A Situation Analysis of Diagnostic and Management Strategies for Gestational Urinary Tract Infections (UTIs) in Kisumu County, Kenya: Maternal Health Implications and Opportunities for Diagnostic Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations and Consent to Participate in the Study
2.2. Study Type
2.3. Study Area
2.4. Study Population and Sample Size Determination
2.5. Inclusion and Exclusion Criteria
2.6. Sampling Technique
2.7. Data Analysis
3. Results
3.1. Demographic Characteristics of Pregnant Women
3.2. Prevalence of Anaemia Among the Study Participants
3.3. Distribution of Gestational Urinary Tract Infection
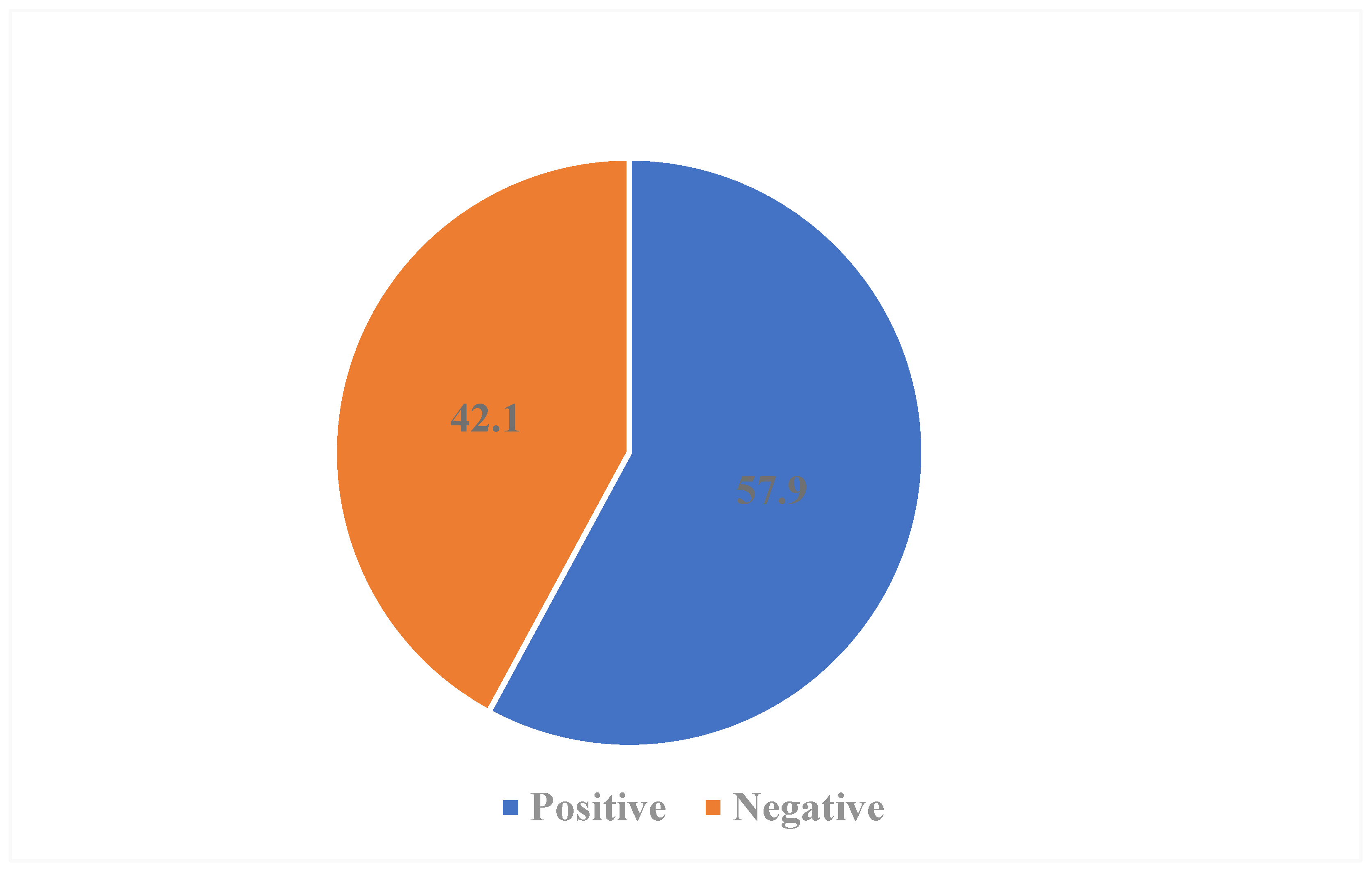
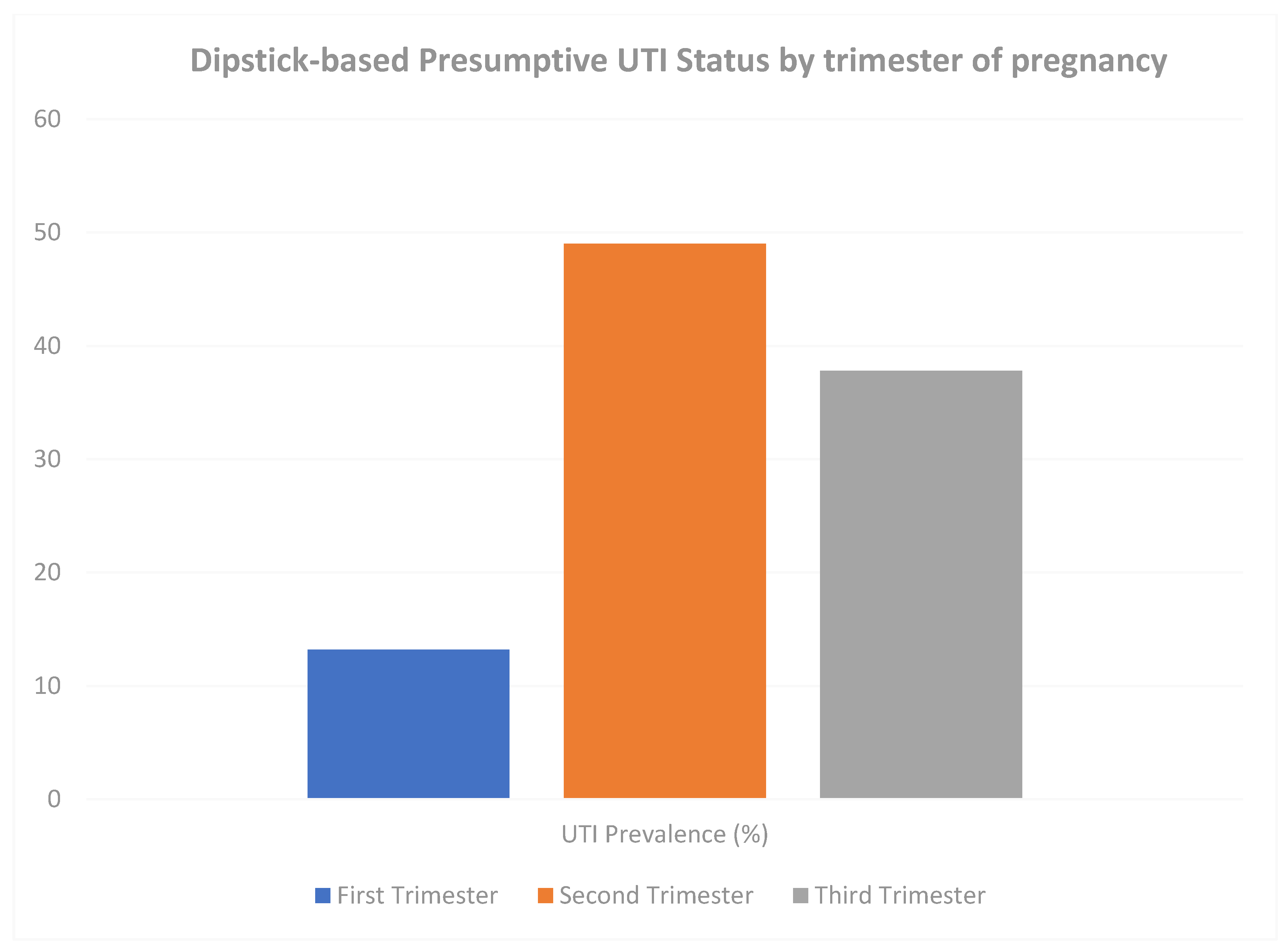
3.4. Risk Factors Associated with Gestational Urinary Tract Infection (UTI)
4. Discussion
4.1. High Dipstick-Positive Presumptive UTI (Screen-Positive) Proportion During Pregnancy
4.2. Inappropriate Gestational UTI Diagnosis
4.3. Notably High Gestational Anaemia
4.4. Regulated Access to Antibiotic Prescriptions
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPATH | Academic Model Providing Access to Healthcare |
| ANC | Antenatal Care |
| ASB | Asymptomatic Bacteriuria |
| CMR | Child Mortality Rate |
| SD | Standard Deviation |
| GCRF | Global Challenge Research Fund |
| IMR | Infant Mortality Rate |
| IPTp | Intermittent Preventive Treatment of Malaria in Pregnancy |
| LMIC | Low- and Middle-Income Countries |
| MCH | Maternal Child Health |
| MDR | Multidrug Resistance |
| MMR | Maternal Mortality Rate |
| MOH | Ministry of Health |
| NMR | Neonatal Mortality Rate |
| MUERC | Maseno University Ethical Review Committee |
| UTI | Urinary Tract Infections |
| WHO | World Health Organisation |
| AWaRe | Access, Watch, and Reserve |
References
- UNICEF DATA. Maternal Mortality Rates and Statistics. Available online: https://data.unicef.org/topic/maternal-health/maternal-mortality/ (accessed on 13 December 2021).
- Koblinsky, M.; Chowdhury, M.E.; Moran, A.; Ronsmans, C. Maternal Morbidity and Disability and Their Consequences: Neglected Agenda in Maternal Health. J. Health Popul. Nutr. 2012, 30, 124–130. [Google Scholar] [CrossRef]
- Gilbert, N.M.; O’Brien, V.P.; Hultgren, S.; Macones, G.; Lewis, W.G.; Lewis, A.L. Urinary Tract Infection as a Preventable Cause of Pregnancy Complications: Opportunities, Challenges, and a Global Call to Action. Glob. Adv. Health Med. 2013, 2, 59–69. [Google Scholar] [CrossRef]
- Lee, A.C.; Mullany, L.C.; Koffi, A.K.; Rafiqullah, I.; Khanam, R.; Folger, L.V.; Rahman, M.; Mitra, D.K.; Labrique, A.; Christian, P.; et al. Urinary tract infections in pregnancy in a rural population of Bangladesh: Population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth 2019, 20, 1. [Google Scholar] [CrossRef]
- Belete, M.A.; Saravanan, M. A Systematic Review on Drug Resistant Urinary Tract Infection Among Pregnant Women in Developing Countries in Africa and Asia; 2005–2016. Infect. Drug Resist. 2020, 13, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Wanja, F.; Ngugi, C.; Omwenga, E.; Maina, J.; Kiiru, J. Urinary Tract Infection among Adults Seeking Medicare at Kiambu Level 5 Hospital, Kenya: Prevalence, Diversity, Antimicrobial Susceptibility Profiles and Possible Risk Factors. Adv. Microbiol. 2021, 11, 360–383. [Google Scholar] [CrossRef]
- Ayoyi, A.O.; Kikuvi, G.; Bii, C.; Kariuki, S. Prevalence, aetiology and antibiotic sensitivity profile of asymptomatic bacteriuria isolates from pregnant women in selected antenatal clinic from Nairobi, Kenya. Pan Afr. Med. J. 2017, 26, 41. [Google Scholar] [CrossRef] [PubMed]
- Masika, W.G.; O’Meara, W.P.; Holland, T.L.; Armstrong, J. Contribution of urinary tract infection to the burden of febrile illnesses in young children in rural Kenya. PLoS ONE 2017, 12, e0174199. [Google Scholar] [CrossRef]
- Sangeda, R.Z.; Paul, F.; Mtweve, D.M. Prevalence of Urinary Tract Infections and Antibiogram of Uropathogens Isolated from Children Under Five Attending Bagamoyo District Hospital in Tanzania: A Cross-Sectional Study. F1000Research 2021, 10, 449. Available online: https://f1000research.com/articles/10-449 (accessed on 14 December 2021). [CrossRef]
- Getaneh, T.; Negesse, A.; Dessie, G.; Desta, M.; Tigabu, A. Prevalence of Urinary Tract Infection and Its Associated Factors among Pregnant Women in Ethiopia: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2021, 2021, 6551526. [Google Scholar] [CrossRef]
- Girma, A.; Aemiro, A.; Workineh, D.; Tamir, D. Magnitude, Associated Risk Factors, and Trend Comparisons of Urinary Tract Infection among Pregnant Women and Diabetic Patients: A Systematic Review and Meta-Analysis. J. Pregnancy 2023, 2023, 8365867. [Google Scholar] [CrossRef]
- Kaduma, J.; Seni, J.; Chuma, C.; Kirita, R.; Mujuni, F.; Mushi, M.F.; van der Meer, F.; Mshana, S.E. Urinary Tract Infections and Preeclampsia among Pregnant Women Attending Two Hospitals in Mwanza City, Tanzania: A 1:2 Matched Case-Control Study. BioMed Res. Int. 2019, 2019, 3937812. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Stephen, B.M.; Joseph, N.; Asiphas, O.; Musa, K.; Taseera, K. Prevalence and bacteriology of culture-positive urinary tract infection among pregnant women with suspected urinary tract infection at Mbarara regional referral hospital, South-Western Uganda. BMC Pregnancy Childbirth 2021, 21, 159. [Google Scholar] [CrossRef]
- Oladeinde, B.H.; Omoregie, R.; Oladeinde, O.B. Asymptomatic Urinary Tract Infection among Pregnant Women Receiving Ante-Natal Care in a Traditional Birth Home in Benin City, Nigeria. Ethiop. J. Health Sci. 2015, 25, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Reda, D.Y.; Ormago, M.D. Prevalence and antimicrobial susceptibility pattern of urinary tract infection among pregnant women attending Hargeisa Group Hospital, Hargeisa, Somaliland. Sci. Rep. 2022, 12, 1419. [Google Scholar] [CrossRef]
- Vicar, E.K.; Acquah, S.E.K.; Wallana, W.; Kuugbee, E.D.; Osbutey, E.K.; Aidoo, A.; Acheampong, E.; Mensah, G.I. Urinary Tract Infection and Associated Factors among Pregnant Women Receiving Antenatal Care at a Primary Health Care Facility in the Northern Region of Ghana. Int. J. Microbiol. 2023, 2023, 3727265. [Google Scholar] [CrossRef]
- Tchente Nguefack, C.; Okalla Ebongue, C.; Nouwe Chokotheu, C.; Ebong Ewougo, C.; Nana Njamen, T.; Mboudou, E. Clinical presentation, risk factors and pathogens involved in bacteriuria of pregnant women attending antenatal clinic of 3 hospitals in a developing country: A cross sectional analytic study. BMC Pregnancy Childbirth 2019, 19, 143. [Google Scholar] [CrossRef]
- Matuszkiewicz-Rowińska, J.; Małyszko, J.; Wieliczko, M. Urinary tract infections in pregnancy: Old and new unresolved diagnostic and therapeutic problems. Arch. Med. Sci. 2015, 11, 67–77. [Google Scholar] [CrossRef]
- Available online: https://www.unfpa.org/sites/default/files/admin-resource/FINALPSAREPORT_0.pdf (accessed on 14 December 2021).
- Available online: https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf (accessed on 14 December 2021).
- Available online: https://kenya.unfpa.org/sites/default/files/pub-pdf/ete_of_rmncah_programme_report_online_2_0.pdf (accessed on 14 December 2021).
- Shrestha, L.B.; Baral, R.; Poudel, P.; Khanal, B. Clinical, etiological and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatr. 2019, 19, 36. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial Resistance in the Context of the Sustainable Development Goals: A Brief Review. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 71–82. [Google Scholar] [CrossRef]
- Ngowi, B.N.; Sunguya, B.; Herman, A.; Chacha, A.; Maro, E.; Rugarabamu, L.F.; Bartlett, J.; Balandya, E.; Mteta, K.A.; Mmbaga, B.T. Prevalence of Multidrug Resistant UTI Among People Living with HIV in Northern Tanzania. Infect. Drug Resist. 2021, 14, 1623–1633. [Google Scholar] [CrossRef]
- Mareș, C.; Petca, R.-C.; Popescu, R.-I.; Petca, A.; Geavlete, B.F.; Jinga, V. Uropathogens’ Antibiotic Resistance Evolution in a Female Population: A Sequential Multi-Year Comparative Analysis. Antibiotics 2023, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- Budd, E.; Cramp, E.; Sharland, M.; Hand, K.; Howard, P.; Wilson, P.; Wilcox, M.; Muller-Pebody, B.; Hopkins, S. Adaptation of the WHO Essential Medicines List for national antibiotic stewardship policy in England: Being AWaRe. J. Antimicrob. Chemother. 2019, 74, 3384–3389. [Google Scholar] [CrossRef] [PubMed]
- Hsia, Y.; Lee, B.R.; Versporten, A.; Yang, Y.; Bielicki, J.; Jackson, C.; Newland, J.; Goossens, H.; Magrini, N.; Sharland, M.; et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health 2019, 7, e861–e871. [Google Scholar] [CrossRef] [PubMed]
- 2021 AWaRe Classification. Available online: https://www.who.int/publications-detail-redirect/2021-aware-classification (accessed on 4 February 2024).
- Sharland, M.; Cappello, B.; Ombajo, L.A.; Bazira, J.; Chitatanga, R.; Chuki, P.; Gandra, S.; Harbarth, S.; Loeb, M.; Mendelson, M.; et al. The WHO AWaRe Antibiotic Book: Providing guidance on optimal use and informing policy. Lancet Infect. Dis. 2022, 22, 1528–1530. [Google Scholar] [CrossRef]
- Mudenda, S.; Daka, V.; Matafwali, S.K. World Health Organization AWaRe framework for antibiotic stewardship: Where are we now and where do we need to go? An expert viewpoint. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e84. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Abubakar, A.; Van Baar, A.; Fischer, R.; Bomu, G.; Gona, J.K.; Newton, C.R. Socio-Cultural Determinants of Health-Seeking Behaviour on the Kenyan Coast: A Qualitative Study. PLoS ONE 2013, 8, e71998. [Google Scholar] [CrossRef]
- Fisher, L.D. Self-designing clinical trials. Stat. Med. 1998, 17, 1551–1562. [Google Scholar] [CrossRef]
- Available online: https://www.kisumu.go.ke/wp-content/uploads/2018/11/KISUMU-COUNTY-FACT-SHEET-1-1.pdf (accessed on 14 December 2021).
- Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available online: https://www.who.int/publications-detail-redirect/WHO-NMH-NHD-MNM-11.1 (accessed on 4 February 2024).
- Cheesbrough, M. District Laboratory Practice in Tropical Countries: Part 2, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; ISBN 978-0-521-67631-1. [Google Scholar]
- H-100—Automatic Urine Analyzer by DIRUI Industrial MedicalExpo. Available online: https://www.medicalexpo.com/prod/dirui-industrial/product-68235-460860.html (accessed on 11 November 2025).
- Kristensen, L.H.; Winther, R.; Colding-Jørgensen, J.T.; Pottegård, A.; Nielsen, H.; Bodilsen, J. Diagnostic accuracy of dipsticks for urinary tract infections in acutely hospitalised patients: A prospective population-based observational cohort study. BMJ Evid.-Based Med. 2025, 30, 36–44. [Google Scholar] [CrossRef]
- Werter, D.E.; Schneeberger, C.; Geerlings, S.E.; de Groot, C.J.M.; Pajkrt, E.; Kazemier, B.M. Diagnostic Accuracy of Urine Dipsticks for Urinary Tract Infection Diagnosis during Pregnancy: A Retrospective Cohort Study. Antibiotics 2024, 13, 567. [Google Scholar] [CrossRef]
- Maina, J.; Mwaniki, J.; Mwiti, F.; Kiiru, S.; Katana, J.; Wanja, F.; Mukaya, J.; Khasabuli, O.; Asiimwe, B.; Gillespie, S.; et al. Evaluation of the diagnostic performance of the urine dipstick test for the detection of urinary tract infections in patients treated in Kenyan hospitals. Access Microbiol. 2023, 5, acmi000483.v3. Available online: https://www.microbiologyresearch.org/content/journal/acmi/10.1099/acmi.0.000483.v3 (accessed on 11 November 2025). [CrossRef]
- Available online: https://kijabehospital.or.ke/uploads/guidelines/national_antibiotics_use_guideline.pdf (accessed on 11 November 2025).
- Antimicrobial Resistance: Global Report on Surveillance. Available online: https://www.who.int/publications/i/item/9789241564748 (accessed on 11 November 2025).
- Neopane, P.; Nypaver, J.; Beqaj, S.S.; Shrestha, R. Rapid Detection of Uropathogens and Antibiotic-resistant Genes Using Open Array Multiplex PCR Technology. Am. J. Clin. Pathol. 2022, 158, S143–S144. [Google Scholar] [CrossRef]
- Asmat, U.; Mumtaz, M.Z.; Malik, A. Rising prevalence of multidrug-resistant uropathogenic bacteria from urinary tract infections in pregnant women. J. Taibah Univ. Med. Sci. 2020, 16, 102–111. [Google Scholar] [CrossRef]
- Eze, U.; Nworie, A. Prevalence and aetiologic agents of urinary tract infections in pregnancy in Abakaliki metropolis, Nigeria. Cont. J. Med. Res. 2010, 4, 18–23. [Google Scholar]
- Ojide, C.; VA, W.; Kalu, E.; UV, N. Asymptomatic bacteriuria among antenatal care women in a tertiary hospital in Benin, Nigeria. Niger. J. Exp. Clin. Biosci. 2014, 2, 79–85. [Google Scholar] [CrossRef]
- Simon-Oke, I.A.; Odeyemi, O.; Afolabi, O.J. Incidence of urinary tract infections and antimicrobial susceptibility pattern among pregnant women in Akure, Nigeria. Sci. Afr. 2019, 6, e00151. [Google Scholar] [CrossRef]
- El-Kashif, M.M.L. Urinary Tract Infection among Pregnant Women and its Associated Risk Factors: A Cross-Sectional Study. Biomed. Pharmacol. J. 2019, 12, 2003–2010. [Google Scholar] [CrossRef]
- Nteziyaremye, J.; Iramiot, S.J.; Nekaka, R.; Musaba, M.W.; Wandabwa, J.; Kisegerwa, E.; Kiondo, P. Asymptomatic bacteriuria among pregnant women attending antenatal care at Mbale Hospital, Eastern Uganda. PLoS ONE 2020, 15, e0230523. [Google Scholar] [CrossRef]
- Karikari, A.B.; Saba, C.K.S.; Yamik, D.Y. Assessment of asymptomatic bacteriuria and sterile pyuria among antenatal attendants in hospitals in northern Ghana. BMC Pregnancy Childbirth 2020, 20, 239. [Google Scholar] [CrossRef]
- McIsaac, W.; Carroll, J.C.; Biringer, A.; Bernstein, P.; Lyons, E.; Low, D.E.; Permaul, J.A. Screening for asymptomatic bacteriuria in pregnancy. J. Obstet. Gynaecol. Can. JOGC 2005, 27, 20–24. [Google Scholar] [CrossRef]
- Tugrul, S.; Oral, O.; Kumru, P.; Köse, D.; Alkan, A.; Yildirim, G. Evaluation and importance of asymptomatic bacteriuria in pregnancy. Clin. Exp. Obstet. Gynecol. 2005, 32, 237–240. [Google Scholar] [PubMed]
- Available online: https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/ghe2019_daly-methods.pdf (accessed on 4 February 2024).
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ubos.org/wp-content/uploads/publications/03_2018Uganda_DHS_2016_KIR.pdf (accessed on 4 February 2024).
- Msuya, S.E.; Hussein, T.H.; Uriyo, J.; Sam, N.E.; Stray-Pedersen, B. Anaemia among pregnant women in northern Tanzania: Prevalence, risk factors and effect on perinatal outcomes. Tanzan. J. Health Res. 2011, 13, 33–39. [Google Scholar] [CrossRef]
- Okia, C.C.; Aine, B.; Kiiza, R.; Omuba, P.; Wagubi, R.; Muwanguzi, E.; Apecu, R.O.; Okongo, B.; Oyet, C. Prevalence, Morphological Classification, And Factors Associated With Anemia Among Pregnant Women Accessing Antenatal Clinic At Itojo Hospital, South Western Uganda. J. Blood Med. 2019, 10, 351–357. [Google Scholar] [CrossRef]
- Ononge, S.; Campbell, O.; Mirembe, F. Haemoglobin status and predictors of anaemia among pregnant women in Mpigi, Uganda. BMC Res. Notes 2014, 7, 712. [Google Scholar] [CrossRef]
- McClure, E.M.; Meshnick, S.R.; Mungai, P.; Malhotra, I.; King, C.L.; Goldenberg, R.L.; Hudgens, M.G.; Siega-Riz, A.M.; Dent, A.E. The Association of Parasitic Infections in Pregnancy and Maternal and Fetal Anemia: A Cohort Study in Coastal Kenya. PLoS Negl. Trop. Dis. 2014, 8, e2724. [Google Scholar] [CrossRef]
- Yakoob, M.Y.; Bhutta, Z.A. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health 2011, 11, S21. [Google Scholar] [CrossRef]
- Ngong, I.N.; Fru-Cho, J.; Yung, M.A.; Akoachere, J.-F.K.T. Prevalence, antimicrobial susceptibility pattern and associated risk factors for urinary tract infections in pregnant women attending ANC in some integrated health centers in the Buea Health District. BMC Pregnancy Childbirth 2021, 21, 673. [Google Scholar] [CrossRef]
- Schneeberger, C.; Geerlings, S.E.; Middleton, P.; Crowther, C.A. Interventions for preventing recurrent urinary tract infection during pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD009279. [Google Scholar] [CrossRef]
- Lee, A.C.; Mullany, L.C.; Quaiyum, M.; Mitra, D.K.; Labrique, A.; Christian, P.; Ahmed, P.; Uddin, J.; Rafiqullah, I.; DasGupta, S.; et al. Effect of population-based antenatal screening and treatment of genitourinary tract infections on birth outcomes in Sylhet, Bangladesh (MIST): A cluster-randomised clinical trial. Lancet Glob. Health 2018, 7, e148–e159. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Chen, S.-F.; Li, H.-C.; Lin, H.-C. No increased risk of adverse pregnancy outcomes in women with urinary tract infections: A nationwide population-based study. Acta Obstet. Gynecol. Scand. 2010, 89, 882–888. [Google Scholar] [CrossRef]
- Schieve, L.A.; Handler, A.; Hershow, R.; Persky, V.; Davis, F. Urinary tract infection during pregnancy: Its association with maternal morbidity and perinatal outcome. Am. J. Public Health 1994, 84, 405–410. [Google Scholar] [CrossRef]
- Meis, P.J.; Michielutte, R.; Peters, T.J.; Wells, H.B.; Sands, R.E.; Coles, E.C.; Johns, K.A. Factors associated with preterm birth in Cardiff, Wales. II. Indicated and spontaneous preterm birth. Am. J. Obstet. Gynecol. 1995, 173, 597–602. [Google Scholar] [CrossRef]
- Walker, E.; Lyman, A.; Gupta, K.; Mahoney, M.V.; Snyder, G.M.; Hirsch, E.B. Clinical Management of an Increasing Threat: Outpatient Urinary Tract Infections Due to Multidrug-Resistant Uropathogens. Clin. Infect. Dis. 2016, 63, 960–965. [Google Scholar] [CrossRef]
- Smith, A.; Anandan, S.; Veeraraghavan, B.; Thomas, N. Colonization of the Preterm Neonatal Gut with Carbapenem-resistant Enterobacteriaceae and Its Association with Neonatal Sepsis and Maternal Gut Flora. J. Glob. Infect. Dis. 2020, 12, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Khawcharoenporn, T.; Vasoo, S.; Singh, K. Urinary Tract Infections due to Multidrug-Resistant Enterobacteriaceae: Prevalence and Risk Factors in a Chicago Emergency Department. Emerg. Med. Int. 2013, 2013, 258517. [Google Scholar] [CrossRef] [PubMed]
- Mutters, N.T.; Mampel, A.; Kropidlowski, R.; Biehler, K.; Günther, F.; Bălu, I.; Malek, V.; Frank, U. Treating urinary tract infections due to MDR E. coli with Isothiocyanates—A phytotherapeutic alternative to antibiotics? Fitoterapia 2018, 129, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Eshetie, S.; Unakal, C.; Gelaw, A.; Ayelign, B.; Endris, M.; Moges, F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral Hospital, Northwest Ethiopia. Antimicrob. Resist. Infect. Control 2015, 4, 12. [Google Scholar] [CrossRef]
- Rashid, M.M.; Akhtar, Z.; Chowdhury, S.; Islam, M.A.; Parveen, S.; Ghosh, P.K.; Rahman, A.; Khan, Z.H.; Islam, K.; Debnath, N.; et al. Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh. Antibiotics 2022, 11, 810. [Google Scholar] [CrossRef]
- Patel, P.K.; Satoh, N.; Narita, M.; Cho, Y.; Oshiro, Y.; Suzuki, T.; Fowler, K.E.; Greene, M.T.; Tokuda, Y.; Kaye, K.S. Inpatient antibiotic prescribing patterns using the World Health Organization (WHO) Access Watch and Reserve (AWaRe) classification in Okinawa, Japan: A point-prevalence survey. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e155. [Google Scholar] [CrossRef]
- Kalungia, A.C.; Mukosha, M.; Mwila, C.; Banda, D.; Mwale, M.; Kagulura, S.; Ogunleye, O.O.; Meyer, J.C.; Godman, B. Antibiotic Use and Stewardship Indicators in the First- and Second-Level Hospitals in Zambia: Findings and Implications for the Future. Antibiotics 2022, 11, 1626. [Google Scholar] [CrossRef]
- Mudenda, S.; Malama, S.; Munyeme, M.; Hang’ombe, B.M.; Mainda, G.; Kapona, O.; Mukosha, M.; Yamba, K.; Bumbangi, F.N.; Mfune, R.L.; et al. Awareness of Antimicrobial Resistance and Associated Factors among Layer Poultry Farmers in Zambia: Implications for Surveillance and Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 383. [Google Scholar] [CrossRef]
- O’Brien, V.P.; Hannan, T.J.; Nielsen, H.V.; Hultgren, S.J. Drug and Vaccine Development for the Treatment and Prevention of Urinary Tract Infections. Microbiol. Spectr. 2016, 4, 10. [Google Scholar] [CrossRef]
- Davenport, M.; Mach, K.E.; Dairiki Shortliffe, L.M.; Banaei, N.; Wang, T.-H.; Liao, J.C. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol. 2017, 14, 296–310. [Google Scholar] [CrossRef]

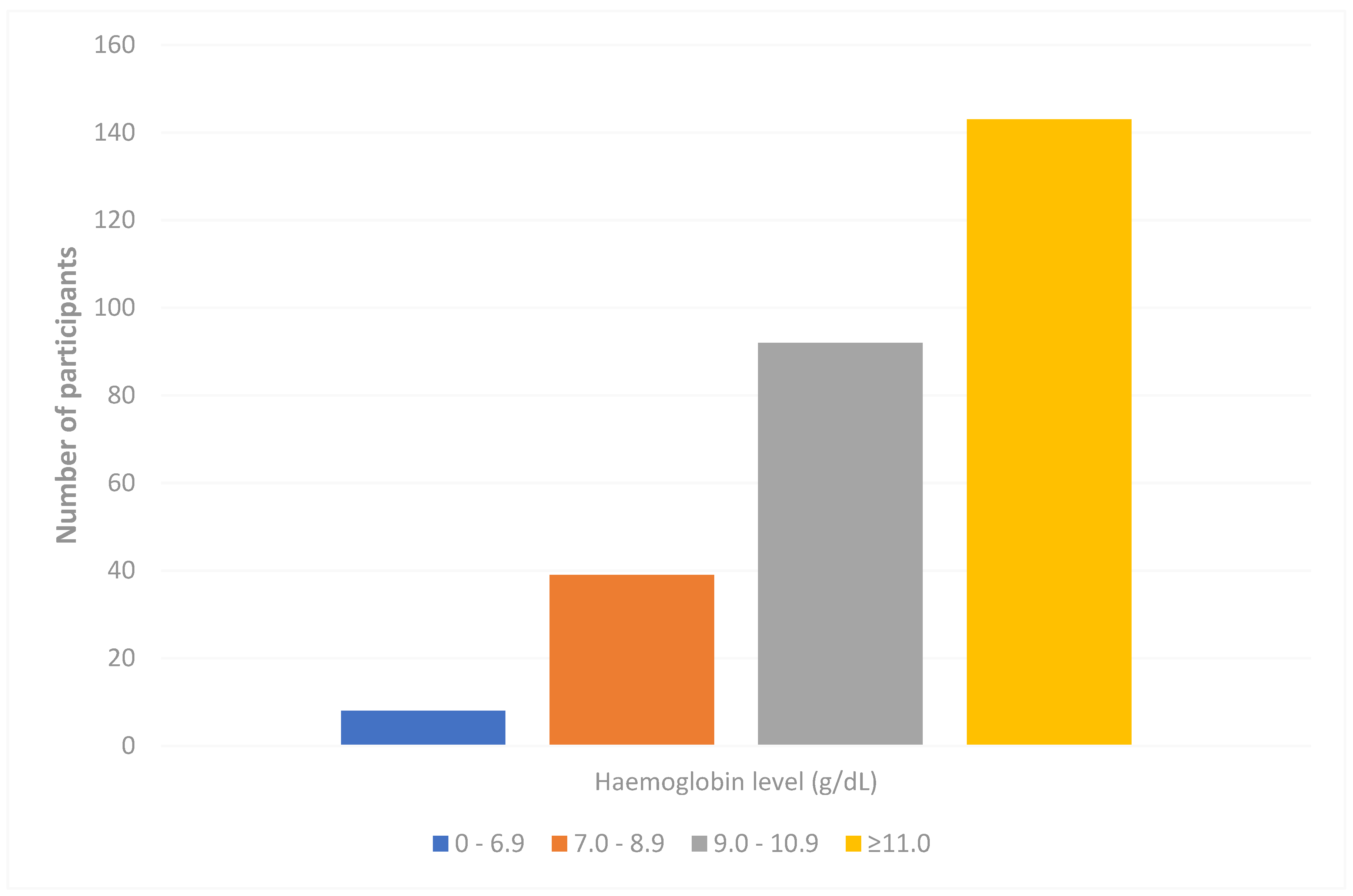
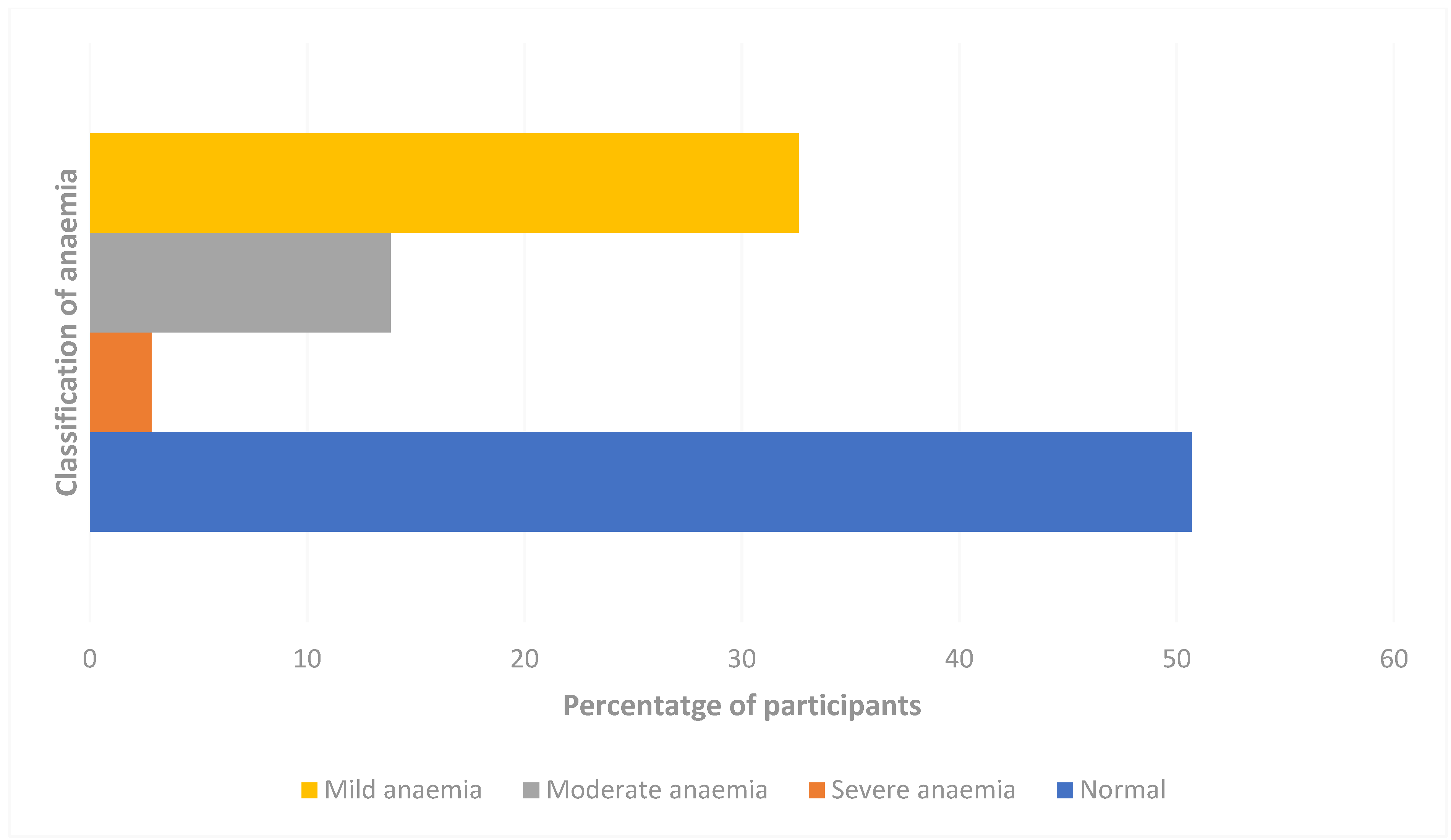
| S/NO | Variable | Categories | Frequency | Percent |
|---|---|---|---|---|
| 1 | First Visit | No | 182 | 43.75 |
| Yes | 234 | 56.25 | ||
| 2 | Marital Status | Married | 296 | 71.15 |
| Single | 120 | 28.85 | ||
| 3 | Gestation (trimesters) | First trimester | 44 | 10.6 |
| Second trimester | 212 | 51.08 | ||
| Third trimester | 159 | 38.31 | ||
| 4 | Gravidae | 1–2 | 261 | 62.74 |
| 3–4 | 108 | 25.96 | ||
| 5–6 | 35 | 8.41 | ||
| 7+ | 12 | 2.88 | ||
| 5 | Parity | 0–2 | 330 | 79.33 |
| 3–5 | 76 | 18.27 | ||
| 6+ | 10 | 2.4 | ||
| 6 | Number of antenatal visits | 1–2 | 337 | 81.01 |
| 3–5 | 74 | 17.79 | ||
| 6+ | 5 | 1.2 | ||
| 7 | Mother’s Age (years) | 10–17 | 46 | 11.1 |
| 18–24 | 209 | 50.2 | ||
| 25–29 | 73 | 17.5 | ||
| 30–34 | 61 | 14.7 | ||
| 35–39 | 24 | 5.8 | ||
| ≥40 | 3 | 0.7 |
| Variable | N | Chi-square (χ2) | p-Value |
|---|---|---|---|
| Haemoglobin level | 282 | 2.34 | 0.504 |
| Number of antenatal visits | 416 | 4.01 | 0.135 |
| Marital status | 416 | 0.30 | 0.587 |
| First visit | 416 | 0.47 | 0.491 |
| Parity | 416 | 3.71 | 0.156 |
| Gravidae | 416 | 7.19 | 0.066 |
| Gestation | 415 | 4.64 | 0.098 |
| Variable | AOR | 95% CI | p-Value |
|---|---|---|---|
| Marital Status | |||
| Single (ref) | 1.00 | – | – |
| Married | 0.89 | [0.48, 1.67] | 0.728 |
| Gestation Trimester | |||
| First trimester (ref) | 1.00 | – | – |
| Second trimester | 0.43 | [0.16, 1.12] | 0.083 |
| Third trimester | 0.50 | [0.18, 1.39] | 0.186 |
| Haemoglobin level | |||
| Non-anaemic (ref) | 1.00 | – | – |
| Severe anaemia | 1.55 | [0.33, 7.27] | 0.575 |
| Moderate anaemia | 1.65 | [0.76, 3.58] | 0.208 |
| Mild anaemia | 1.59 | [0.90, 2.80] | 0.112 |
| First Visit | |||
| No (ref) | 1.00 | – | – |
| Yes | 0.86 | [0.46, 1.62] | 0.643 |
| Mother’s Age | 1.00 | [0.93, 1.09] | 0.900 |
| Number of Visits | |||
| 0–2 visits (ref) | 1.00 | – | – |
| 3–5 visits | 0.58 | [0.26, 1.31] | 0.192 |
| 6+ visits | 0.41 | [0.05, 3.45] | 0.413 |
| Parity | |||
| 0–2 (ref) | 1.00 | – | – |
| 3–4 | 2.33 | [0.74, 7.31] | 0.148 |
| 5+ | 7.85 | [0.48, 129.07] | 0.149 |
| Gravidae | |||
| 0–2 (ref) | 1.00 | – | – |
| 3–4 | 1.00 | [0.44, 2.29] | 1.000 |
| 5–6 | 0.29 | [0.06, 1.41] | 0.125 |
| 7+ | 0.02 | [0.00, 0.70] | 0.031 * |
| Constant | 2.68 | [0.39, 18.64] | 0.318 |
| Observations | 281 | ||
| LR chi2(15) | 15.98 (p = 0.383) | ||
| Pseudo R2 | 0.042 |
| Metric | Value |
|---|---|
| AIC | 395.51 |
| BIC | 453.73 |
| Correctly classified | 60.1% |
| Sensitivity | 86.2% |
| Specificity | 21.9% |
| Positive predictive value | 61.8% |
| Negative predictive value | 52.1% |
| Area under ROC curve (AUC) | 0.633 |
| Hosmer–Lemeshow test | χ2(8) = 7.61, p = 0.472 |
| Variable | VIF | 1/VIF |
|---|---|---|
| Gravidae categories | 3.68 | 0.272 |
| Parity categories | 3.25 | 0.308 |
| Mother’s age | 2.90 | 0.345 |
| First visit | 1.54 | 0.647 |
| Marital status | 1.45 | 0.691 |
| Number of visits | 1.41 | 0.708 |
| Gestation trimesters | 1.19 | 0.839 |
| Hb mild anaemia | 1.17 | 0.853 |
| Hb moderate anaemia | 1.11 | 0.901 |
| Hb severe anaemia | 1.06 | 0.941 |
| Mean VIF | 1.88 |
| Variables | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) |
|---|---|---|---|---|---|---|---|---|---|
| (1) UTI status | 1.000 | ||||||||
| (2) Haemoglobin level | −0.054 | 1.000 | |||||||
| (3) Number of antenatal visits | −0.098 * | −0.064 | 1.000 | ||||||
| (4) Parity | −0.078 | −0.032 | 0.066 | 1.000 | |||||
| (5) Gestation trimester | −0.090 | −0.151 * | 0.262 * | 0.098 * | 1.000 | ||||
| (6) Mother’s age | −0.072 | 0.025 | 0.064 | 0.671 * | 0.090 | 1.000 | |||
| (7) Gravidae | −0.101 * | −0.011 | 0.078 | 0.820 * | 0.127 * | 0.734 * | 1.000 | ||
| (8) First visit | 0.034 | 0.040 | −0.521 * | −0.092 | −0.347 * | −0.156 * | −0.141 * | 1.000 | |
| (9) Marital status | 0.027 | −0.049 | −0.064 | −0.298 * | −0.025 | −0.508* | −0.378 * | 0.144 * | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samarasinghe, S.; Toko, E.N.; Eze, U.A.; Furaha, E.; Anthony, I.S.; Kapasi, T.; Ouma, C.; Ochieng, B. A Situation Analysis of Diagnostic and Management Strategies for Gestational Urinary Tract Infections (UTIs) in Kisumu County, Kenya: Maternal Health Implications and Opportunities for Diagnostic Improvement. Microbiol. Res. 2025, 16, 250. https://doi.org/10.3390/microbiolres16120250
Samarasinghe S, Toko EN, Eze UA, Furaha E, Anthony IS, Kapasi T, Ouma C, Ochieng B. A Situation Analysis of Diagnostic and Management Strategies for Gestational Urinary Tract Infections (UTIs) in Kisumu County, Kenya: Maternal Health Implications and Opportunities for Diagnostic Improvement. Microbiology Research. 2025; 16(12):250. https://doi.org/10.3390/microbiolres16120250
Chicago/Turabian StyleSamarasinghe, Shivanthi, Eunice Namuyenga Toko, Ukpai A. Eze, Esther Furaha, Itodo S. Anthony, Tariq Kapasi, Collins Ouma, and Bertha Ochieng. 2025. "A Situation Analysis of Diagnostic and Management Strategies for Gestational Urinary Tract Infections (UTIs) in Kisumu County, Kenya: Maternal Health Implications and Opportunities for Diagnostic Improvement" Microbiology Research 16, no. 12: 250. https://doi.org/10.3390/microbiolres16120250
APA StyleSamarasinghe, S., Toko, E. N., Eze, U. A., Furaha, E., Anthony, I. S., Kapasi, T., Ouma, C., & Ochieng, B. (2025). A Situation Analysis of Diagnostic and Management Strategies for Gestational Urinary Tract Infections (UTIs) in Kisumu County, Kenya: Maternal Health Implications and Opportunities for Diagnostic Improvement. Microbiology Research, 16(12), 250. https://doi.org/10.3390/microbiolres16120250





