exhibit unique membrane permeability, efflux dynamics, and genetic regulatory pathways, all of which influence ASO internalization and efficacy; and gene expression levels and timing—resistance genes can be regulated by various environmental and cellular signals, potentially affecting their expression profiles and timing. The partial inhibition observed in our study offers an important initial proof of concept that ASO-AuNP conjugates can target and mitigate antibiotic resistance mechanisms in Gram-negative bacteria. Importantly, the modular nature of ASOs—allowing rapid re-design to target different genes—and the robust surface chemistry of AuNPs point to a wide window for further innovation. Possible enhancements include dual-targeting approaches—coupling ASOs aimed at multiple resistance genes (e.g., efflux pumps, beta-lactamases, porin mutations) on a single AuNP scaffold might deliver enhanced or synergistic effects; and in vivo validation—transitioning from in vitro assays to animal models will be essential to gauge pharmacokinetics, tissue distribution, immunogenic responses, and therapeutic efficacy in a more physiologically relevant context.
While this study focused on beta-lactams, the same platform could be used to resensitize bacteria to fluoroquinolones, aminoglycosides, or colistin, potentially broadening the clinical applicability of the approach [60]. Collectively, these findings underscore the feasibility of ASO-mediated strategies to reverse antibiotic resistance, echoing the successes documented for other ASO delivery platforms. These results lay the groundwork for iterative optimization. More refined ASO sequences, enhanced AuNP conjugation techniques, and refined dosing regimens hold the promise of boosting the magnitude of resensitization. In the context of escalating MDR threats worldwide, such incremental gains in antibiotic efficacy—especially for older and less expensive drugs like ampicillin—can have an outsized clinical and economic impact, extending the lifespan of existing antimicrobial agents and delaying the advent of untreatable infections.
4. Conclusions
This study provides compelling proof-of-concept evidence that antisense oligonucleotide-capped gold nanoparticles (ASO-AuNPs) can restore the efficacy of beta-lactam antibiotics against resistant bacterial strains. Our data indicate that the parental Escherichia coli DH5α strain exhibits an ampicillin MIC of 16 ppm, while the engineered ampicillin-resistant PSK strain demonstrates an MIC of 32,768 ppm—a nearly 2000-fold increase. Notably, when the PSK strain was treated with a sub-MIC level (1/3 MIC) of ampicillin, bacterial growth was only partially inhibited; however, the addition of ASO-AuNPs at 0.1 and 0.2 nM resulted in an extra ~30% reduction in growth. These results clearly show that the ASO-AuNP conjugates can sensitize resistant bacteria to ampicillin, effectively lowering the threshold required for antibiotic activity.
Our physicochemical characterization supports these findings. UV–Vis spectroscopy revealed a characteristic surface plasmon resonance (SPR) peak at approximately 523 nm for unmodified AuNPs, which shifted slightly to ~525 nm upon functionalization with thiol-modified ASOs—a change accompanied by a decrease in absorbance intensity. This shift, together with the appearance of a faint halo observed in transmission electron microscopy (TEM) images, confirms the successful conjugation of ASOs to the AuNP surface. TEM analysis further demonstrated that the unmodified AuNPs had a mean diameter of 19.42 ± 2.57 nm, which increased modestly to 21 ± 8.24 nm following ASO attachment. These observations validate our nanoparticle synthesis and functionalization approach and underscore the robustness of the Au-S bonding mechanism.
Our results underscore the therapeutic potential of integrating nanotechnology with antisense strategies to counteract antibiotic resistance. The significant reduction in bacterial growth achieved through the combination of ASO-AuNPs and sub-MIC ampicillin treatment indicates that our platform effectively mitigates beta-lactamase-mediated resistance. This is particularly relevant in light of the global challenge posed by multidrug-resistant pathogens, where novel therapeutic strategies are urgently needed.
Although our findings are promising, several key areas warrant further investigation. First, further optimization of the nanoparticle formulation is required to enhance the loading capacity and intracellular delivery of ASOs. We plan to fine-tune the ASO-to-AuNP ratio to maximize cellular uptake and improve gene silencing efficiency. Second, while our study confirms the proof of concept for ASO-AuNP-mediated resensitization, the precise molecular mechanism—specifically, the relative contributions of steric hindrance versus RNase H-mediated mRNA cleavage—remains to be fully elucidated. Future experiments will focus on quantifying ampR transcript levels using RT-qPCR and assessing RNase H activity to clearly delineate the dominant silencing mechanism. Lastly, it will be essential to extend our evaluation of the ASO-AuNP system to more physiologically relevant models, such as biofilm-associated infections or animal models of chronic bacterial diseases, to determine optimal dosing regimens and assess potential in vivo efficacy.
Our work demonstrates that ASO-AuNP conjugates represent a promising strategy to overcome antibiotic resistance by restoring the activity of conventional antibiotics. By addressing the identified areas for refinement through further mechanistic studies and formulation optimization, ASO-based platforms have the potential to form an integral part of next-generation antimicrobial therapies, ultimately contributing to the preservation of global public health in the face of rapidly evolving multidrug-resistant pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16030070/s1, Table S1: BLAST Alignment Results for the Antisense Oligonucleotide Against Random E. coli Annotations. Alignment categories for the antisense oligonucleotide sequence when queried against random E. coli annotations. The alignments fall into three groups: (1) exact 24-nt matches with the ampR gene; (2) near matches with point mutations (95.83% identity); and (3) shorter exact matches (<24 nt) to unrelated genes. Table S2: BLAST Alignment Results for the Antisense Oligonucleotide Against the E. coli K12 Genome. Potential alignments of the antisense oligonucleotide with the E. coli K12 genome. Most do not meet the criteria (>15 nt overlap, <15% mismatch, and ∆G < −40 kcal/mol) required for significant gene repression, thereby indicating minimal off-target risk.
Author Contributions
Conceptualization, C.R.G.-C., A.L.-B. and J.R.M.-R.; methodology, C.R.G.-C., A.L.-B., A.A.S.-C. and J.R.M.-R.; software, C.R.G.-C. and J.R.M.-R.; validation, C.R.G.-C., A.L.-B. and J.R.M.-R.; formal analysis, C.R.G.-C., A.L.-B., A.A.S.-C. and J.R.M.-R.; investigation, C.R.G.-C. and J.R.M.-R.; resources, J.R.M.-R.; data curation, C.R.G.-C., A.L.-B. and J.R.M.-R.; writing—original draft preparation, C.R.G.-C., A.L.-B. and J.R.M.-R.; writing—review and editing, C.R.G.-C., A.L.-B., A.A.S.-C. and J.R.M.-R.; visualization, C.R.G.-C. and J.R.M.-R.; supervision, J.R.M.-R.; project administration, J.R.M.-R.; funding acquisition, J.R.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Universidad Autonoma de Nuevo León and CONACyT for providing financial support through Paicyt 2019–2020, Paicyt 2020–2021, and Paicyt 2022–2023 Science Grants; CONACyT Grants for: Basic science grant 221332, Fronteras de la Ciencia grant 1502, Infraestructura Grant 279957 and Apoyos a la Ciencia de Frontera grant 316869 y Grant a Ciencia de Frontera CF-2023-I-1327; Garza-Cárdenas C. R for the support from Beca Nacional de Posgrado from CONAHCyT; and León-Buitimea Angel and A. A. Siller-Ceniceros for the support from Becas Nacionales de Postdoctorado from CONAHCyT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We extend our sincere gratitude to Daniel Bahena Uribe (Advanced Laboratory in Electron Nanoscopy, LANE-CINVESTAV-IPN) for his invaluable support and expertise in the HR-TEM electron microscopy analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Medina, E.; Pieper, D.H. Tackling threats and future problems of multidrug-resistant bacteria. In How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3–33. [Google Scholar]
- Van Bambeke, F.; Balzi, E.; Tulkens, P.M. Antibiotic efflux pumps. Biochem. Pharmacol. 2000, 60, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Riu, F.; Ruda, A.; Ibba, R.; Sestito, S.; Lupinu, I.; Piras, S.; Widmalm, G.; Carta, A. Antibiotics and carbohydrate-containing drugs targeting bacterial cell envelopes: An overview. Pharmaceuticals 2022, 15, 942. [Google Scholar] [CrossRef]
- Tabatabaeifar, F.; Isaei, E.; Kalantar-Neyestanaki, D.; Morones-Ramírez, J.R. Antimicrobial and Antibiofilm Effects of Combinatorial Treatment Formulations of Anti-Inflammatory Drugs—Common Antibiotics against Pathogenic Bacteria. Pharmaceutics 2022, 15, 4. [Google Scholar] [CrossRef]
- Hegarty, J.P.; Stewart, D.B. Advances in therapeutic bacterial antisense biotechnology. Appl. Microbiol. Biotechnol. 2018, 102, 1055–1065. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Williamson, P.R.; Zheng, W. Improving therapy of severe infections through drug repurposing of synergistic combinations. Curr. Opin. Pharmacol. 2019, 48, 92–98. [Google Scholar] [CrossRef]
- Meng, J.; He, G.; Wang, H.; Jia, M.; Ma, X.; Da, F.; Wang, N.; Hou, Z.; Xue, X.; Li, M.; et al. Reversion of antibiotic resistance by inhibiting mecA in clinical methicillin-resistant Staphylococci by antisense phosphorothioate oligonucleotide. J. Antibiot. 2015, 68, 158–164. [Google Scholar] [CrossRef]
- Xue, X.Y.; Mao, X.G.; Zhou, Y.; Chen, Z.; Hu, Y.; Hou, Z.; Li, M.K.; Meng, J.R.; Luo, X.X. Advances in the delivery of antisense oligonucleotides for combating bacterial infectious diseases. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 745–758. [Google Scholar] [CrossRef]
- Angrish, N.; Khare, G. Antisense oligonucleotide based therapeutics and its applications against bacterial infections. Med. Drug Discov. 2023, 20, 100166. [Google Scholar] [CrossRef]
- Good, L.; Stach, J.E. Synthetic RNA silencing in bacteria–antimicrobial discovery and resistance breaking. Front. Microbiol. 2011, 2, 185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, J.; Jia, M.; Ma, X.; He, G.; Yu, J.; Wang, R.; Bai, H.; Hou, Z.; Luo, X. oprM as a new target for reversion of multidrug resistance in Pseudomonas aeruginosa by antisense phosphorothioate oligodeoxynucleotides. FEMS Immunol. Med. Microbiol. 2010, 60, 275–282. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Morones, J.R.; Frey, W. Room temperature synthesis of an optically and thermally responsive hybrid PNIPAM–gold nanoparticle. J. Nanoparticle Res. 2010, 12, 1401–1414. [Google Scholar] [CrossRef]
- Morones-Ramírez, J.R. Bioinspired synthesis of optically and thermally responsive nanoporous membranes. NPG Asia Mater. 2013, 5, e52. [Google Scholar] [CrossRef]
- Rafiei, N.; Alishah Aratboni, H.; Alemzadeh, A.; Saavedra-Alonso, S.; Razi, H.; Morones-Ramírez, J.R. Nano-Regulation of Gene Expression in Chlamydomonas reinhardtii: Harnessing AuNPs for Remotely Switchable Lipid Biosynthesis via Antisense Oligonucleotides. ACS Synth. Biol. 2024, 13, 1694–1704. [Google Scholar] [CrossRef]
- Grönbeck, H.; Curioni, A.; Andreoni, W. Thiols and disulfides on the Au (111) surface: The headgroup—Gold interaction. J. Am. Chem. Soc. 2000, 122, 3839–3842. [Google Scholar] [CrossRef]
- Ghosh, S.; Su, Y.H.; Yang, C.J.; Lai, J.Y. Design of Highly Adhesive Urchin-Like Gold Nanostructures for Effective Topical Drug Administration and Symptomatic Relief of Corneal Dryness. Small Struct. 2025, 6, 2400484. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; Rotello, V.M. Functionalized gold nanoparticles for drug delivery. Nanomedicine 2007, 2, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold nanoparticles: Can they be the next magic bullet for multidrug-resistant bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.J.; Lytton-Jean, A.K.; Mirkin, C.A. Maximizing DNA loading on a range of gold nanoparticle sizes. Anal. Chem. 2006, 78, 8313–8318. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef]
- Hammel, M.; Fan, Y.; Sarode, A.; Byrnes, A.E.; Zang, N.; Kou, P.; Nagapudi, K.; Leung, D.; Hoogenraad, C.C.; Chen, T.; et al. Correlating the structure and gene silencing activity of oligonucleotide-loaded lipid nanoparticles using small-angle X-ray scattering. ACS Nano 2023, 17, 11454–11465. [Google Scholar] [CrossRef]
- Sarode, A.; Fan, Y.; Byrnes, A.E.; Hammel, M.; Hura, G.L.; Fu, Y.; Kou, P.; Hu, C.; Hinz, F.I.; Roberts, J.; et al. Predictive high-throughput screening of PEGylated lipids in oligonucleotide-loaded lipid nanoparticles for neuronal gene silencing. Nanoscale Adv. 2022, 4, 2107–2123. [Google Scholar] [CrossRef]
- Ferreira, D.; Fernandes, A.R.; Baptista, P.V. Mild hyperthermia via gold nanoparticles and visible light irradiation for enhanced siRNA and ASO delivery in 2D and 3D tumour spheroids. Cancer Nanotechnol. 2024, 15, 19. [Google Scholar] [CrossRef]
- García-García, P.; Ruiz, M.; Reyes, R.; Delgado, A.; Évora, C.; Riancho, J.A.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Smurf1 silencing using a LNA-ASOs/lipid nanoparticle system to promote bone regeneration. Stem Cells Transl. Med. 2019, 8, 1306–1317. [Google Scholar] [CrossRef]
- Kime, L.; Randall, C.P.; Banda, F.I.; Coll, F.; Wright, J.; Richardson, J.; Empel, J.; Parkhill, J.; O’Neill, A.J. Transient silencing of antibiotic resistance by mutation represents a significant potential source of unanticipated therapeutic failure. MBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Hoynes-O’Connor, A.; Moon, T.S. Development of design rules for reliable antisense RNA behavior in E. coli. ACS Synth. Biol. 2016, 5, 1441–1454. [Google Scholar] [CrossRef]
- Michel, S.; Schirduan, K.; Shen, Y.; Klar, R.; Tost, J.; Jaschinski, F. Using RNA-seq to assess off-target effects of antisense oligonucleotides in human cell lines. Mol. Diagn. Ther. 2021, 25, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, H.; Yoshida, T.; Sasaki, K.; Obika, S.; Inoue, T. Reduction of off-target effects of gapmer antisense oligonucleotides by oligonucleotide extension. Mol. Diagn. Ther. 2022, 26, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Terada, C.; Oh, K.; Tsubaki, R.; Chan, B.; Aibara, N.; Ohyama, K.; Shibata, M.A.; Wada, T.; Harada-Shiba, M.; Yamayoshi, A.; et al. Dynamic and static control of the off-target interactions of antisense oligonucleotides using toehold chemistry. Nat. Commun. 2023, 14, 7972. [Google Scholar] [CrossRef]
- Tu, Q.; Yin, J.; Fu, J.; Herrmann, J.; Li, Y.; Yin, Y.; Stewart, A.F.; Müller, R.; Zhang, Y. Room temperature electrocompetent bacterial cells improve DNA transformation and recombineering efficiency. Sci. Rep. 2016, 6, 24648. [Google Scholar] [CrossRef]
- Ghaffari, E.; Rezatofighi, S.E.; Ardakani, M.R.; Rastegarzadeh, S. Delivery of antisense peptide nucleic acid by gold nanoparticles for the inhibition of virus replication. Nanomedicine 2019, 14, 1827–1840. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV− Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Chávez-Reyes, A.; Castillo, E.C.; García-Rivas, G.; Antonio Ortega-Rivera, O.; Salinas, E.; Ortiz-Martínez, M.; Gómez-Flores, S.L.; Peña-Martínez, J.A.; Pepi-Molina, A.; et al. Synergistic antimicrobial effects of silver/transition-metal combinatorial treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef]
- Tyagi, H.; Kushwaha, A.; Kumar, A.; Aslam, M. A facile pH controlled citrate-based reduction method for gold nanoparticle synthesis at room temperature. Nanoscale Res. Lett. 2016, 11, 362. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Storhoff, J.J.; Elghanian, R.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L. One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J. Am. Chem. Soc. 1998, 120, 1959–1964. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Uscanga-Palomeque, A.C.; Luna Cruz, I.E.; Parra-Saldivar, R.; Morones-Ramirez, J.R. Design of a nanobiosystem with remote photothermal gene silencing in Chlamydomonas reinhardtii to increase lipid accumulation and production. Microb. Cell Factories 2023, 22, 61. [Google Scholar] [CrossRef]
- Allen, N.C.; Chauhan, R.; Bates, P.J.; O’Toole, M.G. Optimization of tumor targeting gold nanoparticles for glioblastoma applications. Nanomaterials 2022, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; Ghalami, M.; Ghaemi, A.; Mosavari, N.; Abdul-Tehrani, H.; Sadeghizadeh, M. Nanodiagnostic method for colorimetric detection of Mycobacterium tuberculosis 16S rRNA. Nanobiotechnology 2008, 4, 28–35. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. In Spherical Nucleic Acids; Jenny Stanford Publishing: Singapore, 2020; pp. 55–90. [Google Scholar]
- Giljohann, D.A.; Seferos, D.S.; Patel, P.C.; Millstone, J.E.; Rosi, N.L.; Mirkin, C.A. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007, 7, 3818–3821. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain Nu Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vázquez-Rodríguez, A.; Morones-Ramírez, J.R. Bacterial exopolysaccharides as reducing and/or stabilizing agents during synthesis of metal nanoparticles with biomedical applications. Int. J. Polym. Sci. 2018, 2018, 7045852. [Google Scholar] [CrossRef]
- Li, Z.; Jin, R.; Mirkin, C.A.; Letsinger, R.L. Multiple thiol-anchor capped DNA–gold nanoparticle conjugates. Nucleic Acids Res. 2002, 30, 1558–1562. [Google Scholar] [CrossRef]
- Narendrakumar, L.; Chakraborty, M.; Kumari, S.; Paul, D.; Das, B. β-Lactam potentiators to re-sensitize resistant pathogens: Discovery, development, clinical use and the way forward. Front. Microbiol. 2023, 13, 1092556. [Google Scholar] [CrossRef]
- Rojas, M.; Del Valle, D. Betalactamasas tipo AmpC: Generalidades y métodos para detección fenotípica. Rev. De La Soc. Venez. De Microbiol. 2009, 29, 78–83. [Google Scholar]
- Then, R. Beta-Lactamase. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–9. [Google Scholar] [CrossRef]
- Brandt, C.; Braun, S.D.; Stein, C.; Slickers, P.; Ehricht, R.; Pletz, M.W.; Makarewicz, O. In silico serine β-lactamases analysis reveals a huge potential resistome in environmental and pathogenic species. Sci. Rep. 2017, 7, 43232. [Google Scholar] [CrossRef]
- Brinas, L.; Zarazaga, M.; Sáenz, Y.; Ruiz-Larrea, F.; Torres, C. β-Lactamases in ampicillin-resistant Escherichia coli isolates from foods, humans, and healthy animals. Antimicrob. Agents Chemother. 2002, 46, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.; Heuer, C.; Hussein, H.; McDougall, S. Minimum inhibitory concentrations of selected antimicrobials against Escherichia coli and Trueperella pyogenes of bovine uterine origin. J. Dairy Sci. 2015, 98, 4427–4438. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Meza-Bustillos, J.F.; Resendiz-Hernández, H.; Suárez-Cantú, I.A.; Ortega-Rivera, O.A.; Salinas, E.; Escárcega-González, C.E.; Morones-Ramírez, J.R. Re-sensitizing ampicillin and kanamycin-resistant E. coli and S. aureus using synergistic metal micronutrients-antibiotic combinations. Front. Bioeng. Biotechnol. 2020, 8, 612. [Google Scholar]
- Olesen, I.; Hasman, H.; Møller Aarestrup, F. Prevalence of β-lactamases among ampicillin-resistant Escherichia coli and Salmonella isolated from food animals in Denmark. Microb. Drug Resist. 2004, 10, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Jani, S.; Ramirez, M.S.; Tolmasky, M.E. Silencing antibiotic resistance with antisense oligonucleotides. Biomedicines 2021, 9, 416. [Google Scholar] [CrossRef]
- Binzel, D.W.; Li, X.; Burns, N.; Khan, E.; Lee, W.J.; Chen, L.C.; Ellipilli, S.; Miles, W.; Ho, Y.S.; Guo, P. Thermostability, tunability, and tenacity of RNA as rubbery anionic polymeric materials in nanotechnology and nanomedicine—Specific cancer targeting with undetectable toxicity. Chem. Rev. 2021, 121, 7398–7467. [Google Scholar] [CrossRef]
- Cela, E.M.; Urquiza, D.; Gómez, M.I.; Gonzalez, C.D. New Weapons to Fight against Staphylococcus aureus Skin Infections. Antibiotics 2023, 12, 1477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).