Environmental Pathogen in Healthcare Settings: Candida auris—The Emerging Threat with a Focus on the Middle East and Infection Control Strategies
Abstract
1. Introduction
2. Environmental Survival and Transmission
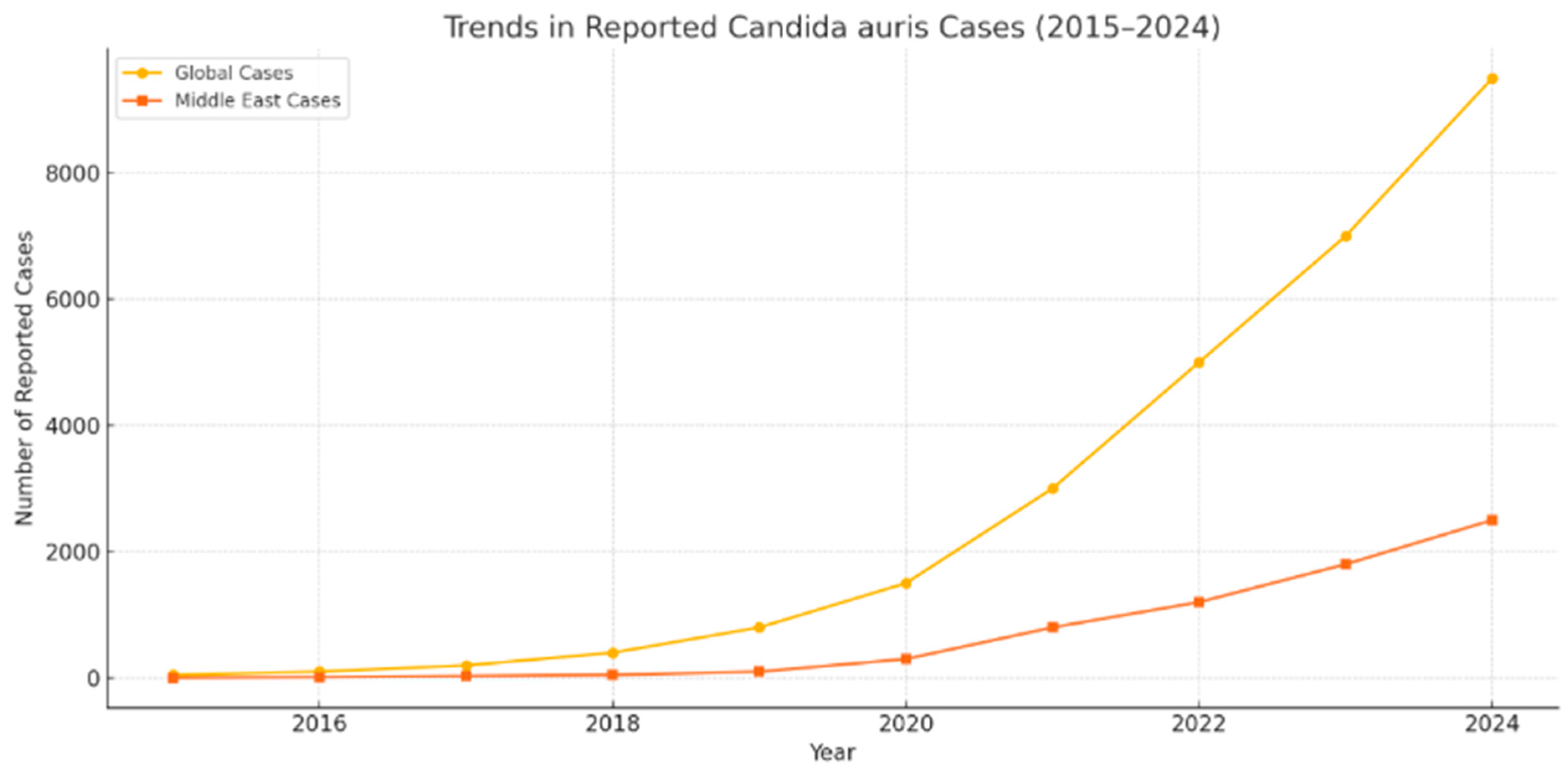
3. Experience and Data in Saudi Arabia and the Middle East
4. Epidemiology and Evolution Post-COVID-19
5. Diagnostic Challenges
6. Treatment and Antifungal Resistance
Cross-Regional Resistance Patterns
7. Infection Control Strategies
7.1. Updated IPC Protocols and Advancements
7.2. Disinfection Materials
7.3. Equipment and Technologies
8. Environmental Monitoring, Surface Culturing, and Staff Compliance Mechanisms
9. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Voidazan, S.; Albu, S.; Toth, R.; Grigorescu, B.; Rachita, A.; Moldovan, I. Healthcare associated infections—A new pathology in medical practice? Int. J. Environ. Res. Public Health 2020, 17, 760. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P. On the emergence, spread and resistance of Candida auris: Host, pathogen and environmental tipping points. J. Med. Microbiol. 2021, 70, 001318. [Google Scholar] [CrossRef] [PubMed]
- Keighley, C.; Garnham, K.; Harch, S.A.; Robertson, M.; Chaw, K.; Teng, J.C.; Chen, S.A. Candida auris: Diagnostic challenges and emerging opportunities for the clinical microbiology laboratory. Curr. Fungal Infect. Rep. 2021, 15, 116–126. [Google Scholar] [CrossRef]
- Hau, P.T.; Shiu, A.; Tam, E.W.; Chau, E.C.; Murillo, M.; Humer, E.; Po, W.W.; Yu, R.C.; Fung, J.; Seto, S.W.; et al. Diversity and Antifungal Susceptibilities of Yeasts from Mangroves in Hong Kong, China—A One Health Aspect. J. Fungi 2024, 10, 728. [Google Scholar] [CrossRef]
- Akinbobola, A.B.; Kean, R.; Hanifi, S.M.; Quilliam, R.S. Environmental reservoirs of the drug-resistant pathogenic yeast Candida auris. PLoS Pathog. 2023, 19, e1011268. [Google Scholar] [CrossRef]
- Tharp, B.; Zheng, R.; Bryak, G.; Litvintseva, A.P.; Hayden, M.K.; Chowdhary, A.; Thangamani, S. Role of microbiota in the skin colonisation of Candida auris. Msphere 2023, 8, e00623-22. [Google Scholar] [CrossRef]
- Sansom, S.E.; Gussin, G.M.; Schoeny, M.; Singh, R.D.; Adil, H.; Bell, P.; Benson, E.C.; Bittencourt, C.E.; Black, S.; Del Mar Villanueva Guzman, M.; et al. Rapid environmental contamination with Candida auris and multidrug-resistant bacterial pathogens near colonised patients. Clin. Infect. Dis. 2024, 78, 1276–1284. [Google Scholar] [CrossRef]
- Motallebirad, T.; Mohammadi, M.R.; Jadidi, A.; Safarabadi, M.; Kerami, A.; Azadi, D.; Hussein, E.S. Tracheal tube infections in critical care: A narrative review of influencing factors, microbial agents, and mitigation strategies in intensive care unit settings. SAGE Open Med. 2024, 12, 20503121241306951. [Google Scholar] [CrossRef]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: Impact and lessons learned. J. Clin. Microbiol. 2020, 58, 10–128. [Google Scholar] [CrossRef]
- Noshili, A.I.; Almutairi, F.A.; Shahbal, S.; Alotaibi, F.A.; Alotaibi, B.; Refaei, R.A.A.; Almutairi, M.M.; Hmed Al Balawi, S.A.; Alshammari, R.T.; Almotairi, A.K.; et al. Systematic Review Are We Ready for New Emerging Infection Candida auris; Review of Preparedness Measure and Strategies for Infection Prevention in the Saudi Arabian Health System. Migr. Lett. 2023, 20, 678–697. [Google Scholar]
- Hata, D.J.; Humphries, R.; Lockhart, S.R.; College of American Pathologists Microbiology Committee. Candida auris: An emerging yeast pathogen posing distinct challenges for laboratory diagnostics, treatment, and infection prevention. Arch. Pathol. Lab. Med. 2020, 144, 107–114. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.R.; Neill, C.; Borman, A.M.; Budd, E.L.; Cummins, M.; Fry, C.; Guy, R.L.; Jeffery, K.; Johnson, E.M.; Manuel, R.; et al. The laboratory investigation, management, and infection prevention and control of Candida auris: A narrative review to inform the 2024 national guidance update in England. J. Med. Microbiol. 2024, 73, 001820. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzaq, N.M.; AlRand, H.; UAE AMR Surveillance Consortium; Elhag Ahmed, A.; Yousef, A.F.; AlBlooshi, A.; Latoom, D.A.; Abdulkareem Al Hammadi, D.A.; Enshasy, D.A.; et al. Surveillance of antimicrobial resistance in the United Arab Emirates: The early implementation phase. Front. Public Health 2023, 11, 1247627. [Google Scholar] [CrossRef]
- Najeeb, H.; Siddiqui, S.A.; Anas, Z.; Ali, S.H.; Usmani, S.U.; Jawed, F.; Jatoi, H.N. The menace of Candida auris epidemic amidst the COVID-19 pandemic: A systematic review. Diseases 2022, 10, 58. [Google Scholar] [CrossRef]
- Siopi, M.; Georgiou, P.C.; Paranos, P.; Beredaki, M.I.; Tarpatzi, A.; Kalogeropoulou, E.; Damianidou, S.; Vasilakopoulou, A.; Karakosta, P.; Pournaras, S.; et al. Increase in candidemia cases and emergence of fluconazole-resistant Candida parapsilosis and C. auris isolates in a tertiary care academic hospital during the COVID-19 pandemic, Greece, 2020 to 2023. Eurosurveillance 2024, 29, 2300661. [Google Scholar] [CrossRef]
- Mohammed, M.A. Fighting cytokine storm and immunomodulatory deficiency: By using natural products therapy up to now. Front. Pharmacol. 2023, 14, 1111329. [Google Scholar] [CrossRef]
- Tavares, L.P.; Galvão, I.; Ferrero, M.R. Novel immunomodulatory therapies for respiratory pathologies. Compr. Pharmacol. 2022, 2022, 554–594. [Google Scholar]
- Christie, M.J.; Irving, A.T.; Forster, S.C.; Marsland, B.J.; Hansbro, P.M.; Hertzog, P.J.; Nold-Petry, C.A.; Nold, M.F. Of bats and men: Immunomodulatory treatment options for COVID-19 guided by the immunopathology of SARS-CoV-2 infection. Sci. Immunol. 2021, 6, eabd0205. [Google Scholar] [CrossRef]
- Huang, C. Pathogenesis of coronaviruses through human monocytes and tissue macrophages. Viral Immunol. 2021, 34, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Novak Babič, M.; Gunde-Cimerman, N. Potable water as a source of intermediate and borderline-resistant Aspergillus and Candida strains. J. Water Health 2025, 23, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Guinea, J.; Arikan-Akdagli, S.; Meijer, E.F.; Meis, J.F.; Buil, J.B.; Dannaoui, E.; Giske, C.G.; Lyskova, P.; Meletiadis, J.; et al. How to interpret MICs of amphotericin B, echinocandins and flucytosine against Candida auris (Candidozyma auris) according to the newly established EUCAST breakpoints. Clin. Microbiol. Infect. 2025; in press. [Google Scholar]
- Garcia-Bustos, V.; Cabanero-Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.Á.; Pemán, J. What do we know about Candida auris? State of the art, knowledge gaps, and future directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Martínez-Álvarez, J.A.; Chávez-Santiago, J.O.; Mora-Montes, H.M. Candida haemulonii complex and Candida auris: Biology, virulence factors, immune response, and multidrug resistance. Infect. Drug Resist. 2023, 16, 1455–1470. [Google Scholar] [CrossRef]
- Foo, P.C.; Nurul Najian, A.B.; Muhamad, N.A.; Ahamad, M.; Mohamed, M.; Yean Yean, C.; Lim, B.H. Loop-mediated isothermal amplification (LAMP) reaction as viable PCR substitute for diagnostic applications: A comparative analysis study of LAMP, conventional PCR, nested PCR (nPCR) and real-time PCR (qPCR) based on Entamoeba histolytica DNA derived from faecal sample. BMC Biotechnol. 2020, 20, 34. [Google Scholar]
- Hu, X.; Deng, Q.; Li, J.; Chen, J.; Wang, Z.; Zhang, X.; Fang, Z.; Li, H.; Zhao, Y.; Yu, P.; et al. Development and clinical application of a rapid and sensitive loop-mediated isothermal amplification test for SARS-CoV-2 infection. MSphere 2020, 5, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Dire, O.; Ahmad, A.; Duze, S.; Patel, M. Survival of Candida auris on environmental surface materials and low-level resistance to disinfectant. J. Hosp. Infect. 2023, 137, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Lexow, F.; Bludau, A.; Köster, A.M.; Misailovski, M.; Seifert, U.; Eggers, M.; Rutala, W.; Dancer, S.J.; Scheithauer, S. How long do bacteria, fungi, protozoa, and viruses retain their replication capacity on inanimate surfaces? A systematic review examining environmental resilience versus healthcare-associated infection risk by “fomite-borne risk assessment”. Clin. Microbiol. Rev. 2024, 37, e00186-23. [Google Scholar] [CrossRef]
- Bäumler, W.; Eckl, D.; Holzmann, T.; Schneider-Brachert, W. Antimicrobial coatings for environmental surfaces in hospitals: A potential new pillar for prevention strategies in hygiene. Crit. Rev. Microbiol. 2022, 48, 531–564. [Google Scholar] [CrossRef]
- Hoenigl, M.; Arastehfar, A.; Arendrup, M.C.; Brüggemann, R.; Carvalho, A.; Chiller, T.; Chen, S.; Egger, M.; Feys, S.; Gangneux, J.P.; et al. Novel antifungals and treatment approaches to tackle resistance and improve outcomes of invasive fungal disease. Clin. Microbiol. Rev. 2024, 37, e00074-23. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of amphotericin B: An overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Emerging fungal infections: New species, new names, and antifungal resistance. Clin. Chem. 2022, 68, 83–90. [Google Scholar] [CrossRef]
- Nivoix, Y.; Ledoux, M.P.; Herbrecht, R. Antifungal therapy: New and evolving therapies. InSeminars Respir. Crit. Care Med. 2020, 41, 158–174. [Google Scholar] [CrossRef]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on current status of echinocandins use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Olivo, M.; Morris, K.N.; Patterson, H.P.; Catano, G.; Patterson, T.F. Ibrexafungerp demonstrates in vitro activity against fluconazole-resistant Candida auris and in vivo efficacy with delayed initiation of therapy in an experimental model of invasive candidiasis. Antimicrob. Agents Chemother. 2021, 65, 10–128. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 12.0. European Committee on Antimicrobial Susceptibility Testing. 2022. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 2 December 2024).
- Lockhart, S.R.; Chowdhary, A.; Gold, J.A. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol. 2023, 21, 818–832. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Friberg, N.; Mares, M.; Kahlmeter, G.; Meletiadis, J.; Guinea, J.; Andersen, C.T.; Arikan-Akdagli, S.; Barchiesi, F.; Chryssanthou, E.; et al. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin. Microbiol. Infect. 2020, 26, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Miranda, I.M.; Costa-de-Oliveira, S. Potential environmental reservoirs of Candida auris: A systematic review. J. Fungi 2024, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Vuichard Gysin, D.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.R.; Filipovic, M.; et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control. 2022, 11, 12. [Google Scholar]
- Yahaya, H.; Sule, H. Candida auris: An emergent virulent and multidrug-resistant yeast associated with serious health implications. Acad. Biol. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- De Melo, C.C.; de Sousa, B.R.; da Costa, G.L.; Oliveira, M.M.; de Lima-Neto, R.G. Colonised patients by Candida auris: Third and largest outbreak in Brazil and impact of biofilm formation. Front. Cell. Infect. Microbiol. 2023, 13, 1033707. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford, K.H.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Ramirez, L.M.; Dias, L.D.; Rivas, L.A.; Ramos, L.S.; Santos, A.L.; Taborda, C.P.; Parra-Giraldo, C.M. Pathogenicity levels of Colombian strains of Candida auris and Brazilian strains of Candida haemulonii species complex in both murine and Galleria mellonella experimental models. J. Fungi 2020, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.; Samaranayake, L.P. Emerging strategies for environmental decontamination of the nosocomial fungal pathogen Candida auris. J. Med. Microbiol. 2022, 71, 001548. [Google Scholar] [CrossRef]
- Douglas, A.P.; Stewart, A.G.; Halliday, C.L.; Chen, S.C. Outbreaks of fungal infections in hospitals: Epidemiology, detection, and management. J. Fungi 2023, 9, 1059. [Google Scholar] [CrossRef]
- Totaro, M.; Casini, B.; Profeti, S.; Tuvo, B.; Privitera, G.; Baggiani, A. Role of hydrogen peroxide vapor (HPV) for the disinfection of hospital surfaces contaminated by multiresistant bacteria. Pathogens 2020, 9, 408. [Google Scholar] [CrossRef]
- Courti, I.; Allix, S. Qualitative comparison of hydrogen peroxide decontamination systems: Vapor vs. aerosol. Laboratories 2024, 1, 124–134. [Google Scholar] [CrossRef]
- Xie, X.; Gao, N.; Zhu, L.; Hunter, M.; Chen, S.; Zang, L. PEDOT: PSS/PEDOT film chemiresistive sensors for hydrogen peroxide vapor detection under ambient conditions. Chemosensors 2023, 11, 124. [Google Scholar] [CrossRef]
- Pereira, A.R.; Braga, D.F.; Vassal, M.; Gomes, I.B.; Simões, M. Ultraviolet C irradiation: A promising approach for the disinfection of public spaces? Sci. Total Environ. 2023, 879, 163007. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Gandhi, H.; Shah, M.; Nagda, M.; Rajpurohit, Y. Ultraviolet light (UV-C): An effective tool to mitigate pandemic spread—A review. Int. J. Eng. Res. Technol. 2021, 10, 26–33. [Google Scholar]
- Zhu, Y.; Kilburn, S.; Kapoor, M.; Chaturvedi, S.; Shaw, K.J.; Chaturvedi, V. In vitro activity of manogepix against multidrug-resistant and panresistant Candida auris from the New York outbreak. Antimicrob. Agents Chemother. 2020, 64, 10–128. [Google Scholar] [CrossRef]
- Thakur, H.; Rao, R. Emphasis of infection prevention and control: A review. J. Popul. Therap. Clin. Pharmacol. 2024, 31, 2238–2249. [Google Scholar]
- Jimenez, Y.A.; Lewis, S.J. Infection prevention and control in the medical imaging environment: A scoping review. Insights Into Imaging 2023, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Godbole, A.A.; Paras; Mehra, M.; Banerjee, S.; Roy, P.; Deb, N.; Jagtap, S. Enhancing Infection Control in ICUS Through AI: A Literature Review. Health Sci. Rep. 2025, 8, e70288. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Pagani, L.; Iannazzo, S.; Moro, M.L.; Viale, P.; Pan, A.; Ansaloni, L.; Coccolini, F.; D’Errico, M.M.; Agreiter, I.; et al. A proposal for a comprehensive approach to infections across the surgical pathway. World J. Emerg. Surg. 2020, 15, 1–26. [Google Scholar] [CrossRef]
- McGinn, C.; Scott, R.; Donnelly, N.; Roberts, K.L.; Bogue, M.; Kiernan, C.; Beckett, M. Exploring the Applicability of Robot-Assisted UV Disinfection in Radiology. Front. Robot. AI 2021, 7, 590306. [Google Scholar] [CrossRef]
- Wood, J.P.; Magnuson, M.; Touati, A.; Gilberry, J.; Sawyer, J.; Chamberlain, T.; McDonald, S.; Hook, D. Evaluation of electrostatic sprayers and foggers for the application of disinfectants in the era of SARS-CoV-2. PLoS ONE 2021, 16, e0257434. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A.; Anderson, D.J.; Chen, L.F.; Sickbert-Bennett, E.E.; Boyce, J.M. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials. Am. J. Infect. Control. 2016, 44, e77–e84. [Google Scholar] [CrossRef]
- Kompatscher, K.; van der Vossen, J.M.; van Heumen, S.P.; Traversari, A.A. Scoping review on the efficacy of filter and germicidal technologies for capture and inactivation of micro-organisms and viruses. J. Hosp. Infect. 2023, 142, 39–48. [Google Scholar] [CrossRef]
- Izadyar, N.; Miller, W. Ventilation strategies and design impacts on indoor airborne transmission: A review. Build. Environ. 2022, 218, 109158. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Roy, S.; Shaikh, N.I.; Malave, P.; Mishra, A.; Alam, M.A.; Ghorpade, Y.; Hasan, M.R.; Nizam, A. Recent advances in multiplex aptasensor detection techniques for food-borne pathogens: A comprehensive review of novel approaches. Biosens. Bioelectron. X 2024, 16, 100417. [Google Scholar] [CrossRef]
- Albers, B.; Verweij, L.; Blum, K.; Oesch, S.; Schultes, M.T.; Clack, L.; Naef, R. Firm, yet flexible: A fidelity debate paper with two case examples. Implement. Sci. 2024, 19, 79. [Google Scholar] [CrossRef] [PubMed]

| Country | Fluconazole (%) | Amphotericin B (%) | Echinocandins (%) |
|---|---|---|---|
| Saudi Arabia | 92 | 35 | 8 |
| USA | 85 | 40 | 12 |
| India | 95 | 30 | 15 |
| UK | 80 | 25 | 10 |
| South Africa | 90 | 33 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlBahrani, S. Environmental Pathogen in Healthcare Settings: Candida auris—The Emerging Threat with a Focus on the Middle East and Infection Control Strategies. Microbiol. Res. 2025, 16, 221. https://doi.org/10.3390/microbiolres16100221
AlBahrani S. Environmental Pathogen in Healthcare Settings: Candida auris—The Emerging Threat with a Focus on the Middle East and Infection Control Strategies. Microbiology Research. 2025; 16(10):221. https://doi.org/10.3390/microbiolres16100221
Chicago/Turabian StyleAlBahrani, Salma. 2025. "Environmental Pathogen in Healthcare Settings: Candida auris—The Emerging Threat with a Focus on the Middle East and Infection Control Strategies" Microbiology Research 16, no. 10: 221. https://doi.org/10.3390/microbiolres16100221
APA StyleAlBahrani, S. (2025). Environmental Pathogen in Healthcare Settings: Candida auris—The Emerging Threat with a Focus on the Middle East and Infection Control Strategies. Microbiology Research, 16(10), 221. https://doi.org/10.3390/microbiolres16100221





