Isolation/Characterization of Colletotrichum gloeosporioides from Tea and MeJA-Induced Antioxidant Defenses
Abstract
1. Introduction
2. Materials and Methods
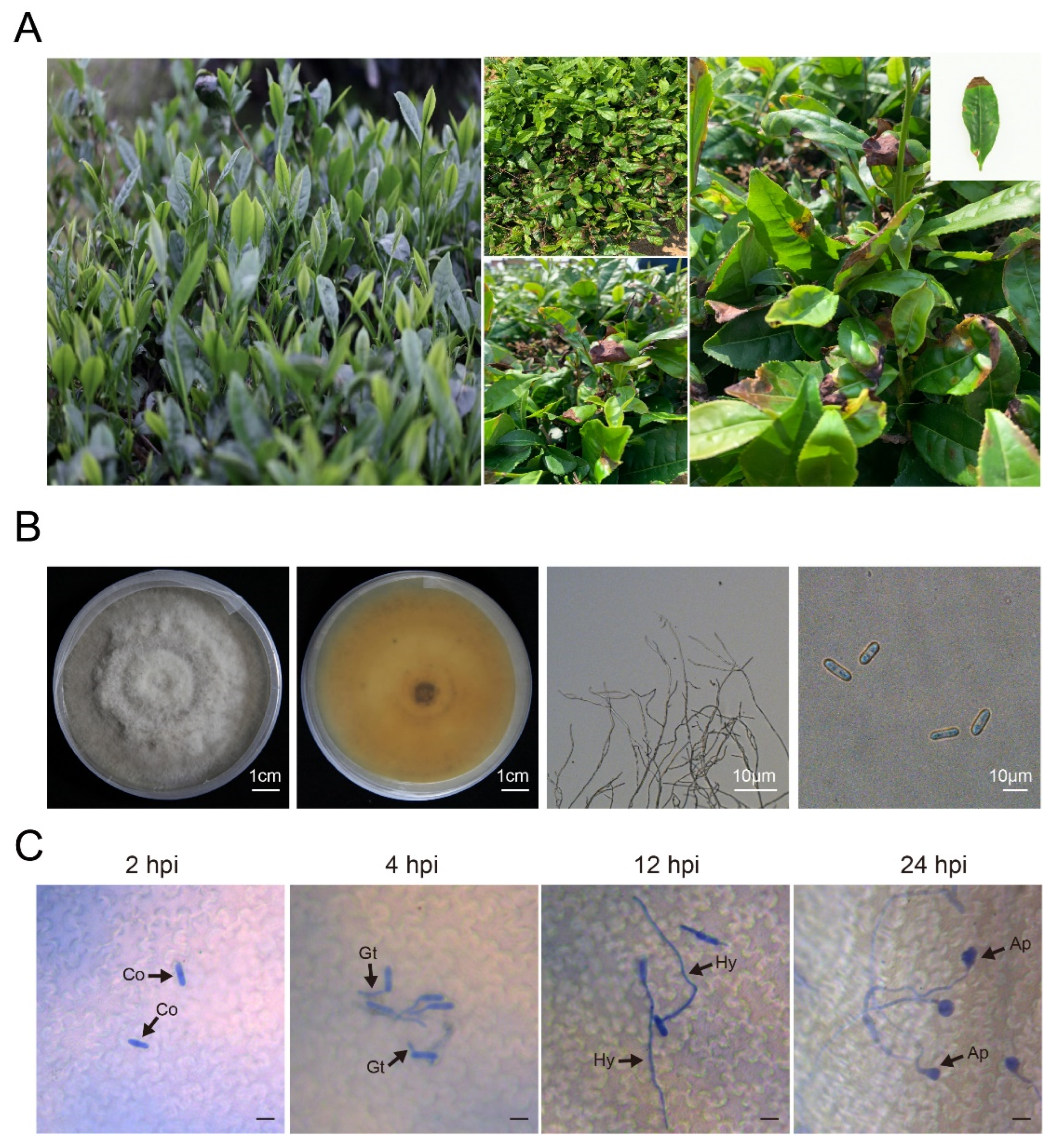
2.1. Isolation and Identification of Pathogen
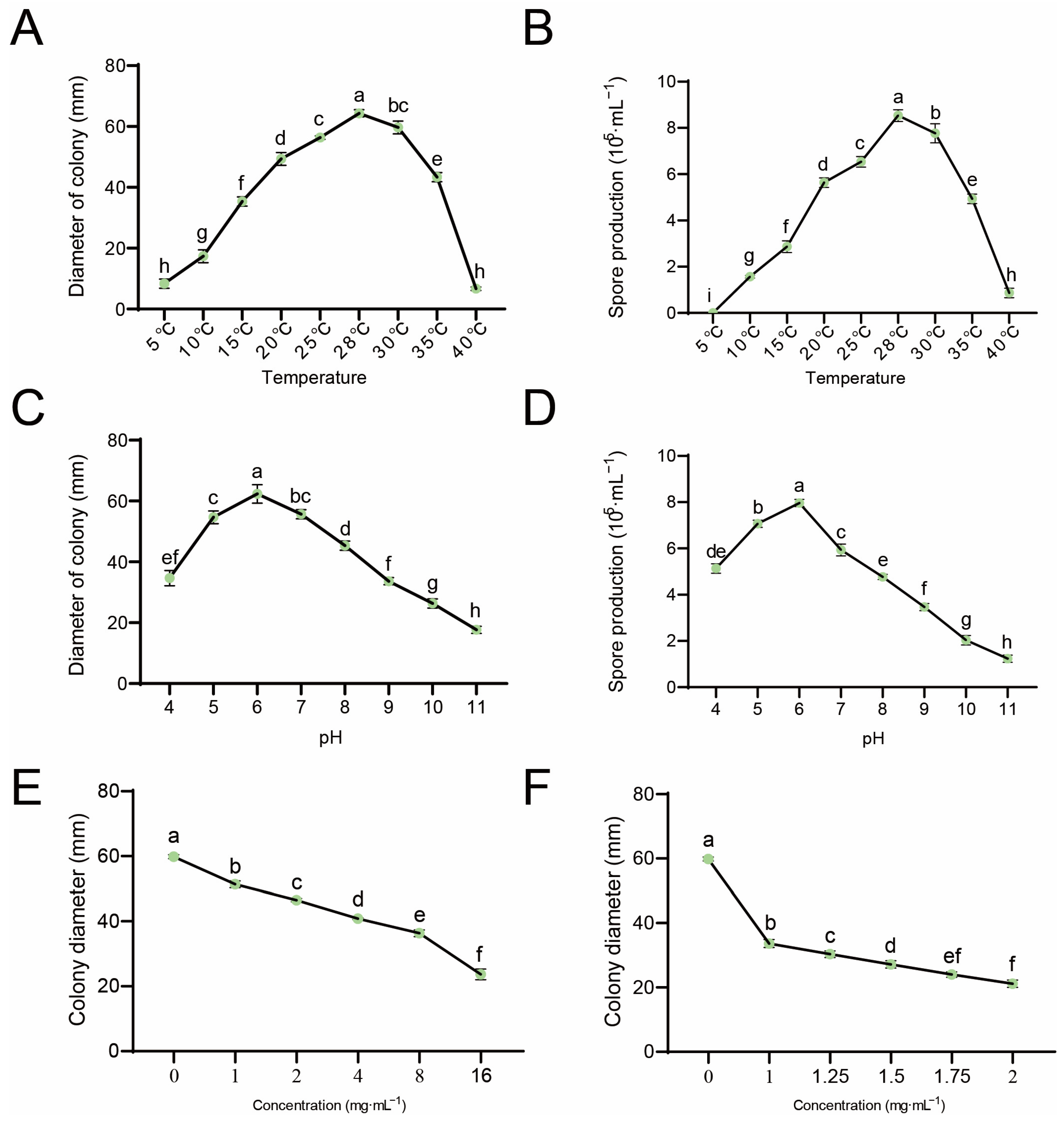
2.2. Characteristics of C. gloeosporioides
2.3. The In Vitro Antifungal Activity Tests
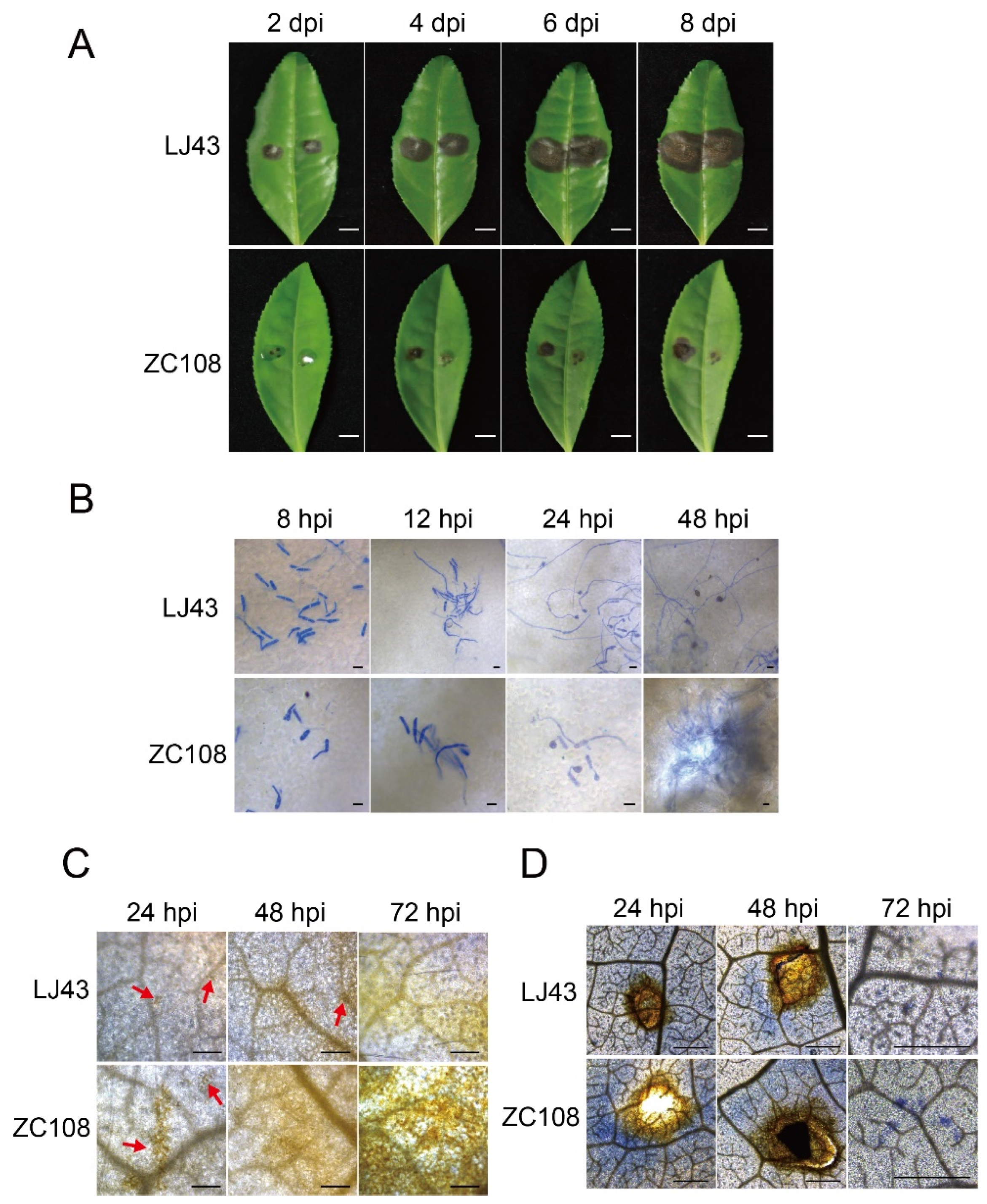
2.4. Infection and Histochemical Staining of Tea Plant Leaves
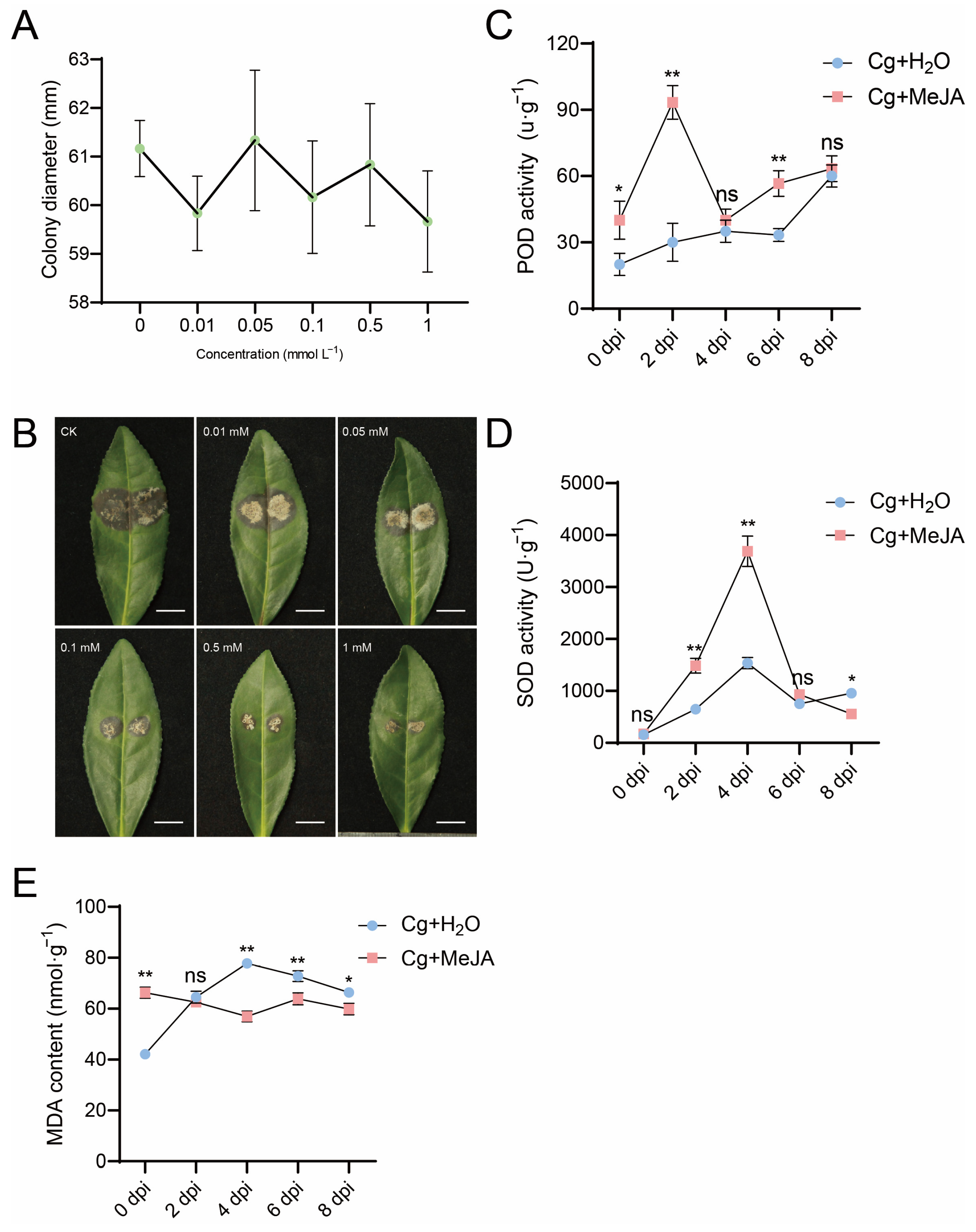
2.5. Exogenous Methyl Jasmonate Treatment
2.6. Enzyme Activity
2.7. Data Statistical Analysis
3. Results
3.1. Characteristics of C. gloeosporioides Strains
3.2. Effects of Environment and Fungicides on C. gloeosporioides Growth
3.3. Response of Different Resistant Tea Leaves to C. gloeosporioides
3.4. Effect of Exogenous Hormone Treatment on C. gloeosporioides
3.5. Changes in Antioxidant Enzyme Activity in Tea Plant Leaves Under Exogenous MeJA Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crous, P.W.; Gams, W.; Stalpers, J.A.; Robert, V.; Stegehuis, G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004, 50, 19–22. [Google Scholar]
- Wei, L.; Ding, Y.; Peng, Y.; Zihao, W.; Guiyi, G. Research Progress on Anthracnose of Tea Plant. Chin. J. Trop. Agric. 2016, 36, 20–26. [Google Scholar]
- Yoshida, K.; Takeda, Y. Evaluation of anthracnose resistance among tea genetic resources by wound-inoculation assay. Jpn. Agric. Res. Q. 2006, 40, 379–386. [Google Scholar] [CrossRef]
- Moriwaki, J.; Sato, T. A new combination for the causal agent of tea anthracnose: Discula theae-sinensis (I. Miyake) Moriwaki & Toy. Sato, comb. nov. J. Gen. Plant Pathol. 2009, 75, 359–361. [Google Scholar] [CrossRef]
- Rabha, A.J.; Naglot, A.; Sharma, G.D.; Gogoi, H.K.; Veer, V. In vitro evaluation of antagonism of endophytic Colletotrichum gloeosporioides against potent fungal pathogens of Camellia sinensis. Indian J. Microbiol. 2014, 54, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H.; Lim, M.-T.; Hur, J.-S.; Yum, K.-J.; Koh, Y.-J. Biological control of tea anthracnose using an antagonistic bacterium of Bacillus subtilis isolated from tea leaves. Plant Pathol. J. 2009, 25, 99–102. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Hao, X.-Y.; Wang, L.; Xiao, B.; Wang, X.-C.; Yang, Y.-J. Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci. Rep. 2016, 6, 35287. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.; Johnston, P.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.; Cai, L.; Cannon, P.; Crouch, J.; Crous, P.; Damm, U.; Goodwin, P.; Chen, H.; Johnston, P.; Jones, E. Colletotrichum—Names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Damm, U.; Cannon, P.F.; Woudenberg, J.; Johnston, P.; Weir, B.; Tan, Y.; Shivas, R.; Crous, P. The Colletotrichum boninense species complex. Stud. Mycol. 2012, 73, 1–36. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.; Woudenberg, J.; Crous, P. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Weir, B.; Damm, U.; Crous, P.W.; Wang, Y.; Liu, B.; Wang, M.; Zhang, M.; Cai, L. Unravelling Colletotrichum species associated with Camellia: Employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia—Mol. Phylogeny Evol. Fungi 2015, 35, 63–86. [Google Scholar] [CrossRef]
- Wan, Y.; Zou, L.; Zeng, L.; Tong, H.; Chen, Y. A new Colletotrichum species associated with brown blight disease on Camellia sinensis. Plant Dis. 2021, 105, 1474–1481. [Google Scholar] [CrossRef]
- Dickens, J.; Cook, R. Glomerella cingulata on Camellia. Plant Pathol. 1989, 38, 75–85. [Google Scholar] [CrossRef]
- Guo, M.; Pan, Y.; Dai, Y.; Gao, Z. First report of brown blight disease caused by Colletotrichum gloeosporioides on Camellia sinensis in Anhui Province, China. Plant Dis. 2014, 98, 284. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, L.; Zhang, Z.; Peng, C.; Ke, D.; Gan, P.; Wang, Z.; Wei, R.; Liu, W.; Yang, J. Isolation and identification of Colletotrichum as fungal pathogen from tea and preliminary fungicide screening. Qual. Assur. Saf. Crops Foods 2022, 14, 92–101. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.-M.; Wen, J.-J.; Bian, X.-L. Study on residue of difenoconazole in tea and its transfer from made tea to infusion. Chin. J. Pestic. Sci. 2010, 12, 299–302. [Google Scholar]
- Li, X.; Ahammed, G.J.; Li, Z.; Tang, M.; Yan, P.; Han, W. Decreased biosynthesis of jasmonic acid via lipoxygenase pathway compromised caffeine-induced resistance to Colletotrichum gloeosporioides under elevated CO2 in tea seedlings. Phytopathology 2016, 106, 1270–1277. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Qian, W.-J.; Li, N.-N.; Hao, X.-Y.; Wang, L.; Xiao, B.; Wang, X.-C.; Yang, Y.-J. Metabolic changes of caffeine in tea plant (Camellia sinensis (L.) O. Kuntze) as defense response to Colletotrichum fructicola. J. Agric. Food Chem. 2016, 64, 6685–6693. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Hyde, K.; Taylor, P.; Weir, B.; Waller, J.; Abang, M.; Zhang, J.; Yang, Y.; Phoulivong, S.; Liu, Z. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Templeton, M.D.; Rikkerink, E.H.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Guerber, J.C.; Liu, B.; Correll, J.C. Characterization of diversity in Colletotrichum acutatum sensu lato by mating compatibility, mtDNA and intron RFLPs, and sequence analysis of two gene introns. Mycologia 2003, 95, 872–895. [Google Scholar] [CrossRef]
- Jeyaraj, A.; Elango, T.; Yu, Y.; Chen, X.; Zou, Z.; Ding, Z.; Zhao, Z.; Chen, X.; Li, X.; Chen, L. Impact of exogenous caffeine on regulatory networks of microRNAs in response to Colletotrichum gloeosporioides in tea plant. Sci. Hortic. 2021, 279, 109914. [Google Scholar] [CrossRef]
- Abe, T.; Kono, M. Studies on the anthracnose of Tea bush. II. Physiological characters and pathogenicities of anthracnose fungi isolated from apparently healthy leaves. Sci. Rep. Fac. Agric. Saikyo Univ. 1956, 8, 81–88. [Google Scholar]
- Lu, Q.; Wang, Y.; Li, N.; Ni, D.; Yang, Y.; Wang, X. Differences in the characteristics and pathogenicity of Colletotrichum camelliae and C. fructicola isolated from the tea plant [Camellia sinensis (L.) O. Kuntze]. Front. Microbiol. 2018, 9, 3060. [Google Scholar] [CrossRef]
- Mao, G.; Tian, Y.; Shi, J.; Liao, C.; Huang, W.; Wu, Y.; Wen, Z.; Yu, L.; Zhu, X.; Li, J. Design, synthesis, antibacterial, and antifungal evaluation of phenylthiazole derivatives containing a 1, 3, 4-thiadiazole thione moiety. Molecules 2024, 29, 285. [Google Scholar] [CrossRef]
- Wang, C.-F.; Huang, L.-L.; Buchenauer, H.; Han, Q.-M.; Zhang, H.-C.; Kang, Z.-S. Histochemical studies on the accumulation of reactive oxygen species (O2− and H2O2) in the incompatible and compatible interaction of wheat—Puccinia striiformis f. sp. tritici. Physiol. Mol. Plant Pathol. 2007, 71, 230–239. [Google Scholar] [CrossRef]
- Han, R.; Yin, W.; Ahmad, B.; Gao, P.; Li, Z.; Wang, X. Pathogenesis and immune response in resistant and susceptible cultivars of grapevine (Vitis spp.) against Elsinoe ampelina infection. Phytopathology 2021, 111, 799–807. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Yang, Z.; Tang, S.; Jin, P.; Wang, K.; Wang, X. Effect of methyl jasmonate on the inhibition of Colletotrichum acutatum infection in loquat fruit and the possible mechanisms. Postharvest Biol. Technol. 2008, 49, 301–307. [Google Scholar] [CrossRef]
- Glowacz, M.; Roets, N.; Sivakumar, D. Control of anthracnose disease via increased activity of defence related enzymes in ‘Hass’ avocado fruit treated with methyl jasmonate and methyl salicylate. Food Chem. 2017, 234, 163–167. [Google Scholar] [CrossRef]
- Yamada, K.; Sonoda, R. A fluorescence microscopic study of the infection process of Discula theae-sinensis in tea. Jpn. Agric. Res. Q. 2014, 48, 399–402. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Huang, Y.; Yue, C.; Wang, B.; Cao, H.; Wang, L.; Wang, X.; Yang, Y.; Xiao, B. Phylogenetic study of Colletotrichum species associated with Camellia sinensis from the major tea areas in China. Sci. Agric. Sin. 2015, 48, 4924–4935. [Google Scholar]
- Li, X.-l.; Luo, L.-b.; Hu, X.-q.; Lou, B.-g.; He, Y. Revealing the chemical changes of tea cell wall induced by anthracnose with confocal Raman microscopy. Spectrosc. Spectr. Anal. 2014, 34, 1571–1576. [Google Scholar]
- Abe, T.; Kono, M. Studies on the anthracnose of Tea bush. IV. On the sporulation, germination, and infection of conidia of the anthracnose fungi. Sci. Rep. Kyoto Univ. Agric. 1959, 11, 38–43. [Google Scholar]
- He, S.; An, T.; Liu, S. Validation of reliable reference genes for RT-qPCR studies of target gene expression in Colletotrichum camelliae during spore germination and mycelial growth and interaction with host plants. Front. Microbiol. 2019, 10, 2055. [Google Scholar] [CrossRef]
- Liu, W. Anthracnose Pathogens Identification and the Genetic Diversity of Tea Plant; Fujian Agriculture and Forestry University: Fuzhou, China, 2013. [Google Scholar]
- MacKenzie, D.; Jeenes, D.; Belshaw, N.; Archer, D. Regulation of secreted protein production by filamentous fungi: Recent developments and perspectives. Microbiology 1993, 139, 2295–2307. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Sun, Q.; Ma, N.; Zhang, F.J.; Zhang, S.; Zhang, Z.Q.; Wang, X.-F.; Sun, P.; You, C.-X.; Zhang, Z. Inhibitory effect of tea saponin on major apple-disease-inducing fungi. Phytopathology 2023, 113, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Kakiki, K.; Misato, T. Mechanism of action of the antifugal agent polyoxin D. J. Bacteriol. 1970, 104, 189–196. [Google Scholar] [CrossRef]
- Meng, X.; Li, J.; Bi, F.; Zhu, L.; Ma, Z. Antifungal activities of crude extractum from Camellia semiserrata Chi (Nanshancha) seed cake against Colletotrichum musae, Colletotrichum gloeosporioides and Penicillium italicum in vitro and in vivo fruit test. Plant Pathol. J. 2015, 31, 414. [Google Scholar] [CrossRef]
- Suda, K.; Kochi, S.I. Biological efficacy of polyoxin D in crop protection. J. Pestic. Sci. 2025, 50, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, E. Susceptibility of Japanese and Assam tea cultivars to the tea anthracnose fungus. Ann. Phytopathol. Soc. Jpn. 1981, 47, 406. [Google Scholar]
- Takeda, Y. Studies on variations in genetic resources of tea in Japan and application to tea [Camellia sinensis] breeding. Bull. Natl. Inst. Veg. Tea Sci. 2002, 1, 97–180. [Google Scholar]
- Yoshida, K.; Takeda, Y. Assay method for screening of tea plants with resistance to anthracnose caused by Colletotrichum theae-sinensis by use of novel wound-inoculation. Bull. Natl. Inst. Veg. Tea Sci. 2004, 137–146. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Q.; Xiong, F.; Hao, X.; Wang, L.; Zheng, M.; Li, N.; Ding, C.; Wang, X.; Yang, Y. Genome-wide identification, characterization, and expression analysis of nucleotide-binding leucine-rich repeats gene family under environmental stresses in tea (Camellia sinensis). Genomics 2020, 112, 1351–1362. [Google Scholar] [CrossRef]
- Shi, Y.L.; Sheng, Y.Y.; Cai, Z.Y.; Yang, R.; Zheng, X.Q. Involvement of Salicylic Acid in Anthracnose Infection in Tea Plants Revealed by Transcriptome Profiling. Int. J. Mol. Sci. 2019, 20, 2439. [Google Scholar] [CrossRef]
- Chen, J.S.; Thseng, F.M.; Ko, W.H. Improvement of Survival and Subsequent Growth of Tea Cuttings. HortScience 1990, 25, 305–306. [Google Scholar] [CrossRef]
- Takeda, Y. Cross compatibility of tea (Camellia sinensis) and its allied species in the genus Camellia. Jpn. Agric. Res. Q. 1990, 24, 111–116. [Google Scholar]
- Nesumi, A.; Ogino, A.; Yoshida, K.; Taniguchi, F.; Murakami, A. ‘Sunrouge’, a New Tea Cultivar with High Anthocyanin. Jpn. Agric. Res. Q. 2012, 46, 321–328. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, S.; Yang, Y.; Zeng, J. Investigation on the breeding of new tea cultivar, Zhongcha 108, with early-sprouting, superior-quality and suitable for manufacturing high-qulity green tea. China Tea 2003, 25, 12–14. [Google Scholar]
- Wang, L.; Wang, Y.; Cao, H.; Hao, X.; Zeng, J.; Yang, Y.; Wang, X. Transcriptome analysis of an anthracnose-resistant tea plant cultivar reveals genes associated with resistance to Colletotrichum camelliae. PLoS ONE 2016, 11, e0148535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Fang, J.; Yin, W.; Yan, X.; Tu, M.; Liu, H.; Zhang, Z.; Li, Z.; Gao, M. VqWRKY56 interacts with VqbZIPC22 in grapevine to promote proanthocyanidin biosynthesis and increase resistance to powdery mildew. New Phytol. 2023, 237, 1856–1875. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Thirugnanasambantham, K.; Mandal, A.K.A. Suppressive subtractive hybridization approach revealed differential expression of hypersensitive response and reactive oxygen species production genes in tea (Camellia sinensis (L.) O. Kuntze) leaves during Pestalotiopsis thea infection. Appl. Biochem. Biotechnol. 2012, 168, 1917–1927. [Google Scholar] [CrossRef]
- Rashid, A. Defense responses of plant cell wall non-catalytic proteins against pathogens. Physiol. Mol. Plant Pathol. 2016, 94, 38–46. [Google Scholar] [CrossRef]
- Shetty, N.P.; Jrgensen, H.J.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Li, A.; Wang, M.; Zhou, R.; Kong, X.; Huo, N.; Wang, W.S.; Jia, J. Comparative analysis of early H2O2 accumulation in compatible and incompatible wheat–powdery mildew interactions. Plant Pathol. 2005, 54, 308–316. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Lu, Q.; Wang, L.; Qian, W.; Li, N.; Ding, C.; Wang, X.; Yang, Y. Transcriptional analysis and histochemistry reveal that hypersensitive cell death and H2O2 have crucial roles in the resistance of tea plant (Camellia sinensis (L.) O. Kuntze) to anthracnose. Hortic. Res. 2018, 5, 18. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Zhang, S. The relationship between active oxygen metabolism and resistance to late blight in potato. Potato Res. 2018, 61, 365–373. [Google Scholar] [CrossRef]
- Kępczyńska, E.; Kępczyński, J. Inhibitory effect of methyl jasmonate on development of phytopathogen Alternaria alternata (Fr.) Keissl. and its reversal by ethephon and ACC. Acta Physiol. Plant. 2005, 27, 491–496. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X.; Liu, Q.; Wang, F.; Feng, W.; Wan, N. Combination effect of chitosan and methyl jasmonate on controlling Alternaria alternata and enhancing activity of cherry tomato fruit defense mechanisms. Crop Prot. 2014, 56, 31–36. [Google Scholar] [CrossRef]
- Quiróz-López, E.P.; Rentería-Martínez, M.E.; Ramírez-Bustos, I.I.; Moreno-Salazar, S.F.; Martínez-Ruíz, F.E.; Villar-Luna, E.; Fernández-Herrera, E. Efecto del ácido salicílico y metil jasmonato sobre Colletotrichum sp. en frutos de mango. Trop. Subtrop. Agroecosyst. 2021, 24, 44. [Google Scholar] [CrossRef]
- Goodrich-Tanrikulu, M.; Mahoney, N.E.; Rodriguez, S.B. The plant growth regulator methyl jasmonate inhibits aflatoxin production by Aspergillus flavus. Microbiology 1995, 141, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ma, B. Benzothiadiazole-or methyl jasmonate-induced resistance to Colletotrichum musae in harvested banana fruit is related to elevated defense enzyme activities. J. Hortic. Sci. Biotechnol. 2007, 82, 500–506. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, X.; Chen, M.; Fu, Y.; Xiang, M.; Chen, J. Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chem. 2020, 305, 125483. [Google Scholar] [CrossRef]
- Darras, A.I.; Terry, L.A.; Joyce, D.C. Methyl jasmonate vapour treatment suppresses specking caused by Botrytis cinerea on cut Freesia hybrida L. flowers. Postharvest Biol. Technol. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, N.; Shamsi, I.H.; Jabeen, Z.; Siddiqui, M.H.; Jiang, L.-x. Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. J. Zhejiang Univ. Sci. B 2018, 19, 130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Wang, Y.; Zhou, X.; Ma, S.; Shan, Z.; Wan, S.; Xue, Z.; Mei, H.; Tang, Y.; Liu, S.; et al. Isolation/Characterization of Colletotrichum gloeosporioides from Tea and MeJA-Induced Antioxidant Defenses. Microbiol. Res. 2025, 16, 220. https://doi.org/10.3390/microbiolres16100220
Peng C, Wang Y, Zhou X, Ma S, Shan Z, Wan S, Xue Z, Mei H, Tang Y, Liu S, et al. Isolation/Characterization of Colletotrichum gloeosporioides from Tea and MeJA-Induced Antioxidant Defenses. Microbiology Research. 2025; 16(10):220. https://doi.org/10.3390/microbiolres16100220
Chicago/Turabian StylePeng, Chunju, Yu Wang, Xuan Zhou, Shifu Ma, Zhiguo Shan, Shuai Wan, Zekun Xue, Huiling Mei, Yan Tang, Shujing Liu, and et al. 2025. "Isolation/Characterization of Colletotrichum gloeosporioides from Tea and MeJA-Induced Antioxidant Defenses" Microbiology Research 16, no. 10: 220. https://doi.org/10.3390/microbiolres16100220
APA StylePeng, C., Wang, Y., Zhou, X., Ma, S., Shan, Z., Wan, S., Xue, Z., Mei, H., Tang, Y., Liu, S., Han, R., Li, X., & Zeng, G. (2025). Isolation/Characterization of Colletotrichum gloeosporioides from Tea and MeJA-Induced Antioxidant Defenses. Microbiology Research, 16(10), 220. https://doi.org/10.3390/microbiolres16100220





