The Growing Antibiotic Resistance of Campylobacter Species: Is There Any Link with Climate Change?
Abstract
1. Introduction
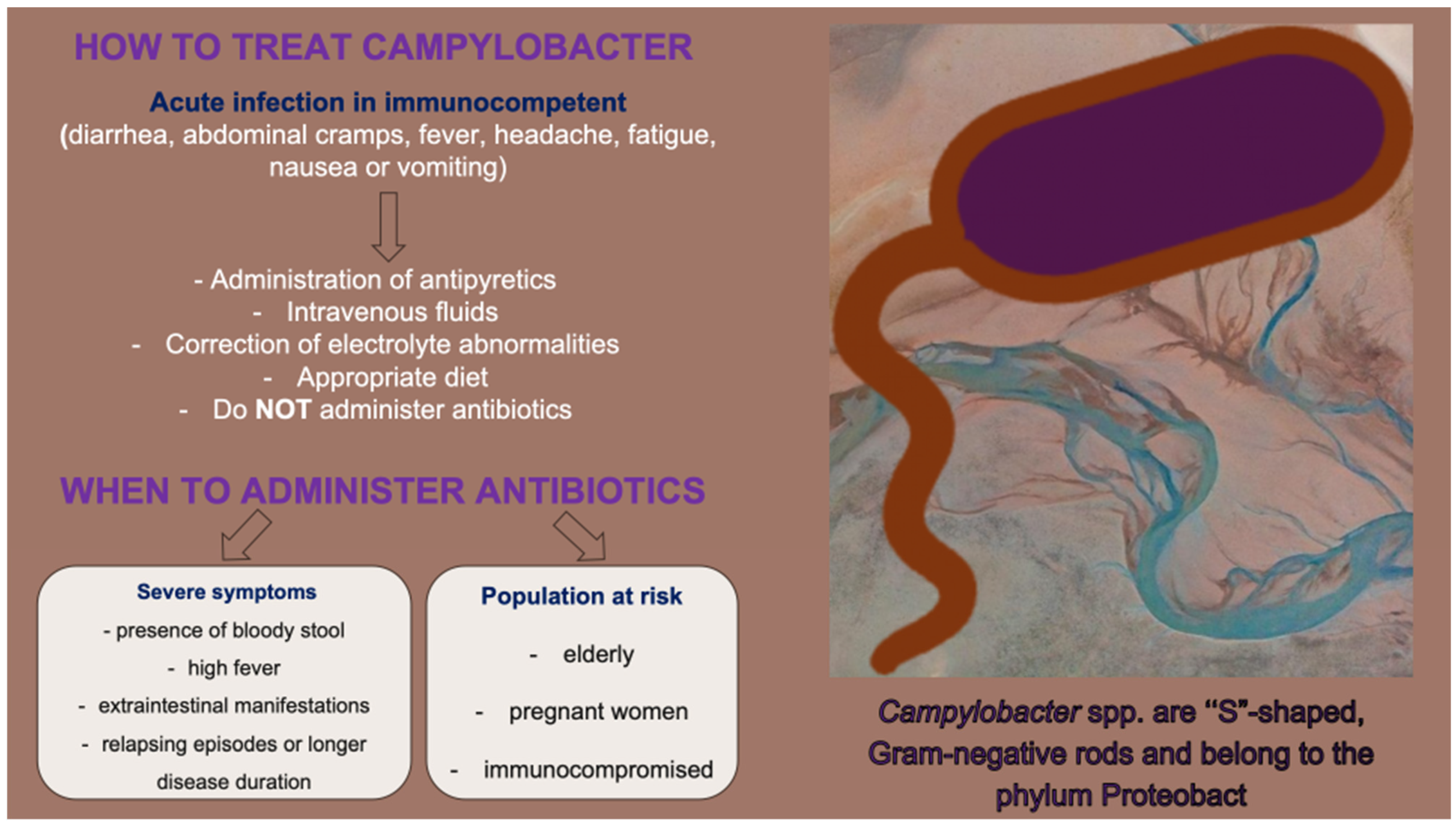
2. Treatment Indications
3. AMR: Changing the World Map
4. Mechanisms of Antibiotic Resistance in Campylobacter Species
4.1. Campylobacter spp. and Resistance to Macrolides
4.2. Campylobacter spp. and Resistance to Fluoroquinolones
4.3. Campylobacter spp. and Resistance to Tetracyclines
4.4. Campylobacter and Resistance to Aminoglycosides
4.5. Campylobacter spp. and Resistance to Phenicols
4.6. Campylobacter spp. and Resistance to β-Lactams
4.7. Campylobacter spp. and Resistance to Fosfomycin
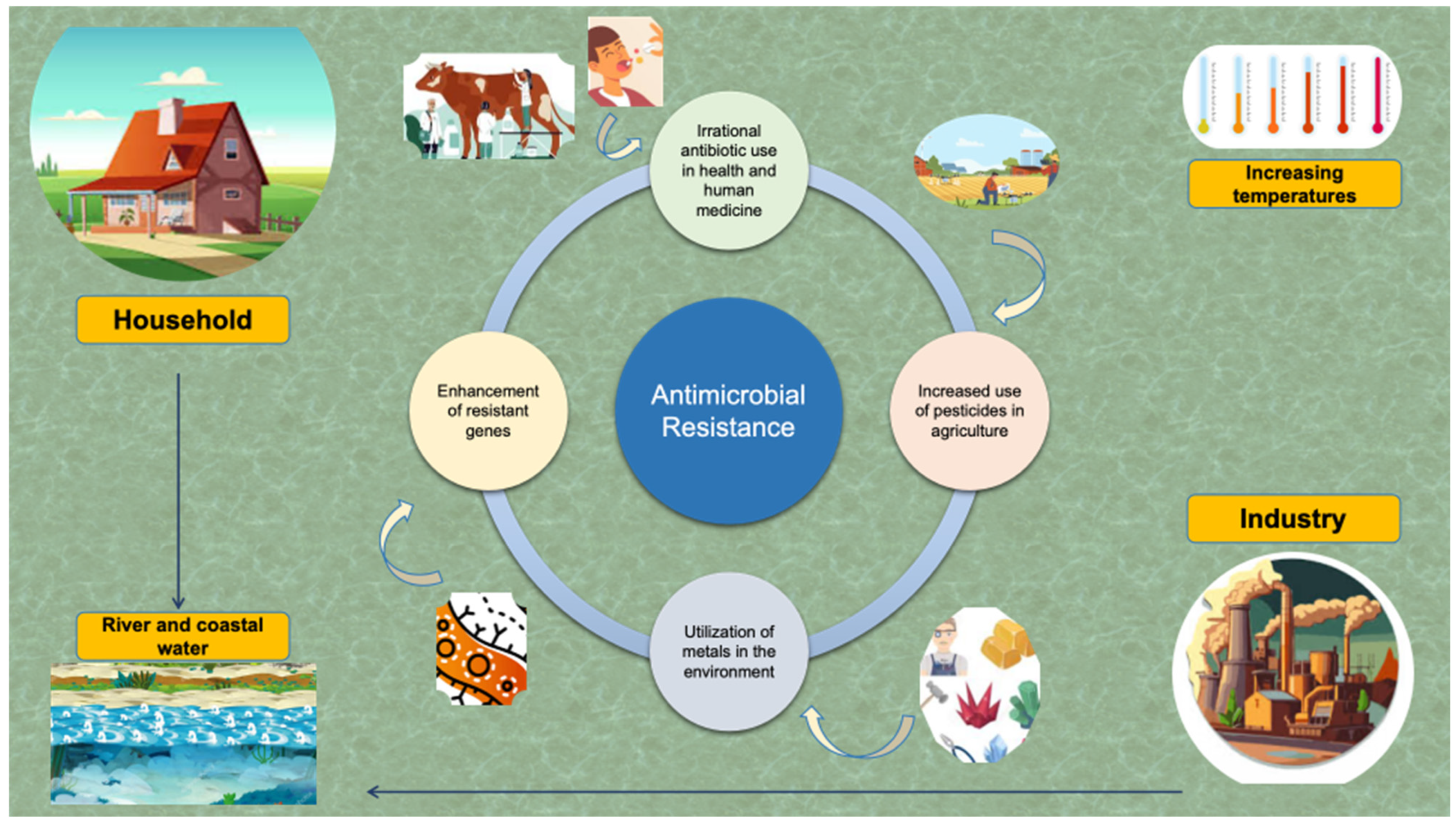
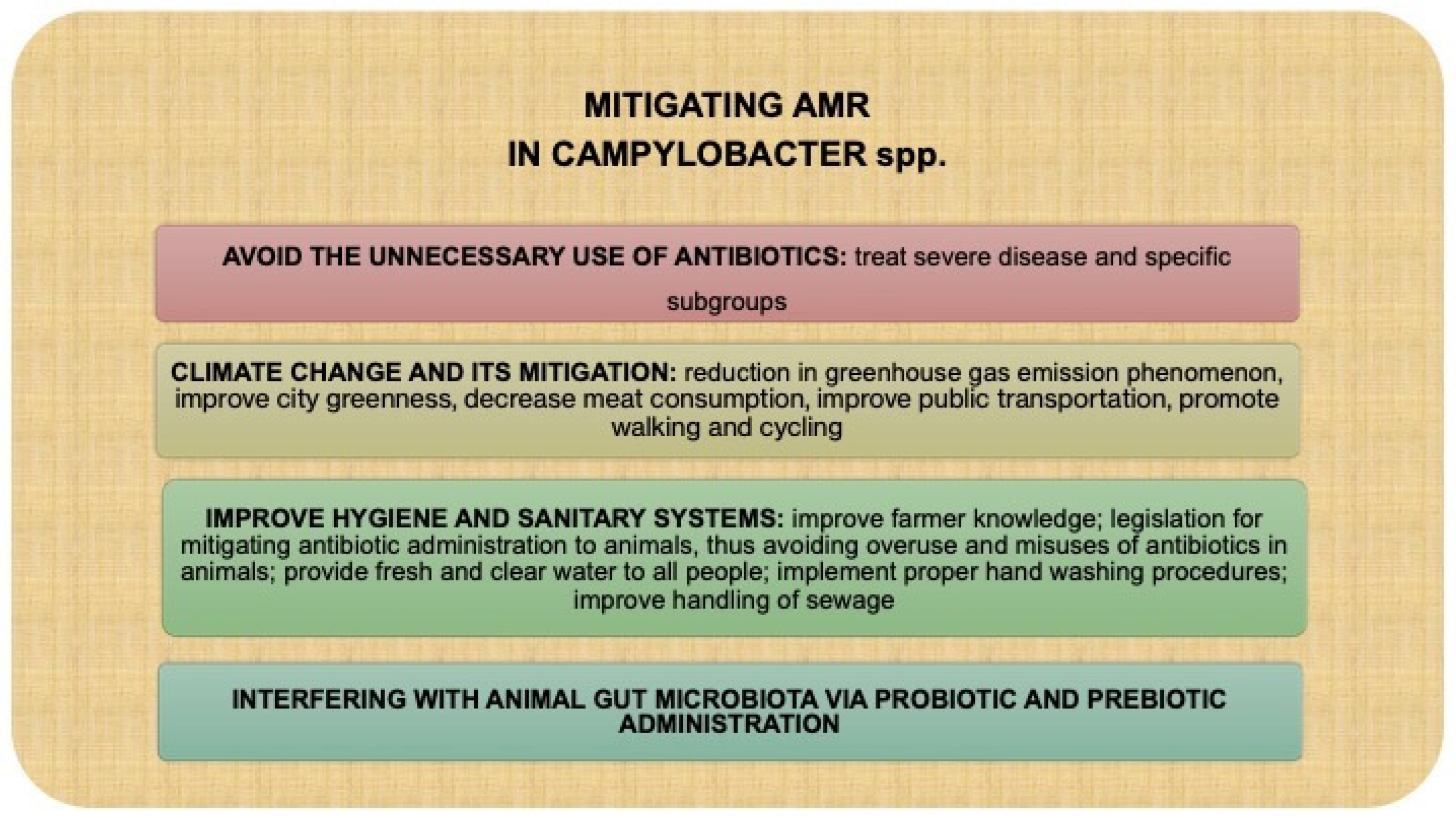
5. The Link Between Climate Crisis and Development of Campylobacter AMR
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Man, S.M. The clinical importance of emerging Campylobacter species. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 669–685. [Google Scholar] [CrossRef]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a Foodborne Pathogen: A Review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef]
- Hakeem, M.J.; Lu, X. Survival and Control of Campylobacter in Poultry Production Environment. Front. Cell Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Gulhan, T. Campylobacter in Wild Birds: Is It an Animal and Public Health Concern? Front. Microbiol. 2022, 12, 812591. [Google Scholar] [CrossRef]
- Al Hakeem, W.G.; Fathima, S.; Shanmugasundaram, R.; Selvaraj, R.K. Campylobacter jejuni in Poultry: Pathogenesis and Control Strategies. Microorganisms 2022, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Vinzent, R.; Dumas, J.; Picard, N. Septicemie grave au cours de la grossesse, due a un vibrian. Avortement consecutif. Bull. Acad. Nat. Med. 1947, 131, 90–92. [Google Scholar]
- Costa, D.; Iraola, G. Pathogenomics of Emerging Campylobacter Species. Clin. Microbiol. Rev. 2019, 32, e00072-18. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Yanestria, S.M.; Effendi, M.H.; Moses, I.; Kusala, M.; Fauzia, K.; Ayuti, S.; Fauziah, I.; Silaen, O.; Riwu, K.; et al. Campylobacteriosis: A rising threat in foodborne illnesses. Open Vet. J. 2024, 14, 1733–1750. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Arguello, Y.M.; Perdoncini, G.; Rodrigues, L.B.; dos Santos, L.R.; Borges, K.A.; Furian, T.Q.; Salle, C.T.P.; Moraes, H.L.d.S.; Gomes, M.J.P.; Nascimento, V.P.D. Identification of pathogenic genes in Campylobacter jejuni isolated from broiler carcasses and broiler slaughterhouses. Sci. Rep. 2021, 11, 4588. [Google Scholar] [CrossRef]
- Veronese, P.; Dodi, I. Campylobacter jejuni/coli Infection: Is It Still a Concern? Microorganisms 2024, 12, 2669. [Google Scholar] [CrossRef] [PubMed]
- Austhof, E.; Warner, S.; Helfrich, K.; Pogreba-Brown, K.; Brown, H.E.; Klimentidis, Y.C.; Walter, E.S.; Jervis, R.H.; White, A. Exploring the association of weather variability on Campylobacter—A systematic review. Environ. Res. 2024, 252, 118796. [Google Scholar] [CrossRef] [PubMed]
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef]
- Tam, C.C.; Rodrigues, L.C.; O’Brien, S.J.; Hajat, S. Temperature dependence of reported Campylobacter infection in England, 1989-1999. Epidemiol Infect. 2006, 134, 119–125. [Google Scholar] [CrossRef]
- Tam, C.C.; Rodrigues, L.C.; Viviani, L.; Dodds, J.P.; Evans, M.R.; Hunter, P.R.; Gray, J.J.; Letley, L.H.; Rait, G.; Tompkins, D.S.; et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): Incidence in the community and presenting to general practice. Gut 2012, 61, 69–77. [Google Scholar] [CrossRef]
- Goddard, M.R.; O’Brien, S.; Williams, N.; Guitian, J.; Grant, A.; Cody, A.; Colles, F.; Buffet, J.-C.; Adlen, E.; Stephens, A.; et al. A restatement of the natural science evidence based regarding the source, spread and control of Campylobacter species causing human disease. Proc. Biol. Sci. 2022, 289, 20220400. [Google Scholar] [CrossRef]
- Awofisayo-Okuyelu, A.; Hall, I.; Adak, G.; Hawker, J.I.; Abbott, S.; McCARTHY, N. A systematic review and meta-analysis on the incubation period of Campylobacteriosis. Epidemiol. Infect. 2017, 145, 2241–2253. [Google Scholar] [CrossRef]
- Guo, Y.T.; Hsiung, C.A.; Wu, F.T.; Chi, H.; Huang, Y.-C.; Liu, C.-C.; Huang, Y.-C.; Lin, H.-C.; Shih, S.-M.; Huang, C.-Y.; et al. Clinical manifestations and risk factors of Campylobacter gastroenteritis in children in Taiwan. Biomed. J. 2023, 46, 100590. [Google Scholar] [CrossRef]
- White, A.E.; Ciampa, N.; Chen, Y.; Kirk, M.; Nesbitt, A.; Bruce, B.B.; Walter, E.S. Characteristics of Campylobacter and Salmonella Infections and Acute Gastroenteritis in Older Adults in Australia, Canada, and the United States. Clin. Infect. Dis. 2019, 69, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C.; Stone, S.M.; Sutton, S.H.; Flaherty, J.P. Cutaneous manifestations of Campylobacter jejuni infection: A case report and review of the literature. Infect. Dis. Clin. Pract. 2020, 28, 61–63. [Google Scholar] [CrossRef]
- Hannu, T.; Kauppi, M.; Tuomala, M.; Laaksonen, I.; Klemets, P.; Kuusi, M. Reactive arthritis following an outbreak of Campylobacter jejuni infection. J. Rheumatol. 2004, 31, 528–530. [Google Scholar] [PubMed]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter species and Guillain-Barré syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [CrossRef]
- Jacobs, B.C.; Hazenberg, M.P.; van Doorn, P.A.; Endtz, H.P.; van der Meché, F.G. Cross-reactive antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in patients with Guillain-Barré or Miller Fisher syndrome. J. Infect. Dis. 1997, 175, 729–733. [Google Scholar] [CrossRef]
- Rosenberg, A.; Weinberger, M.; Paz, S.; Valinsky, L.; Agmon, V.; Peretz, C. Ambient temperature and age-related notified Campylobacter infection in Israel: A 12-year time series study. Environ. Res. 2018, 164, 539–545. [Google Scholar] [CrossRef]
- Queenan, K.; Häsler, B. Climate change and campylobacteriosis from chicken meat: The changing risk factors and their importance. Food Control. 2025, 173, 111193. [Google Scholar] [CrossRef]
- Rushton, S.P.; Sanderson, R.A.; Diggle, P.J.; Shirley, M.D.F.; Blain, A.P.; Lake, I.; Maas, J.A.; Reid, W.D.K.; Hardstaff, J.; Williams, N.; et al. Climate, human behaviour or environment: Individual-based modelling of Campylobacter seasonality and strategies to reduce disease burden. J. Transl. Med. 2019, 17, 34. [Google Scholar] [CrossRef]
- Dietrich, J.; Hammerl, J.A.; Johne, A.; Kappenstein, O.; Loeffler, C.; Nöckler, K.; Rosner, B.; Spielmeyer, A.; Szabo, I.; Richter, M.H. Impact of climate change on foodborne infections and intoxications. J. Health Monit. 2023, 8 (Suppl. S3), 78–92. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Portes, A.B.; Panzenhagen, P.; Pereira Dos Santos, A.M.; Junior, C.A.C. Antibiotic Resistance in Campylobacter: A Systematic Review of South American Isolates. Antibiotics 2023, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Bukari, Z.; Emmanuel, T.; Woodward, J.; Ferguson, R.; Ezughara, M.; Darga, N.; Lopes, B.S. The Global Challenge of Campylobacter: Antimicrobial Resistance and Emerging Intervention Strategies. Trop. Med. Infect. Dis. 2025, 10, 25. [Google Scholar] [CrossRef]
- Ahmed, A.O.; Ameh, J.A.; Abubakar, M.B.; Raufu, I.A. Campylobacteriosis in Africa: A neglected demon. Microbe 2025, 7, 100409. [Google Scholar] [CrossRef]
- Tracz, D.M.; Keelan, M.; Ahmed-Bentley, J.; Gibreel, A.; Kowalewska-Grochowska, K.; Taylor, D.E. pVir and bloody diarrhea in Campylobacter jejuni enteritis. Emerg. Infect. Dis. 2005, 11, 838–843. [Google Scholar] [CrossRef]
- Grzybowska-Chlebowczyk, U.; Kalita, B.; Flak-Wancerz, A.; Jasielska, M.; Więcek, S.; Wojcieszyn, M.; Horowska-Ziaja, S.; Chlebowczyk, W.; Woś, H. Clinical course of Campylobacter infections in children. Pediatria Polska. 2013, 88, 329–334. [Google Scholar] [CrossRef]
- Ternhag, A.; Asikainen, T.; Giesecke, J.; Ekdahl, K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin. Infect. Dis. 2007, 44, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, G.M. The health burden of Campylobacter infection and the impact of antimicrobial resistance: Playing chicken. Clin. Infect. Dis. 2007, 44, 701–703. [Google Scholar] [CrossRef]
- Najjar, I.; Paluca, F.; Loukidis, K.; Tarr, P.E. Recurrent Campylobacter Enteritis in Patients with Hypogammaglobulinemia: Review of the Literature. J. Clin. Med. 2020, 9, 553. [Google Scholar] [CrossRef]
- Gerritsen van der Hoop, A.; Veringa, E.M. Cholecystitis caused by Campylobacter jejuni. Clin. Infect. Dis. 1993, 17, 133. [Google Scholar] [CrossRef]
- Lang, C.L.; Chiang, C.K.; Hung, K.Y.; Wu, K.D. Campylobacter jejuni peritonitis and bacteremia in a patient undergoing continuous ambulatory peritoneal dialysis. Clin. Nephrol. 2009, 71, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Monselise, A.; Blickstein, D.; Ostfeld, I.; Segal, R.; Weinberger, M. A case of cellulitis complicating Campylobacter jejuni subspecies bacteremia and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Pisipati, S.; Zafar, A.; Zafar, Y. Campylobacter coli bacteraemia: How common is it? BMJ Case Rep. 2020, 13, e236634. [Google Scholar] [CrossRef]
- Kusulja, M.; Santini, M.; Margetić, K.; Guzvinec, M.; Šoprek, S.; Butić, I.; Andrašević, A.T. Meningitis caused by Campylobacter jejuni: A case presentation and literature review. Acta Clin. 2021, 76, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Roan, J.N.; Ko, W.C.; Luo, C.Y. Abdominal septic aortic pseudoaneurysm caused by Campylobacter jejuni infection: Report of a case. Surg. Today 2009, 39, 137–140. [Google Scholar] [CrossRef]
- Hannu, T.; Mattila, L.; Rautelin, H.; Siitonen, A.; Leirisalo-Repo, M. Three cases of cardiac complications associated with Campylobacter jejuni infection and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 619–622. [Google Scholar] [CrossRef]
- Roa-Bautista, A.; Brown, L.K.; Tadros, S.; Burns, S.O.; Godbole, G.; Lowe, D.M. Clinical Features, Immunological Characteristics, and Treatment Outcomes of Campylobacter spp. Infections in Patients with Common Variable Immunodeficiency. J. Allergy Clin. Immunol. Pract. 2023, 11, 3493–3501.e4. [Google Scholar] [CrossRef]
- Pang, J.; Beyi, A.F.; Looft, T.; Zhang, Q.; Sahin, O. Fecal Microbiota Transplantation Reduces Campylobacter jejuni Colonization in Young Broiler Chickens Challenged by Oral Gavage but Not by Seeder Birds. Antibiotics 2023, 12, 1503. [Google Scholar] [CrossRef]
- de la Torre, R.G.; Morís, G.; Martínez, D.P.; Montes, I.C. Guillain-Barré syndrome, tuberculosis and inflammatory bowel disease: A multiple association. Int. Arch. Med. 2010, 3, 15. [Google Scholar] [CrossRef]
- Shang, P.; Feng, J.; Wu, W.; Zhang, H.L. Intensive Care and Treatment of Severe Guillain-Barré Syndrome. Front. Pharmacol. 2021, 12, 608130. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.A.; Iftikhar, H.; Najam, M.; Masood, M.; Al-Marri, N.D.R.; Elgassim, M.A.M.; Fayed, M.; Shaban, E.E. Plasma exchange (PE) versus intravenous immunoglobulin (IVIG) for the treatment of Guillain-Barré syndrome (GBS) in patients with severe symptoms: A systematic review and meta-analysis. eNeurologicalSci 2023, 31, 100468. [Google Scholar] [CrossRef]
- Pope, J.E.; Krizova, A.; Garg, A.X.; Thiessen-Philbrook, H.; Ouimet, J.M. Campylobacter reactive arthritis: A systematic review. Semin. Arthritis Rheum. 2007, 37, 48–55. [Google Scholar] [CrossRef]
- Janssen, R.; Krogfelt, K.A.; Cawthraw, S.A.; van Pelt, W.; Wagenaar, J.A.; Owen, R.J. Host-pathogen interactions in Campylobacter infections: The host perspective. Clin. Microbiol. Rev. 2008, 21, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, D.; Salpynov, Z.; Gusmanov, A.; Khuanbai, Y.; Mukhatayev, Z.; Kunz, J. Enteric Infection-Associated Reactive Arthritis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3433. [Google Scholar] [CrossRef]
- Berumen, A.; Lennon, R.; Breen-Lyles, M.; Griffith, J.; Patel, R.; Boxrud, D.; Decuir, M.; Farrugia, G.; Smith, K.; Grover, M. Characteristics and Risk Factors of Post-Infection Irritable Bowel Syndrome After Campylobacter Enteritis. Clin. Gastroenterol. Hepatol. 2021, 19, 1855–1863.e1. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Osek, J. Antimicrobial resistance mechanisms among Campylobacter. Biomed. Res. Int. 2013, 2013, 340605. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Kang, M. In vitro activity of fosfomycin against Campylobacter isolates from poultry and wild birds. PLoS ONE 2018, 13, e0200853. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Coque, T.M.; Cantón, R.; Pérez-Cobas, A.E.; Fernández-de-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef]
- Ferraz, M.P. Antimicrobial Resistance: The Impact from and on Society According to One Health Approach. Societies 2024, 14, 187. [Google Scholar] [CrossRef]
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.A.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar]
- Li, Z.; Cai, H.; Xu, B.; Dong, Q.; Jia, K.; Lin, Z.; Wang, X.; Liu, Y.; Qin, X. Prevalence, antibiotic resistance, resistance and virulence determinants of Campylobacter jejuni in China: A systematic review and meta-analysis. One Health. 2025, 20, 100990. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Sellera, F.P.; Lincopan, N.; Debone, D.; Miraglia, S.G.E.K.; Tavella, R.A. Catastrophic floods and antimicrobial resistance: Interconnected threats with wide-ranging impacts. One Health 2024, 19, 100891. [Google Scholar] [CrossRef]
- Fernández Salgueiro, M.; Cernuda Martínez, J.A.; Gan, R.K.; Arcos González, P. Climate change and antibiotic resistance: A scoping review. Environ. Microbiol. Rep. 2024, 16, e70008. [Google Scholar] [CrossRef]
- Dalhoff, A. Global fluoroquinolone resistance epidemiology and implications for clinical use. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 976273. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Healy, J.M.; Cui, Z.; Ahart, L.; Medalla, F.; Ray, L.C.; Reynolds, J.; E Laughlin, M.; Vugia, D.J.; Hanna, S.; et al. Epidemiology and Antimicrobial Resistance of Campylobacter Infections in the United States, 2005–2018. Open Forum Infect. Dis. 2023, 10, ofad378. [Google Scholar] [CrossRef] [PubMed]
- Sodagari, H.R.; Agrawal, I.; Sohail, M.N.; Yudhanto, S.; Varga, C. Monitoring antimicrobial resistance in Campylobacter isolates of chickens and turkeys at the slaughter establishment level across the United States, 2013–2021. Epidemiol. Infect. 2024, 152, e41. [Google Scholar] [CrossRef]
- Kenyon, C. Positive Association between the Use of Quinolones in Food Animals and the Prevalence of Fluoroquinolone Resistance in E. coli and K. pneumoniae, A. baumannii and P. aeruginosa: A Global Ecological Analysis. Antibiotics 2021, 10, 1193. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Zhang, Q.; Shen, J. Antimicrobial Resistance in Campylobacter spp. Microbiol. Spectr. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Kiskó, G.; Bajramović, B.; Elzhraa, F.; Erdei-Tombor, P.; Dobó, V.; Mohácsi-Farkas, C.; Taczman-Brückner, A.; Belák, Á. The Invisible Threat of Antibiotic Resistance in Food. Antibiotics 2025, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Burmeister, A.R. Horizontal Gene Transfer. Evol. Med. Public. Health 2015, 2015, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Chawla, M.; Ahrodia, T.; Narendrakumar, L.; Das, B. Antibiotic Potentiation as a Promising Strategy to Combat Macrolide Resistance in Bacterial Pathogens. Antibiotics 2023, 12, 1715. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, S.J.; Leśniczak-Staszak, M.; Gowin, E.; Szaflarski, W. Mechanistic Insights into Clinically Relevant Ribosome-Targeting Antibiotics. Biomolecules 2024, 14, 1263. [Google Scholar] [CrossRef]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Jehanne, Q.; Bénéjat, L.; Ducournau, A.; Aptel, J.; Pivard, M.; Gillet, L.; Jauvain, M.; Lehours, P. Increasing rates of erm(B) and erm(N) in human Campylobacter coli and Campylobacter jejuni erythromycin-resistant isolates between 2018 and 2023 in France. Antimicrob Agents Chemother. 2025, 69, e0166824. [Google Scholar] [CrossRef]
- Wei, B.; Kang, M. Molecular Basis of Macrolide Resistance in Campylobacter Strains Isolated from Poultry in South Korea. Biomed. Res. Int. 2018, 2018, 4526576. [Google Scholar] [CrossRef] [PubMed]
- Bolinger, H.; Kathariou, S. The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms. Appl. Environ. Microbiol. 2017, 83, e00416-17. [Google Scholar] [CrossRef]
- Gao, F.; Tu, L.; Chen, M.; Chen, H.; Zhang, X.; Zhuang, Y.; Luo, J.; Chen, M. Erythromycin resistance of clinical Campylobacter jejuni and Campylobacter coli in Shanghai, China. Front. Microbiol. 2023, 14, 1145581. [Google Scholar] [CrossRef]
- Gibreel, A.; Wetsch, N.M.; Taylor, D.E. Contribution of the CmeABC efflux pump to macrolide and tetracycline resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 2007, 51, 3212–3216. [Google Scholar] [CrossRef] [PubMed]
- Cagliero, C.; Mouline, C.; Payot, S.; Cloeckaert, A. Involvement of the CmeABC efflux pump in the macrolide resistance of Campylobacter coli. J. Antimicrob. Chemother. 2005, 56, 948–950. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Future Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Shen, Z.; Wang, Y.; Deng, F.; Liu, D.; Naren, G.; Dai, L.; Su, C.-C.; Wang, B.; Wang, S.; et al. Emergence of a Potent Multidrug Efflux Pump Variant That Enhances Campylobacter Resistance to Multiple Antibiotics. mBio 2016, 7, e01543-16. [Google Scholar] [CrossRef]
- Luo, N.; Sahin, O.; Lin, J.; Michel, L.O.; Zhang, Q. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob. Agents Chemother. 2003, 47, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef]
- Li, W.; Atkinson, G.C.; Thakor, N.S.; Allas, Ü.; Lu, C.-C.; Chan, K.-Y.; Tenson, T.; Schulten, K.; Wilson, K.S.; Hauryliuk, V.; et al. Mechanism of tetracycline resistance by ribosomal protection protein Tet(O). Nat. Commun. 2013, 4, 1477. [Google Scholar] [CrossRef]
- Connell, S.R.; Tracz, D.M.; Nierhaus, K.H.; Taylor, D.E. Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrob. Agents Chemother. 2003, 47, 3675–3681. [Google Scholar] [CrossRef]
- Lynch, C.T.; Lynch, H.; Burke, S.; Hawkins, K.; Buttimer, C.; Mc Carthy, C.; Egan, J.; Whyte, P.; Bolton, D.; Coffey, A.; et al. Antimicrobial Resistance Determinants Circulating among Thermophilic Campylobacter Isolates Recovered from Broilers in Ireland Over a One-Year Period. Antibiotics 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Pacífico, C.; Wösten, M.M.S.M.; Hilbert, F. Low-Level Tetracycline Resistance Gene tet(O)_3 in Campylobacter jejuni. Antibiotics 2023, 12, 426. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Shiraliyev, R.; Orman, M.A. Metabolic disruption impairs ribosomal protein levels, resulting in enhanced aminoglycoside tolerance. Elife 2024, 13, RP94903. [Google Scholar] [CrossRef]
- Jana, S.; Deb, J.K. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 2006, 70, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Magnet, S.; Blanchard, J.S. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 2005, 105, 477–498. [Google Scholar] [CrossRef]
- Doi, Y.; Arakawa, Y. 16S ribosomal RNA methylation: Emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 2007, 45, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.; Gerbaud, G.; Trieu-Cuot, P.; Courvalin, P. Structural relationship between the genes encoding 3′-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann. Inst. Pasteur Microbiol. 1985, 136B, 135–150. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Update 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. Medchemcomm 2016, 7, 11–27. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Tang, M.; Pu, J.; Zhang, J.; Lu, J.; Zhang, Y.; Gao, Y. Aminoglycoside Resistance and Possible Mechanisms in Campylobacter Spp. Isolated from Chicken and Swine in Jiangsu, China. Front. Microbiol. 2021, 12, 716185. [Google Scholar] [CrossRef]
- Zárate, S.G.; De la Cruz Claure, M.L.; Benito-Arenas, R.; Revuelta, J.; Santana, A.G.; Bastida, A. Overcoming Aminoglycoside Enzymatic Resistance: Design of Novel Antibiotics and Inhibitors. Molecules 2018, 23, 284. [Google Scholar] [CrossRef]
- Guirado, P.; Miró, E.; Iglesias-Torrens, Y.; Navarro, F.; Campoy, S.; Alioto, T.S.; Gómez-Garrido, J.; Madrid, C.; Balsalobre, C. A New Variant of the aadE-sat4-aphA-3 Gene Cluster Found in a Conjugative Plasmid from an MDR Campylobacter jejuni Isolate. Antibiotics 2022, 11, 466. [Google Scholar] [CrossRef]
- Qin, X.; Zerr, D.M.; McNutt, M.A.; Berry, J.E.; Burns, J.L.; Kapur, R.P. Pseudomonas aeruginosa syntrophy in chronically colonized airways of cystic fibrosis patients. Antimicrob. Agents Chemother. 2012, 56, 5971–5981. [Google Scholar] [CrossRef]
- Nirdnoy, W.; Mason, C.J.; Guerry, P. Mosaic structure of a multiple-drug-resistant, conjugative plasmid from Campylobacter jejuni. Antimicrob. Agents Chemother. 2005, 49, 2454–2459. [Google Scholar] [CrossRef]
- Werner, G.; Hildebrandt, B.; Witte, W. Aminoglycoside-streptothricin resistance gene cluster aadE-sat4-aphA-3 disseminated among multiresistant isolates of Enterococcus faecium. Antimicrob. Agents Chemother. 2001, 45, 3267–3269. [Google Scholar] [CrossRef]
- Gharbi, M.; Kamoun, S.; Hkimi, C.; Ghedira, K.; Béjaoui, A.; Maaroufi, A. Relationships between Virulence Genes and Antibiotic Resistance Phenotypes/Genotypes in Campylobacter spp. Isolated from Layer Hens and Eggs in the North of Tunisia: Statistical and Computational Insights. Foods 2022, 11, 3554. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Petridou, E.; Papageorgiou, K.; Giantsis, I.A.; Delis, G.; Economou, V.; Frydas, I.; Papadopoulos, G.; Hatzistylianou, M.; Kritas, S.K. Phenotypic and Molecular Patterns of Resistance among Campylobacter coli and Campylobacter jejuni Isolates, from Pig Farms. Animals 2021, 11, 2394. [Google Scholar] [CrossRef] [PubMed]
- Lysitsas, M.; Triantafillou, E.; Spyrou, V.; Billinis, C.; Valiakos, G. Phenotypic Investigation of Florfenicol Resistance and Molecular Detection of floR Gene in Canine and Feline MDR Enterobacterales. Vet. Sci. 2024, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Long, K.S.; Poehlsgaard, J.; Kehrenberg, C.; Schwarz, S.; Vester, B. The Cfr rRNA methyltransferase confers resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A antibiotics. Antimicrob. Agents Chemother. 2006, 50, 2500–2505. [Google Scholar] [CrossRef]
- Biswas, T.; Houghton, J.L.; Garneau-Tsodikova, S.; Tsodikov, O.V. The structural basis for substrate versatility of chloramphenicol acetyltransferase CATI. Protein Sci. 2012, 21, 520–530. [Google Scholar] [CrossRef]
- Gharbi, M.; Tiss, R.; Hamdi, C.; Hamrouni, S.; Maaroufi, A. Occurrence of Florfenicol and Linezolid Resistance and Emergence of optrA Gene in Campylobacter coli Isolates from Tunisian Avian Farms. Int. J. Microbiol. 2024, 2024, 1694745. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, L.; Sahin, O.; Wu, Z.; Liu, M.; Zhang, Q. Emergence of a plasmid-borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter. J. Antimicrob. Chemother. 2017, 72, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Verdino, A.; Soriente, A.; Marabotti, A. The Odd Couple(s): An Overview of Beta-Lactam Antibiotics Bearing More Than One Pharmacophoric Group. Int. J. Mol. Sci. 2021, 22, 617. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Griggs, D.J.; Peake, L.; Johnson, M.M.; Ghori, S.; Mott, A.; Piddock, L.J. Beta-lactamase-mediated beta-lactam resistance in Campylobacter species: Prevalence of Cj0299 (bla OXA-61) and evidence for a novel beta-Lactamase in C. jejuni. Antimicrob. Agents Chemother. 2009, 53, 3357–3364. [Google Scholar] [CrossRef]
- Castañeda-García, A.; Blázquez, J.; Rodríguez-Rojas, A. Molecular Mechanisms and Clinical Impact of Acquired and Intrinsic Fosfomycin Resistance. Antibiotics 2013, 2, 217–236. [Google Scholar] [CrossRef]
- Dijkmans, A.C.; Zacarías, N.V.O.; Burggraaf, J.; Mouton, J.W.; Wilms, E.B.; Van Nieuwkoop, C.; Touw, D.J.; Stevens, J.; Kamerling, I.M.C. Fosfomycin: Pharmacological, Clinical and Future Perspectives. Antibiotics 2017, 6, 24. [Google Scholar] [CrossRef]
- Mattioni Marchetti, V.; Hrabak, J.; Bitar, I. Fosfomycin resistance mechanisms in Enterobacterales: An increasing threat. Front. Cell Infect. Microbiol. 2023, 13, 1178547. [Google Scholar] [CrossRef]
- Arca, P.; Rico, M.; Braña, A.F.; Villar, C.J.; Hardisson, C.; Suárez, J.E. Formation of an adduct between fosfomycin and glutathione: A new mechanism of antibiotic resistance in bacteria. Antimicrob. Agents Chemother. 1988, 32, 1552–1556. [Google Scholar] [CrossRef]
- Magnano San Lio, R.; Favara, G.; Maugeri, A.; Barchitta, M.; Agodi, A. How Antimicrobial Resistance Is Linked to Climate Change: An Overview of Two Intertwined Global Challenges. Int. J. Environ. Res. Public Health 2023, 20, 1681. [Google Scholar] [CrossRef] [PubMed]
- Sweileh, W.M. Global research publications on irrational use of antimicrobials: Call for more research to contain antimicrobial resistance. Glob. Health 2021, 17, 94. [Google Scholar] [CrossRef]
- Kuhn, K.G.; Nygård, K.M.; Guzman-Herrador, B.; Sunde, L.S.; Rimhanen-Finne, R.; Trönnberg, L.; Jepsen, M.R.; Ruuhela, R.; Wong, W.K.; Ethelberg, S. Campylobacter infections expected to increase due to climate change in Northern Europe. Sci. Rep. 2020, 10, 13874. [Google Scholar] [CrossRef]
- Damtew, Y.T.; Tong, M.; Varghese, B.M.; Anikeeva, O.; Hansen, A.; Dear, K.; Driscoll, T.; Zhang, Y.; Capon, T.; Bi, P. The impact of temperature on non-typhoidal Salmonella and Campylobacter infections: An updated systematic review and meta-analysis of epidemiological evidence. EBioMedicine 2024, 109, 105393. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-J.; Khant, N.A.; Kim, H.; Namkoong, S. Impact of Climate Change on Waterborne Diseases: Directions towards Sustainability. Water 2023, 15, 1298. [Google Scholar] [CrossRef]
- Semenza, J.C. Climate Change and Contagion: The Circuitous Impacts from Infectious Diseases. J. Infect. Dis. 2024, 229, 928–930. [Google Scholar] [CrossRef]
- Skarp, C.P.A.; Hänninen, M.L.; Rautelin, H.I.K. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef]
- Strakova, N.; Shagieva, E.; Ovesna, P.; Korena, K.; Michova, H.; Demnerova, K.; Kolackova, I.; Karpiskova, R. The effect of environmental conditions on the occurrence of Campylobacter jejuni and Campylobacter coli in wastewater and surface waters. J. Appl. Microbiol. 2022, 132, 725–735. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, F.F.; Hu, J.Y.; Mohammed, A.; Cheng, H.W. Bacillus subtilis-based probiotic improves skeletal health and immunity in broiler chickens exposed to heat stress. Animals 2021, 11, 1494. [Google Scholar] [CrossRef]
- Balta, I.; Lemon, J.; Murnane, C.; Pet, I.; Vintila, T.; McCleery, D.; Callaway, T.; Douglas, A.; Stef, L.; Corcionivoschi, N. The One Health aspect of climate events with impact on foodborne pathogens transmission. One Health 2024, 19, 100926. [Google Scholar] [CrossRef]
- USDA. Global Market Analysis. United States Department of Agriculture. Livestock and Poultry: World Markets and Trade; Foreign Agricultural Service: Washington, DC, USA, 2023. [Google Scholar]
- OECD; FAO. Agricultural Outlook 2021–2030. (Chapter 6): Meat; OECD Publishing: Paris, France, 2021. [Google Scholar]
- Bundurus, I.A.; Balta, I.; Pet, I.; Stef, L.; Popescu, C.A.; McCleery, D.; Lemon, J.; Callaway, T.; Douglas, A.; Corcionivoschi, N. Mechanistic concepts involved in biofilm associated processes of Campylobacter jejuni: Persistence and inhibition in poultry environments. Poult. Sci. 2024, 10, 104328. [Google Scholar] [CrossRef]
- Awad, D.A.; Masoud, H.A.; Hamad, A. Climate changes and food-borne pathogens: The impact on human health and mitigation strategy. Clim. Change 2024, 177, 92. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention Strategies to Control Campylobacter at Different Stages of the Food Chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef]
- Neelawala, R.N.; Edison, L.K.; Kariyawasam, S. Pre-Harvest Non-Typhoidal Salmonella Control Strategies in Commercial Layer Chickens. Animals 2024, 14, 3578. [Google Scholar] [CrossRef]
- Naeem, M.; Bourassa, D. Probiotics in Poultry: Unlocking Productivity Through Microbiome Modulation and Gut Health. Microorganisms 2025, 13, 257. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Alsayeqh, A.F.; Aqib, A.I. Role of Bacteriophages for Optimized Health and Production of Poultry. Animals 2022, 12, 3378. [Google Scholar] [CrossRef] [PubMed]
- Baars, C.; Barbir, J.; Paulino Pires Eustachio, J.H. How Can Climate Change Impact Human Health via Food Security? A Bibliometric Analysis. Environments 2023, 10, 196. [Google Scholar] [CrossRef]
- Mohamed, M.I.; Khalifa, H.O.; Habib, I. The role of virulence genes in Campylobacter pathogenicity: A perspective from the Gulf Cooperation Council countries. Front. Cell Infect. Microbiol. 2025, 15, 1584835. [Google Scholar] [CrossRef]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Danasekaran, R. One Health: A Holistic Approach to Tackling Global Health Issues. Indian. J. Community Med. 2024, 49, 260–263. [Google Scholar] [CrossRef] [PubMed]
| Resistance Genes | Point Mutations | Active Efflux | Other Mechanisms | Study [Ref.] | |
|---|---|---|---|---|---|
| Macrolides | Erm(B), MDRGIs loci; aacA-aphD, sat4, aphA-3, fosXCC, aad9 and tet(O) | domain V of the 23S rRNA; A2074C, A2074G, A2075G | CmeABC; synergy with point mutations in the ribosomal proteins L4 (G74D) and L22 (inserted at position 86 or 98) | Zhang et al. [75] Jehanne et al. [76] Bolinger et al. [78] Gibreel et al. [80] Cagliero et al. [81] | |
| Fluoroquinolones | GyrA; fluoroquinolone resistance in Campylobacter spp. | T86I, T86K, A70T and D90N | CmeABC; synergy with point mutations in GyrA genes | Shariati et al. [84] Luangtongkum et al. [85] Yao et al. [86] | |
| Tetracyclines | Tet(O) | - | CmeABC CmeG; synergy with mutations in Tet(O) gene | Grossman et al. [89] Li et al. [90] Connell et al. [91] | |
| Aminoglycosides | aadE-sat4-aphA-3 cluster aacA-aphD-aac cluster sat4 gene aph(2″)-Ib, Ic, If1, If3, Ih aac(6′)-Ie/aph(2″)-If2 | binding sites of rRNA | - | modification of the aminoglycoside structure by enzymes such as aminoglycoside acetyltransferases, aminoglycoside phosphotransferases and aminoglycoside nucleotidyltransferases | Guirado et al. [104] Werner et al. [107] Gharbi et al. [100] Papadopoulos et al. [101] |
| Phenicols | 23S rRNA cfr gene cfr(C) gene; Campylobacter coli isolates of cattle origin | G2073A mutation Methylation of the adenine at position 2503 in the 23S rRNA | variant RE-CmeABC; synergy with point mutations | Long et al. [103] Gharbi et al. [108] Tang et al. [114] | |
| β-Lactams | - | - | CmeABC CmeDEF; synergy with β-lactamase OXA-61 | OXA-61 (Cj0299); β-lactamase present in C. jejuni and coli | Reygaert et al. [116] Griggs et al. [117] |
| Fosfomycin | fosXCC gene; part of the MDRGI loci | - | - | Castaneda–Garcia et al. [118] Mattioni Marchetti et al. [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geladari, E.V.; Kounatidis, D.; Margellou, E.; Evangelopoulos, A.; Jahaj, E.; Adamou, A.; Sevastianos, V.; Geladari, C.V.; Vallianou, N.G. The Growing Antibiotic Resistance of Campylobacter Species: Is There Any Link with Climate Change? Microbiol. Res. 2025, 16, 226. https://doi.org/10.3390/microbiolres16110226
Geladari EV, Kounatidis D, Margellou E, Evangelopoulos A, Jahaj E, Adamou A, Sevastianos V, Geladari CV, Vallianou NG. The Growing Antibiotic Resistance of Campylobacter Species: Is There Any Link with Climate Change? Microbiology Research. 2025; 16(11):226. https://doi.org/10.3390/microbiolres16110226
Chicago/Turabian StyleGeladari, Eleni V., Dimitris Kounatidis, Evangelia Margellou, Apostolos Evangelopoulos, Edison Jahaj, Andreas Adamou, Vassilios Sevastianos, Charalampia V. Geladari, and Natalia G. Vallianou. 2025. "The Growing Antibiotic Resistance of Campylobacter Species: Is There Any Link with Climate Change?" Microbiology Research 16, no. 11: 226. https://doi.org/10.3390/microbiolres16110226
APA StyleGeladari, E. V., Kounatidis, D., Margellou, E., Evangelopoulos, A., Jahaj, E., Adamou, A., Sevastianos, V., Geladari, C. V., & Vallianou, N. G. (2025). The Growing Antibiotic Resistance of Campylobacter Species: Is There Any Link with Climate Change? Microbiology Research, 16(11), 226. https://doi.org/10.3390/microbiolres16110226






