Abstract
Gray mold, caused by Botrytis cinerea Pers. Fr. (teleomorph: Botryotinia fuckeliana), is a major disease affecting Moroccan vineyards. However, limited information is available on the natural populations of this pathogen. In this study, 82 single-spore isolates collected from vineyards in two major wine-growing regions were evaluated for phenotypic, physiological, and molecular variability. The isolates exhibited differences in morphotypes, conidial size, and sclerotia production on PDA medium. Temperature significantly affected mycelial growth rate (mm d−1). All isolates were virulent on grapevine leaves, showing varying levels of aggressiveness. Among the representative isolates, 20 were heterothallic and 2 were homothallic. Mating-type analysis revealed that 12% belonged to MAT1-1 and 75% to MAT1-2. Transposable element genotyping showed that the population was composed of 41.7% transposa, 29.2% vacuma, 16.7% Flipper-only, and 12.5% Boty-only. This work represents the first report on genotypic variation in B. cinerea populations from Moroccan vineyards. The findings provide new insights into the morphenotypic and genetic diversity of the pathogen and may support the development of improved strategies for disease management.
1. Introduction
Botrytis cinerea Pers. (teleomorph Botryotinia fuckeliana (de Bary) Whetzel) is a haploid, heterothallic filamentous ascomycete that causes gray mold disease and exhibits a necrotrophic lifestyle [1], being capable of infecting numerous host plants, including fruits, vegetables, ornamental, and wild plants [2]. Gray mold infestations lead to significant losses in both yield and quality, positioning it as the second most impactful phytopathogenic fungus [3]. B. cinerea is a polycyclic pathogen; it can extract nutrients saprophytically or from a living cells after penetration through natural openings or injuries [4]. B. cinerea can be isolated from diverse plant organs, and infection can be reproduced in the laboratory on various hosts [5].
Botrytis species show high diversity in colony morphology, mycelial growth rate, sclerotia form, and spore shape and size. Paul [6] defined three morphological types, sclerotial (Sc), sporulating (Sp), and mycelial (M), through the observation of five B. cinerea strains. Martinez [7] then defined eight distinct morphological types, encompassing four mycelial and four sclerotial forms. The strains were classified into these categories. Morphological traits exhibit significant resemblance across various species within the Botrytis genus, and internal factors and external conditions can readily influence them. Consequently, identifying B. cinerea from certain other species solely based on morphological features is exceedingly challenging. For example, B. cinerea closely resembles B. fabae and B. calthae regarding their cultural characteristics.
However, these phenotypic evaluations can now be strengthened by integrating molecular characteristics. Developing specialized primers using RAPDs targeting B. cinerea has facilitated straightforward identification at the species level [8]. Botrytis cinerea is no longer considered a monophyletic but rather a set of cryptic species coexisting in sympatry [9]. This was made possible by developing PCR-RFLP for locus Bc-hch, which divided B. cinerea into two groups, group I and group II [10]. In recent years, B. cinerea has been identified as a species complex consisting of at least two separate phylogenetic species: B. cinerea sensu stricto and B. pseudocinerea [11]. While B. pseudocinerea naturally shows resistance to the hydroxyanilide (HA) fungicide, its occurrence is negatively correlated with fungicide applications [12]. Moreover, Leroch et al. [13] verified the existence of a new clade termed Botrytis group S, which shares close affinity with B. cinerea.
The morphological and physiological distinctions imply genetic diversity among individuals within these communities. Various studies have focused on detecting and assessing genetic diversity in natural populations of B. cinerea. Developments in molecular techniques and markers have been used to study the genetic structure of this fungus, such as RAPDs, RFLPs, AFLPs, SSRs, and transposons, and together with access to vast sequence datasets, have facilitated the assessment of this variation [14,15,16,17,18].
The presence or absence of two transposable elements (TEs), Boty and Flipper, categorizes B. cinerea into four transposon types: transposa (both TEs present), vacuma (both TEs absent), Boty only, and Flipper only [15]. While initially thought to have limited classification value, these TEs are linked to diverse adaptations in host range and seasonal frequencies [12,19]. Initially discovered by Diolez et al. [20], Boty, a retrotransposon related to the gypsy-like long terminal repeat (LTR)-containing transposon, was identified in B. cinerea. Its presence or absence led to the proposal by these authors that Boty-containing and Boty-deficient groups represent distinct lineages in the B. cinerea population. Subsequently, Levis et al. [21] found Flipper, another TE, a mobile Fot1-like transposon carrying an insertion sequence within the nitrate reductase gene’s coding region. Notably, even when both transposa and vacuma coexist on the same plant, their relative frequencies differ significantly [22].
There are two mating-type genes found in ascomycetes: MAT1-1 and MAT1-2. Mating type (MAT) is a key regulatory factor for sexual compatibility in ascomycetes [23]. Research indicates that the sexual recombination of B. cinerea involving MAT1-1 and MAT1-2 is widespread in nature [24]. Further research has shown that the two forms of MAT1 have identical chromosomal localizations with nonhomologous sequences [25]. In natural populations, rare ascospore progeny of field isolates were homothallic (both MAT1-1 and MAT1-2), with the rest of the isolates being heterothallic (either MAT1-1 or MAT1-2).
Viticulture is an important commercial area in global agriculture. With a cultivated area of 6.59 million hectares and a total production of 72 million tons in 2023 [26], Morocco’s cultivated area amounts to 43,367 hectares. Production topped 319 thousand tons in 2023, placing the country fourth in Africa [27]. B. cinerea is present in all the areas where grapevines are grown in Morocco, although the incidence of fungal diseases is lower compared with other viticulture regions of the world. However, when rain occurs soon before the harvest, and temperatures are moderate, significant losses can occur. Generally, producers apply an average of three to four preventive treatments against B. cinerea in the extensive vineyards [28]. There has been no previous study describing the natural populations of B. cinerea in the vineyards in Morocco.
Being aware of the problems derived from infections caused by B. cinerea in Moroccan vineyards, this work aimed to (i) obtain information about the morphological and physiological diversity of B. cinerea populations in vineyards, and (ii) assess the molecular diversity of B. cinerea isolates collected from vineyards in two regions in Morocco.
2. Materials and Methods
2.1. Botrytis Cinerea Sampling and Morphological Identification
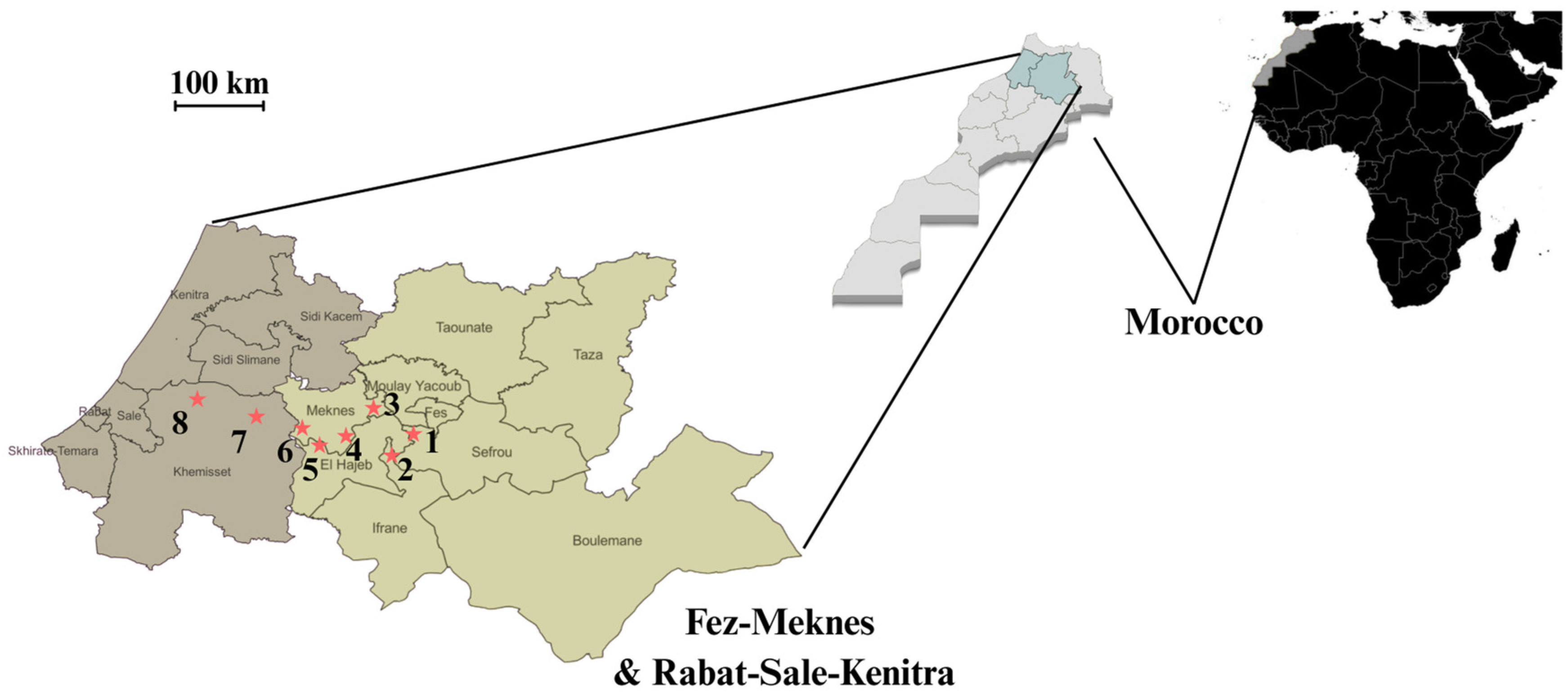
Isolates of B. cinerea were collected from grape (Vitis vinifera) bunches exhibiting typical gray mold symptoms. Sampling was conducted between June and December during 2022 growing seasons, across eight commercial vineyards located in the Fez–Meknes and Rabat–Sale–Kenitra regions of Morocco (Figure 1). Symptomatic grape bunches were collected in the field, individually placed in plastic bags, and transported in coolers to the laboratory to maintain sample integrity. Upon arrival, infected berries were selected from each bunch and used for fungal isolation. Representative photos of symptomatic grape bunches and infected berries are provided in Supplementary Figure S1.
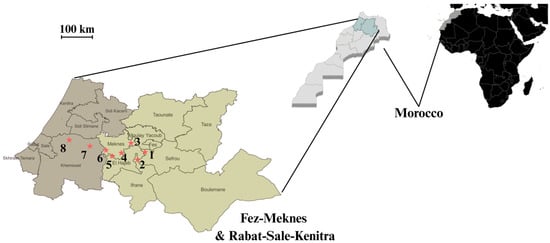
Figure 1.
Sampling sites of representative vineyards in the Fez–Meknes (light gray) and Rabat–Sale–Kenitra (dark gray) wine-producing regions of Morocco. Numbers correspond to 1—Douar Laqsir; 2—Boufekrane; 3—Souk Elgour; 4—Haj Kaddour; 5—Souk Sabt Jahjouh; 6—Ait Ouallal; 7—Khemisset; 8—Tiffelt. * Location of sampled vineyards. The exact geographical coordinates of each vineyard are provided in Table S1.
Symptomatic host tissues were plated on potato dextrose agar (PDA) medium supplemented with chloramphenicol (0.2 mg/L) to inhibit bacterial growth and incubated at 20 °C for five days. Preliminary identification of B. cinerea isolates was based on characteristic colony morphology. To obtain genetically homogeneous cultures, single-spore isolation was performed. Conidia were gently scraped from sporulating cultures and suspended in 1 mL of sterile distilled water (SDW). A 50 µL aliquot of the conidial suspension was spread onto 55 mm water agar plates and incubated at room temperature for 18 h. Under a stereomicroscope, individual germinated conidia were transferred onto fresh PDA plates and incubated for 36 h at room temperature to allow mycelial development. Monospore isolates (Table S1) were maintained on PDA at 4 °C for short-term use and preserved long-term in 20% glycerol at −80 °C.
2.2. DNA Extraction
Genomic DNA was extracted from the mycelium of B. cinerea isolates using the cetyltrimethylammonium bromide (CTAB) method described by Conlon et al. [29], with minor modifications. Fungal material was mechanically homogenized in CTAB extraction buffer using a tissue lyzer and metal beads. The homogenate was incubated overnight at 65 °C in the presence of Proteinase K, RNase A, and β-mercaptoethanol to ensure complete lysis and degradation of proteins and RNA. DNA was subsequently purified through sequential extraction with phenol–chloroform–isoamyl alcohol (25:24:1, v/v) followed by chloroform–isoamyl alcohol (24:1, v/v). Precipitation was carried out using NaCl and ice-cold isopropanol, followed by washing with 70% ethanol. The resulting DNA pellets were air-dried and resuspended in Tris-EDTA (TE) buffer. The quality and integrity of the extracted genomic DNA were assessed by electrophoresis on 1% (w/v) agarose gels, and DNA concentrations were determined using a BioDROP µlite+ spectrophotometer (BioDrop, Cambridge, UK). DNA extraction was performed in triplicate for each isolate and used for subsequent PCR analyses.
2.3. Molecular Confirmation
Species confirmation of B. cinerea was performed using the species-specific primer pair C729+/−, as described by Rigotti et al. [8] (Table S3). PCR was conducted according to the conditions detailed in Table S4, using a Prime thermocycler (Techne, Bibby Scientific, Staffordshire, UK). Each PCR assay was repeated twice to ensure reproducibility. Amplified products were analyzed by electrophoresis on 1% agarose gels prepared in 0.5× Tris-borate-EDTA (TBE) buffer and stained with ethidium bromide. The successful amplification of a fragment approximately 700 bp in size confirmed the identity of the isolate as B. cinerea.
2.4. Morphological Diversity and Culture Conditions
B. cinerea strains were grown on PDA at 20 °C in darkness (three replicates per strain). After 3 weeks, morphological traits including mycelial color, sclerotia production and pattern, and sporulation were assessed following Martinez et al. [7]. Growth rate was measured on PDA at 15, 21, and 25 °C using 6 mm mycelial plugs placed on 9 cm Petri dishes. Colonies were incubated in darkness, and two perpendicular diameters were measured daily. Growth rate was expressed as the increase in colony area per day.
2.5. Virulence Characterization of B. cinerea
Virulence of B. cinerea isolates representing different morphotypes was assessed on detached leaves of Vitis vinifera cv. ‘Cabernet Sauvignon’ and ‘Chardonnay’. Leaves were surface-sterilized (0.5% sodium hypochlorite, 2 min), rinsed, wounded with a sterile needle, and inoculated with 5 mm PDA plugs of actively growing mycelium. Four inoculations were performed per leaf, with experiments in duplicate. Inoculated leaves were incubated in humid chambers (100% RH, 25 °C, darkness) for 96 h. Lesion diameters were measured with a caliper (precision 0.2 mm).
2.6. Transposon Detection
The presence of the retrotransposon Boty and the Fot1-like element Flipper was assessed by PCR using primers F300/F1550 for Flipper [21] and Boty-F/R for Boty [30] (Table S3). PCR products were resolved on 1% agarose gels in 0.5× TBE buffer and visualized with ethidium bromide staining. Isolates carrying both elements were classified as transposa, those with only one element as Boty-only or Flipper-only, and those lacking both as vacuma.
2.7. Mating-Type Determination
Mating types MAT1-1 and MAT1-2 were identified by PCR using primers described by van Kan, J. et al. [31] (Table S3). Amplification conditions are provided in Table S4. Isolates carrying only one mating-type gene were classified as heterothallic, while those with both MAT1-1 and MAT1-2 were considered homothallic. A chi-squared test was used to assess deviations from the expected 1:1 mating-type ratio.
2.8. Nuclear Gene Sequencing and Phylogenetic Analysis
Genomic DNA was extracted from fungal isolates grown in YPG medium (7 g/L yeast extract, 7 g/L peptone, 10 g/L glucose) at 30 °C for 3 days, following the Moroccan standard NM ISO 20837-2008 [32]. DNA quality and concentration were assessed by Nanodrop spectrophotometry. The G3PDH gene was amplified using primers G3PDHfor/rev under standard PCR conditions. Amplification products were verified on agarose gel, purified with ExoSAP-IT™, and sequenced using the BigDye™ Terminator v3.1 kit on an Applied Biosystems SeqStudio analyzer (All from Applied Biosystems, Foster City, CA, USA). Sequences were aligned using MAFFT v7.526 with the auto setting. Phylogenetic analysis was performed in R using the phangorn and ggtree packages. An initial tree was generated via neighbor joining based on maximum likelihood distances. The best substitution model was selected using BIC. A maximum likelihood tree was then inferred with optimized base frequencies, substitution matrix, and rate heterogeneity (+I, +G, k = 4). Bootstrap analysis was conducted with 1000 replicates. The tree was rooted using the MRCA of two outgroups (M. fructigena and S. sclerotiorum) and visualized with the ggtree package.
2.9. Statistical Analysis
Statistical analyses were performed using analysis of variance (ANOVA) after verifying the normality of residuals and homogeneity of variance. Post hoc comparisons were conducted using Tukey’s Honest Significant Difference (HSD) test. All analyses were carried out in RStudio (R version 2025.05.0). Statistical significance was considered at p < 0.05.
3. Results
3.1. Morphological and Molecular Identification of B. cinerea
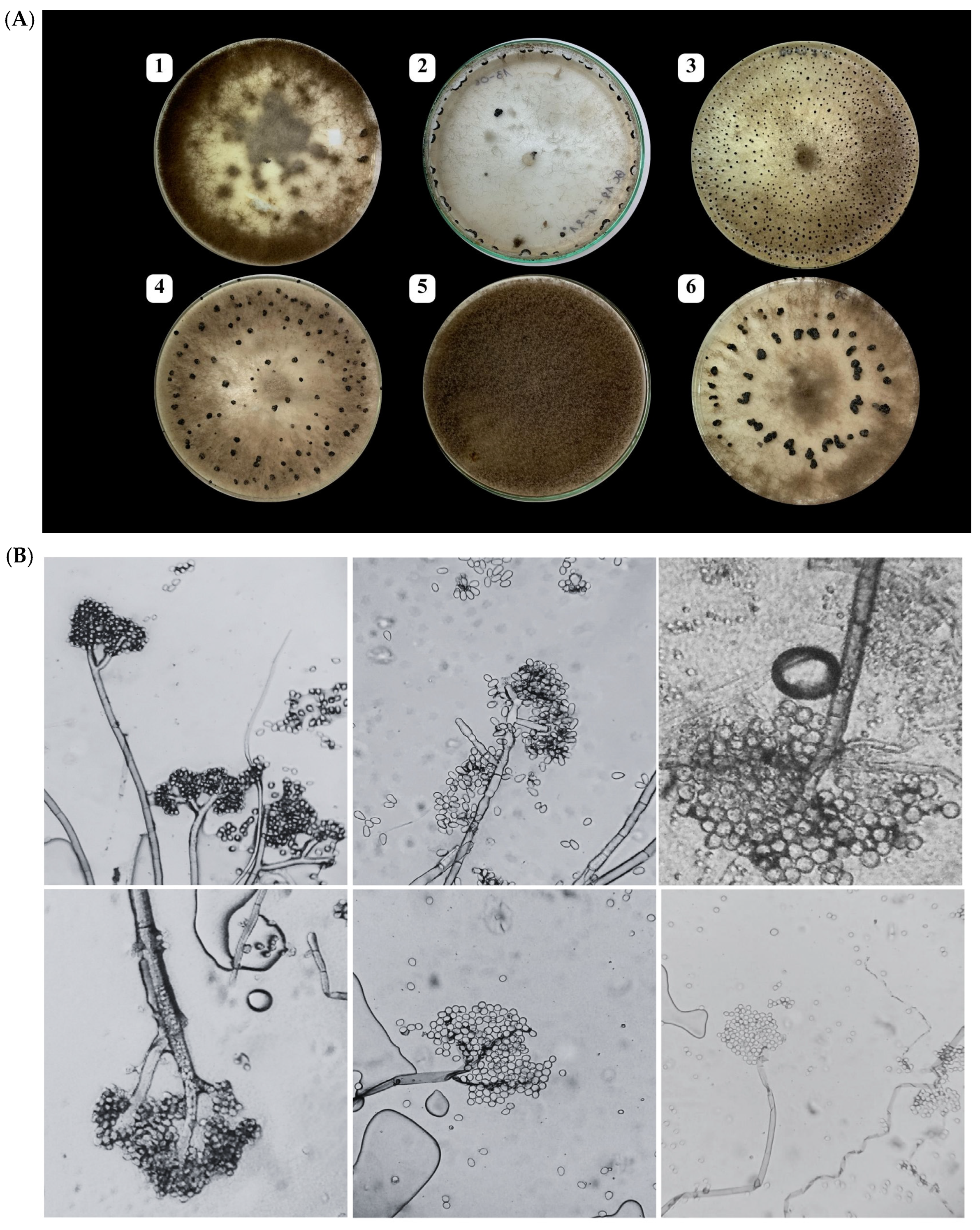
A total of 82 fungal isolates were obtained from symptomatic grapevine berries collected across eight vineyards in Morocco during the 2021–2022 growing seasons (Table S1). Following incubation on PDA medium at 21 °C, colonies initially exhibited a white to ashy-white appearance, gradually turning grey to greyish-brown over time. Microscopic examination of the isolates (Figure 2B) revealed characteristic asexual structures of Botrytis cinerea. The images show hyaline, septate hyphae giving rise to branched, brown conidiophores. The conidiophores are mostly straight and repeatedly branched at the apex, with each terminal branch bearing clusters of conidia. The conidia are unicellular, smooth-walled, and arranged in grape-like clusters at the tips of the conidiophores. They exhibit a range of shapes from ellipsoidal to globose, with some appearing ovate, and vary in color from hyaline to pale brown.
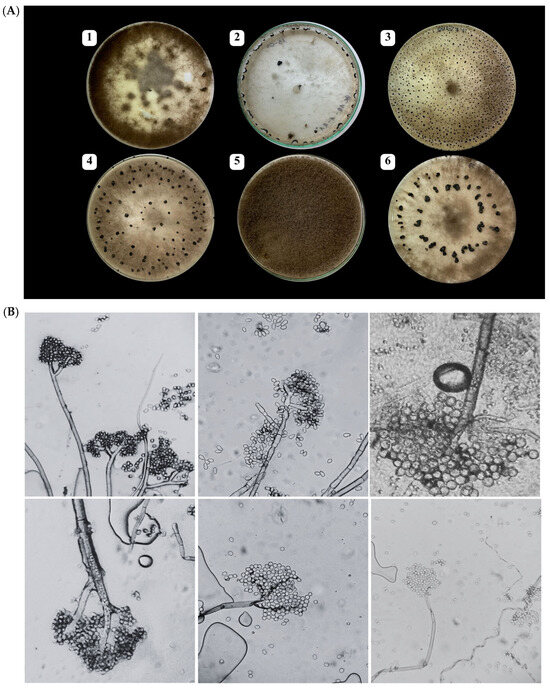
Figure 2.
(A) Macroscopic features of Botrytis cinerea isolates on PDA medium after 14 days at 21 °C, showing (1) mycelial masses, (2) sclerotia forming at the edge of the Petri dish, (3–4) numerous small and scattered sclerotia, (5) thick and woolly colonies, and (6) large sclerotia often arranged in concentric circles; and (B) microscopic (×400) characteristics.
Based on these macroscopic and microscopic morphological features, all isolates were preliminarily identified as B. cinerea.
To confirm species identity, 24 representative isolates were subjected to PCR using the B. cinerea-specific primer pair C729+/−, which targets a unique genomic region of the species. All tested isolates yielded a single amplicon of approximately 700 bp, consistent with the expected product size (Figure S3). These results confirmed that all 24 isolates belonged to B. cinerea.
3.2. Morphological and Cultural Diversity
The morphology of B. cinerea isolates collected from Moroccan vineyards was assessed on potato dextrose agar (PDA) medium incubated at 21 °C in the dark. Macroscopic traits, including mycelial appearance, sporulation, and sclerotia production, were recorded after three weeks of incubation.
Following the classification criteria proposed by Martinez et al. [7], the colonies of the tested B. cinerea isolates were visually grouped into eight distinct morphotypes (Table 1). These morphotypes were assigned to two main morphological categories: a mycelial type, characterized by the absence of sclerotia, and a sclerotial type, characterized by variable but generally abundant production of sclerotia.

Table 1.
Morphological classification of 82 selected Botrytis cinerea isolates.
Among the 82 isolates, the sclerotial type was predominant (n = 60), with morphotype S3 being the most frequent (n = 34). This morphotype was distinguished by dense mycelial growth and abundant, large sclerotia covering the surface of the Petri dish. The remaining isolates (n = 22) were classified as mycelial type, with morphotype M3 being the most common, characterized by clustered mycelial tufts and moderate to abundant sporulation.
The influence of fungal isolate on spore size was evaluated using an aligned rank transformation ANOVA (ART-ANOVA). The analysis revealed a highly significant effect of isolate on spore area (F(45, 17,361) = 171.69, p < 0.0001), indicating that spore size varies substantially between the 46 tested isolates. Post hoc pairwise comparisons (Bonferroni-adjusted) grouped isolates into multiple statistically distinct clusters based on their average spore area. The smallest average spore size was observed in isolate BC14 (10.7 µm2, group a), while the largest was recorded in isolate BC79 (65.4 µm2, group r). These findings highlight a strong genetic effect of isolate identity on spore morphology in this population.
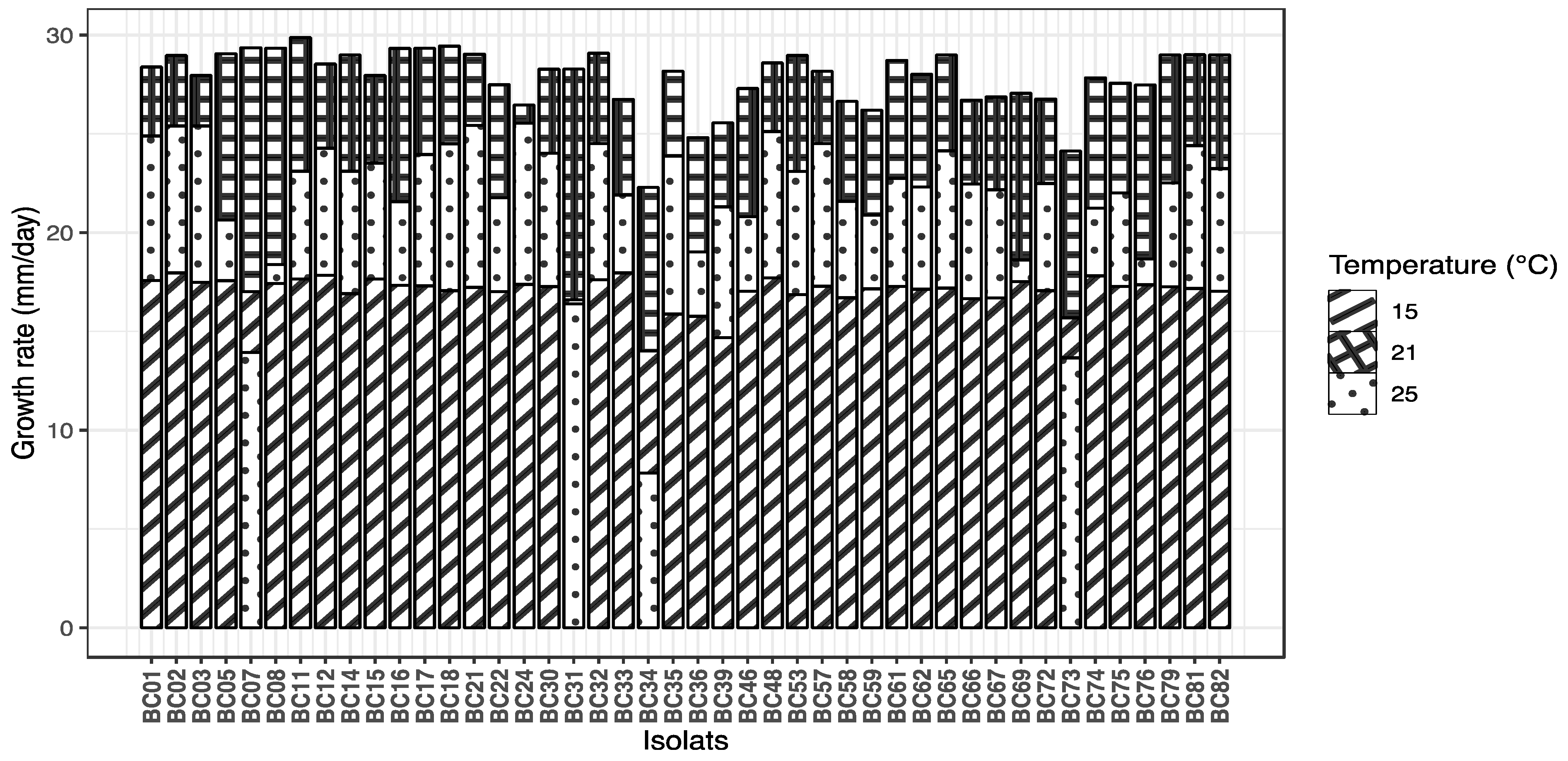
The growth rate of B. cinerea isolates was significantly affected by temperature and isolate identity, as well as their interaction. A linear mixed-effects model revealed a significant effect of temperature on growth rate (p < 0.001). Growth rate was highest at 21 °C (mean estimate = 27.21 mm/day), and significantly lower at both 15 °C and 25 °C, with decreases of 10.45 mm/day and 5.29 mm/day, respectively (Tukey-adjusted pairwise comparisons, all p < 0.0001). The random effect of isolate accounted for substantial variability among isolates (variance = 4.20), indicating differences in growth potential across isolates. Figure 3 presents the growth rates of each isolate at different temperatures.
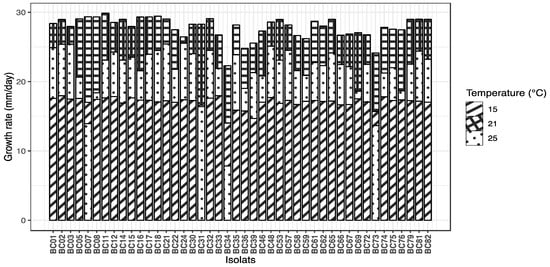
Figure 3.
Mycelial growth rate (mm/day) of 44 Botrytis cinerea isolates cultured on PDA medium at three temperatures (15, 21, and 25 °C).
The ranking of Botrytis cinerea isolates based on their mean growth rates revealed distinct growth performances across temperatures. At the optimal temperature of 21 °C, isolate BC11 exhibited the highest growth rate (29.87 mm/day), closely followed by BC18 and BC07, with the slowest isolates showing growth rates around 14 mm/day. At 25 °C, the fastest growth was observed for isolate BC24 (25.54 mm/day), while several isolates displayed notably reduced growth, with the slowest isolate BC34 growing at only 7.84 mm/day. At the lowest temperature of 15 °C, isolate BC33 led the ranking (17.96 mm/day), with most isolates showing substantially slower growth rates compared to higher temperatures. This ranking highlights both the temperature-dependent growth variability and significant isolate-specific differences in growth capacity. A table presenting the adjusted means and statistical groupings for all isolates is provided in Table S2.
3.3. Isolates’ Aggressivity
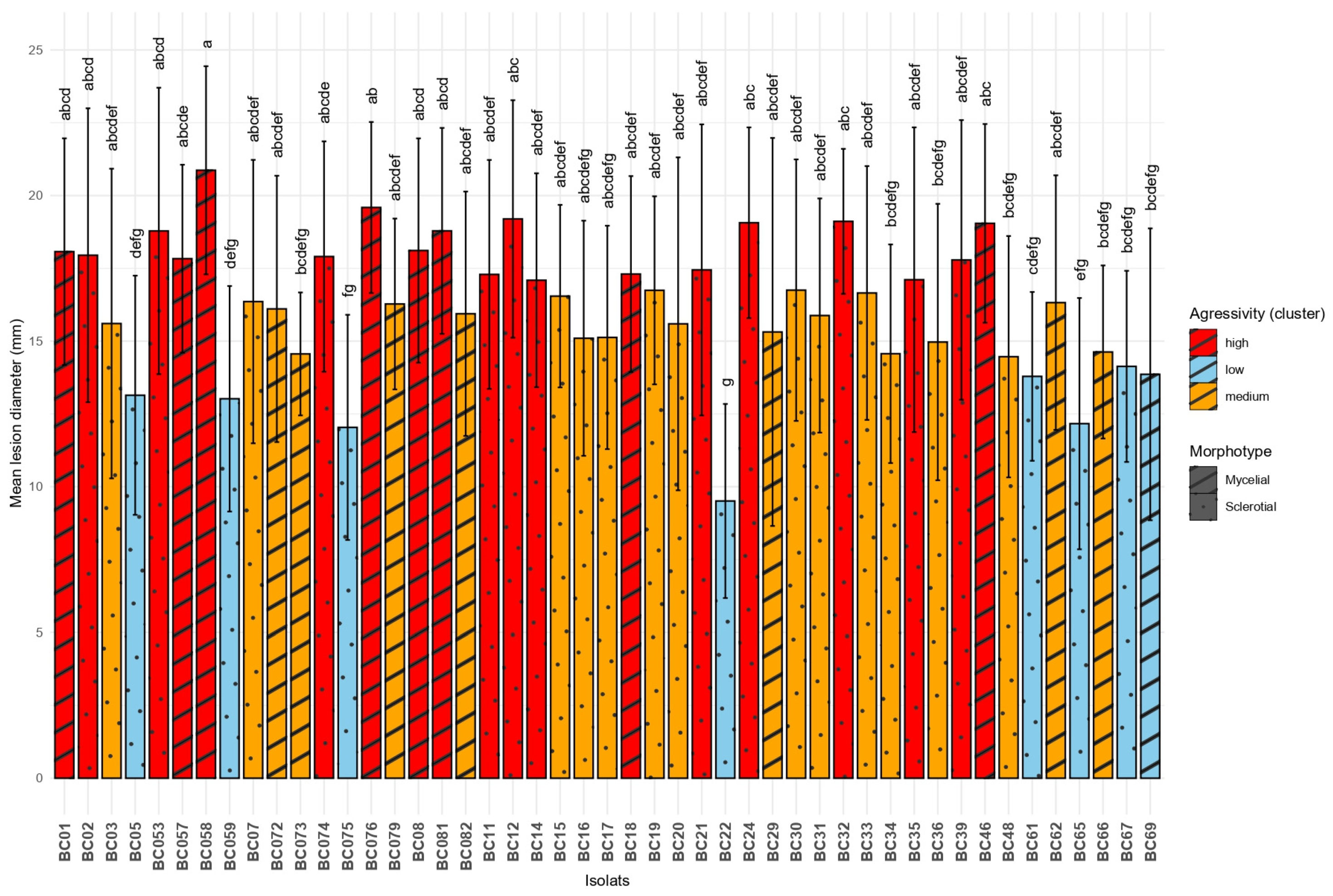
All Botrytis cinerea isolates tested were able to induce lesions on both Vitis vinifera cultivars, Chardonnay and Cabernet Sauvignon. However, significant differences in aggressiveness were observed among isolates (ANOVA, F = 4.991, df = 23, p < 0.0001). The most aggressive isolate was BC58 (mean = 20.87 mm), followed by BC76 and BC12, whereas the least aggressive was BC22 (mean = 9.51 mm), forming a significantly distinct group according to Tukey’s HSD test. Isolates clustered into seven statistical groups (from “a” to “g”), highlighting a broad range of virulence across the tested population. These results are presented in Figure 4. Morphotype was significantly associated with aggressiveness (F = 12.75, p = 0.000379). Aggressiveness levels among B. cinerea isolates were further characterized by hierarchical clustering analysis (Ward’s method) performed on the mean lesion diameter of each isolate, averaged across both cultivars. This approach partitioned the isolates into three statistically distinct clusters, representing low, medium, and high virulence classes. The “high” virulence group contained 19 isolates with the greatest mean lesion diameters, while the “low” group contained 8 isolates with the smallest lesions. The “medium” aggressivity cluster comprised 20 isolates with intermediate lesion diameters.
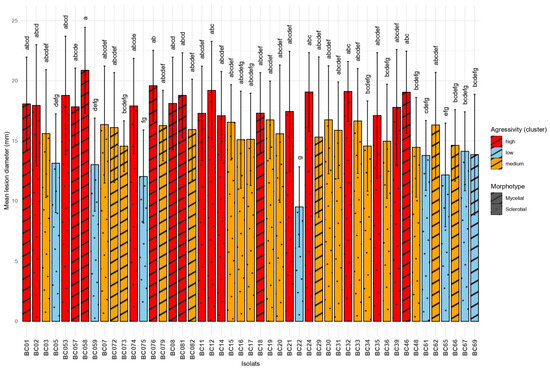
Figure 4.
Comparison of mean lesion diameters caused by different Botrytis cinerea isolates on grapevine leaves. Means sharing the same letter are not significantly different according to Tukey’s HSD test at p = 0.05.
Isolates of the mycelial morphotype (mean lesion diameter = 17.0 mm, n = 256) were significantly more aggressive than those of the sclerotial morphotype (mean = 15.8 mm, n = 496), as indicated by a Welch’s t-test (t = 3.55, p = 0.0004; 95% CI [0.54, 1.87] mm). These results further support the hypothesis that morphological differentiation in Botrytis cinerea is linked to pathogenic potential.
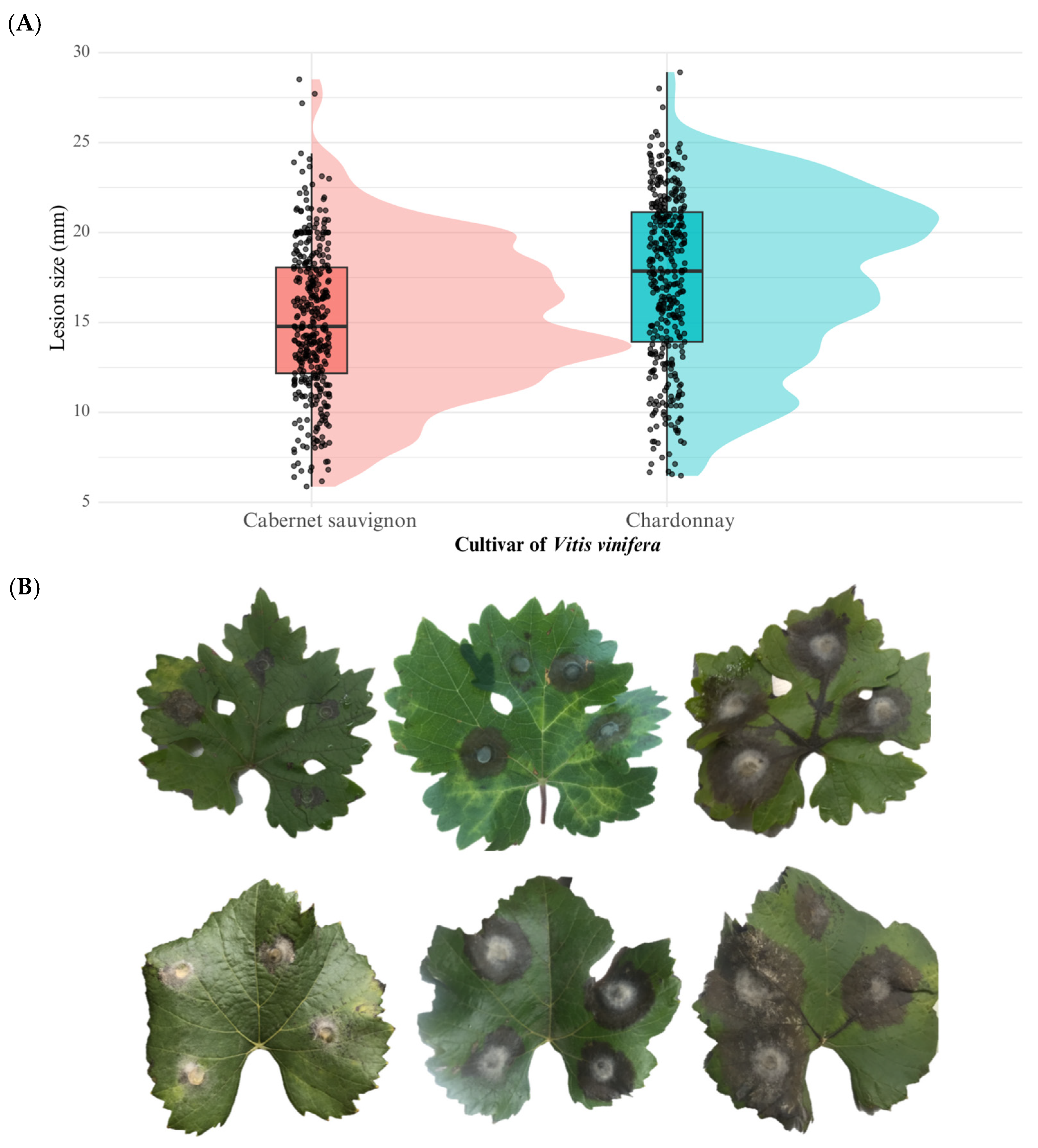
The host cultivar had also a highly significant effect on lesion development (F = 52.32, p = 1.17 × 10−12). The cultivar ‘Chardonnay’ exhibited significantly higher lesion diameters (mean = 17.4 mm) compared to ‘Cabernet Sauvignon’ (mean = 15.0 mm), suggesting that ‘Chardonnay’ is more susceptible to B. cinerea infection. These results are illustrated in Figure 5.
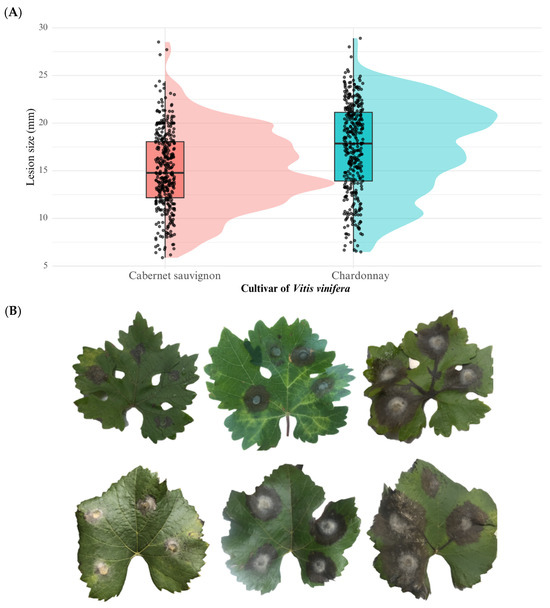
Figure 5.
(A) Boxplot showing the variation in aggressiveness phenotypes of Botrytis cinerea field isolates tested on Vitis vinifera leaves of two grapevine cultivars (Cabernet Sauvignon and Chardonnay). (B) Macroscopic symptoms on detached leaves: the upper row corresponds to Cabernet Sauvignon and the lower row to Chardonnay. From right to left, lesions were caused by hypovirulent, virulent, and hypervirulent isolates of B. cinerea.
3.4. Transposable Element
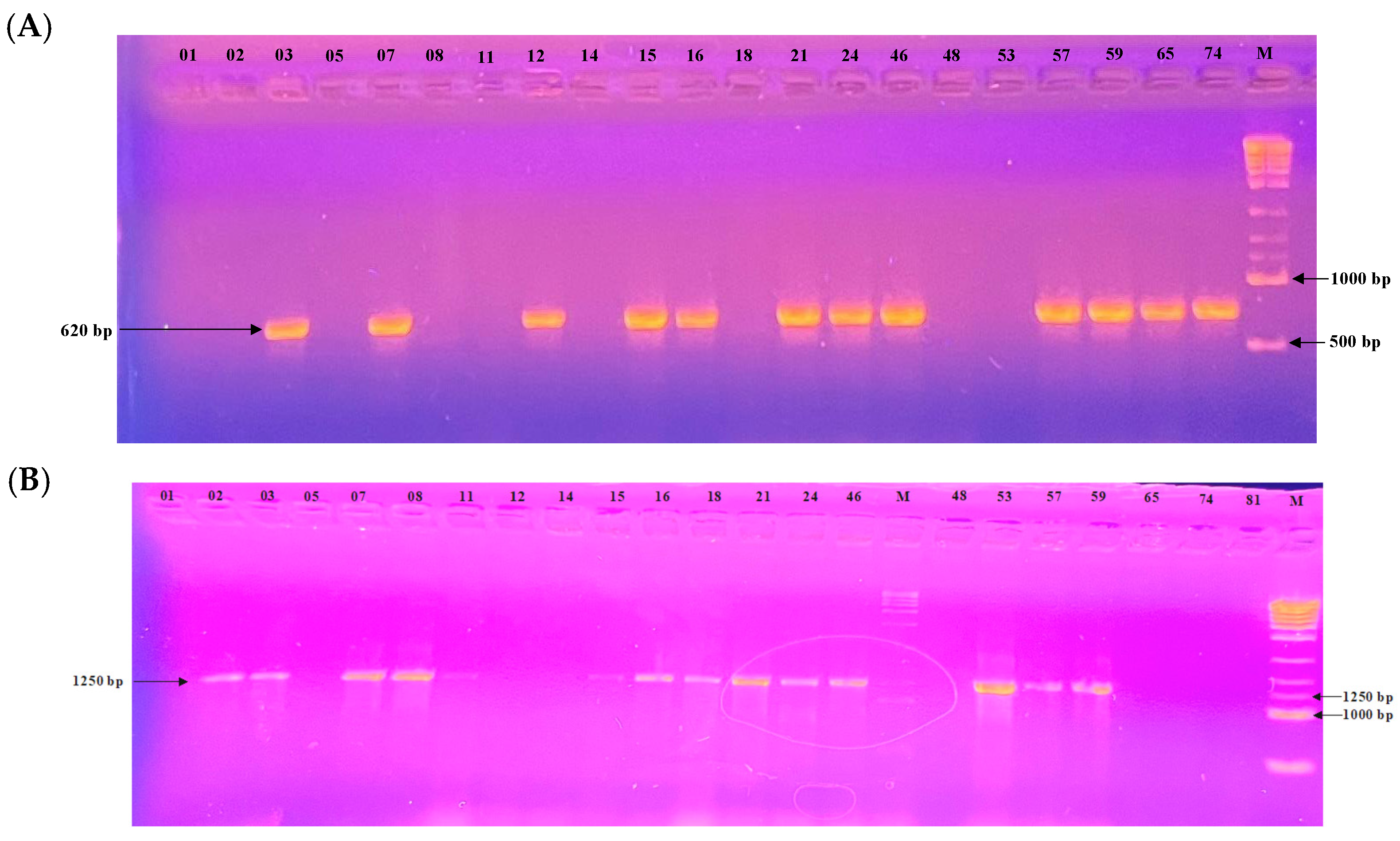
All 24 Botrytis cinerea isolates were screened for the presence of transposable elements (TEs) using PCR with specific primers targeting the Boty and Flipper sequences. PCR profiles are shown in Figure 6. Based on the presence or absence of these elements, isolates were classified into four types: transposa (both Boty and Flipper present), Boty-only, Flipper -only, and vacuma (neither element detected).
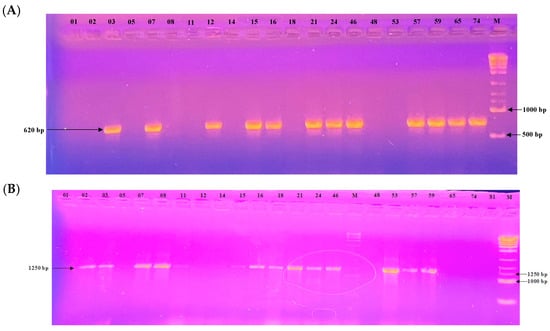
Figure 6.
Electrophoretic gel patterns depicting PCR amplification of a subset of isolates with primer sets targeting (A) Boty (620 bp) and (B) Flipper (1250 bp). Numbers above the lanes indicate isolate identifiers; M denotes the molecular size marker.
Among the 24 isolates analyzed, 10 (41.7%) were classified as transposa-type, 3 isolates (12.5%) carried only Boty (Boty-only), 4 isolates (16.7%) carried only Flipper (Flipper-only), and 7 isolates (29.2%) were of the vacuma type.
These results highlight a predominance of the transposa type within the tested population, while vacuma and Flipper-only types were also relatively frequent. Boty-only isolates were the least represented group. The relationship between transposable element (TE) types and the major morphotype of Botrytis cinerea isolates (mycelial vs. sclerotial) was evaluated using Fisher’s exact test. Although no statistically significant association was observed (p = 0.096), the distribution showed that transposa-type isolates were more frequently associated with the sclerotial morphotype (9 out of 10), while Flipper-only and Vacuma types showed a more balanced distribution across both morphotypes.
3.5. Mating-Type Distribution
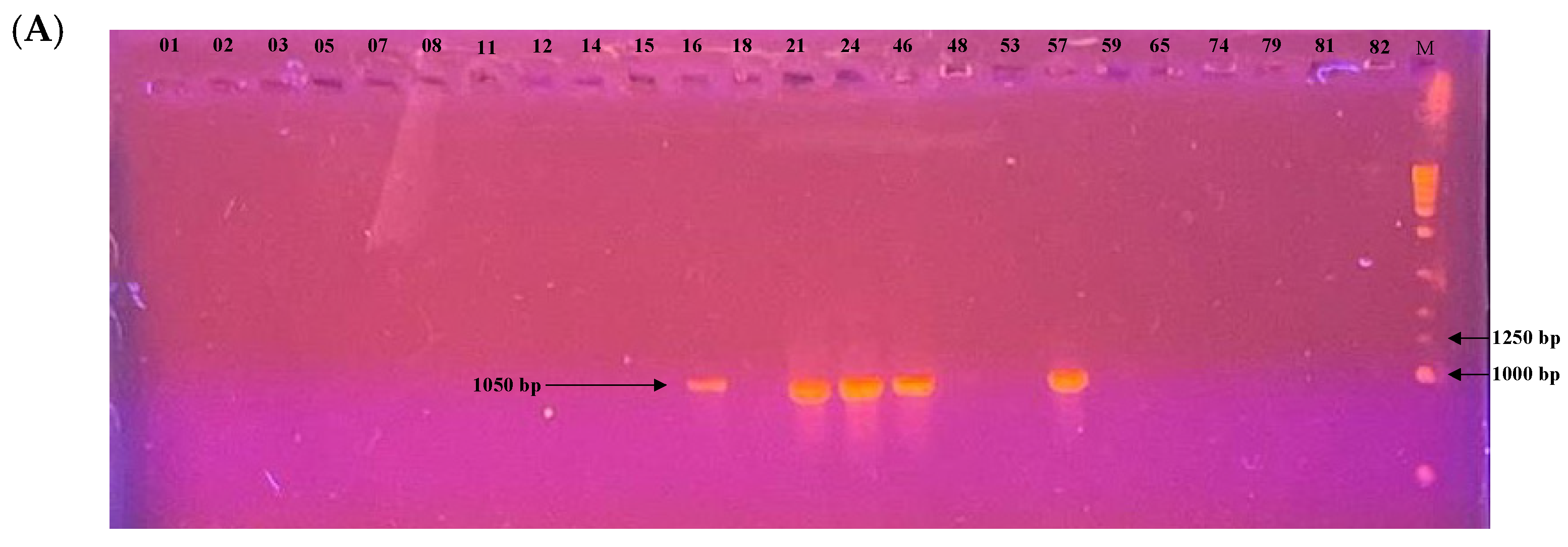
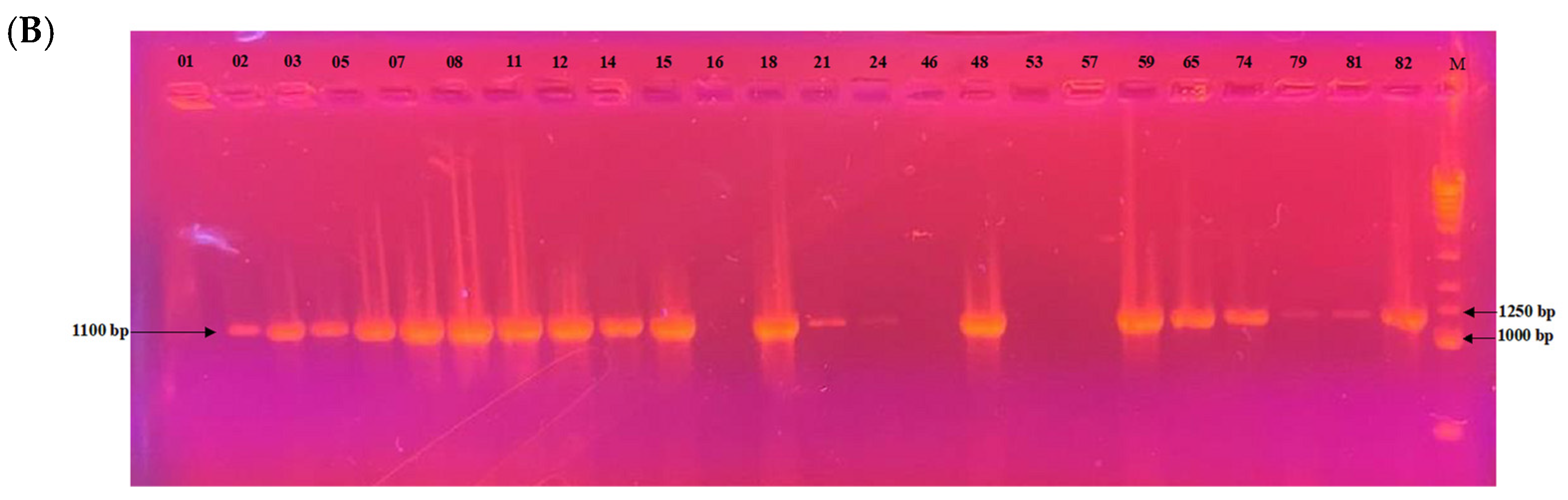
Among the 24 isolates analyzed, the mating type could be determined for 22 of them. PCR profiles are shown in Figure 7. Of these, 17 isolates (77.3%) belonged to the MAT1-2 type and 5 isolates (22.7%) to the MAT1-1 type. A chi-squared goodness-of-fit test was performed to assess whether the observed distribution deviated from the expected 1:1 ratio. The result was significant (χ2 = 6.55, df = 1, p = 0.0105), indicating a significant deviation from the expected distribution. The MAT1-2 type was thus significantly more prevalent in this population.
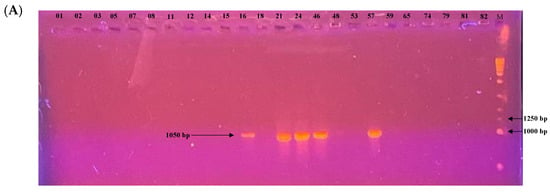
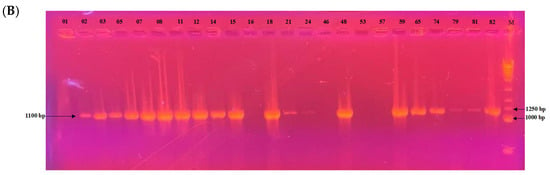
Figure 7.
Electrophoretic gel patterns depicting PCR amplification of a subset of isolates with primer sets targeting (A) MAT1-1 (1050 bp) and (B) MAT1-2 (1100 bp). Numbers above the lanes indicate isolate identifiers; M denotes the molecular size marker.
To examine the potential association between the major morphotype and the mating type, Fisher’s exact test was applied. The result was not significant (p = 1; odds ratio = 0.88, 95% CI = [0.06, 10.76]), suggesting no association between the two variables.
3.6. Phylogenetic Analysis
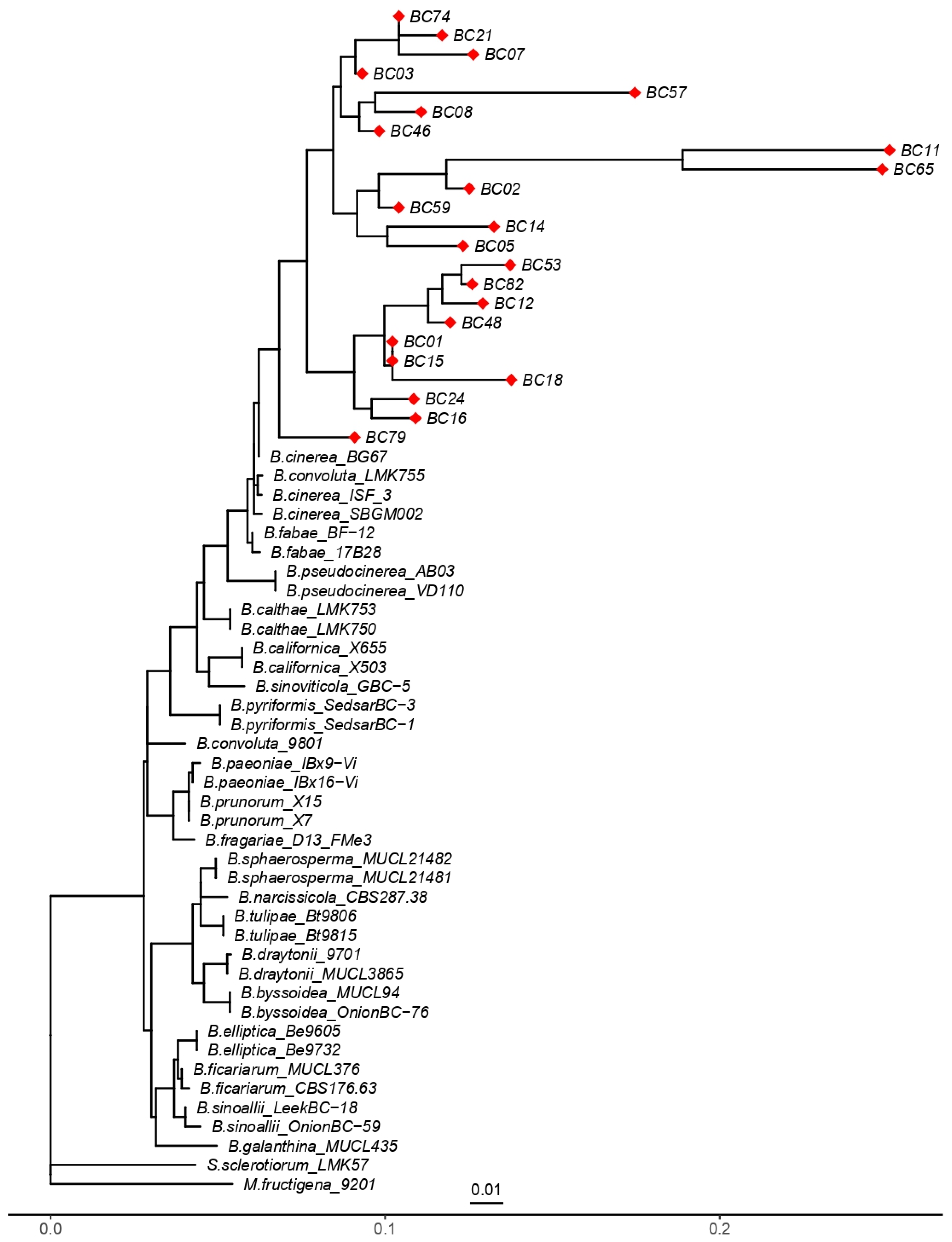
The maximum likelihood (ML) phylogenetic analysis based on partial sequences of the G3PDH gene clearly separated the isolates of Botrytis cinerea from other Botrytis species (Figure 8). All B. cinerea isolates clustered, indicating their close genetic relationship, while also revealing substantial intra-specific diversity. Several well-defined subgroups were observed, such as isolates BC74–BC21–BC07, BC01–BC15-BC18, and BC24–BC16, suggesting the presence of distinct genetic lineages within the B. cinerea population. Some isolates (e.g., BC11 and BC65) exhibited relatively long branches, reflecting higher levels of divergence, which may be associated with local selective pressures.
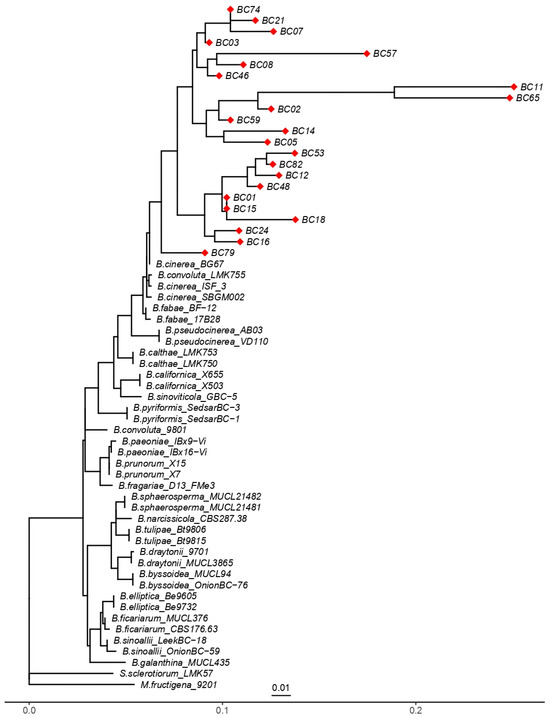
Figure 8.
Phylogenetic tree inferred from partial DNA sequences of the G3PDH gene using the maximum likelihood method. “◆” indicates the 23 Botrytis cinerea isolates analyzed in this study (BC01–BC82). Reference B. cinerea strains were included for comparison, B. pseudocinerea isolates were used to highlight interspecific divergence, and Sclerotinia sclerotiorum together with Monilinia fructigena served as outgroups. The scale bar represents 0.01 substitutions per site.
B. pseudocinerea isolates clustered into a separate but closely related clade, supporting the recognition of this taxon as a cryptic sibling species of B. cinerea. In contrast, other specialized Botrytis species, such as B. calthae, B. paeoniae, B. tulipae, and B. elliptica, were placed in distinct clades, consistent with their evolutionary divergence and host specialization. The outgroups Monilinia fructigena and Sclerotinia sclerotiorum were positioned outside the Botrytis clade, thereby validating the monophyly of the genus.
The reference isolates used for tree construction are listed in Table S5 of the Supplementary Files, ensuring robust taxonomic placement of the studied isolates relative to well-characterized representatives of the genus. Overall, these findings confirm that the G3PDH gene is a suitable molecular marker for discriminating Botrytis species and for detecting intra-specific variation within B. cinerea.
4. Discussion
In this study, 82 isolates of B. cinerea were collected from symptomatic gray mold lesions on grape berries in two major viticultural regions of Morocco. The phenotypic and genetic diversity of B. cinerea isolates has been extensively investigated worldwide [33,34,35]. However, in Morocco, few studies have addressed this diversity. Prior investigations have characterized B. cinerea isolates from faba bean across seven Moroccan regions [36], from saffron in the Taliouine province [37], from Mamora pear in the Maamora forest [38], and from strawberry in Larache province [39]. These works employed either morphological characterization, SRAP (Sequence-Related Amplified Polymorphism) analysis, or ITS region sequencing. To our knowledge, the present study is the first comprehensive assessment of morphological, physiological, and molecular diversity of B. cinerea isolates associated with grapevine in Moroccan vineyards.
Botrytis cinerea is present in all areas where grapevines are cultivated in Morocco, but the damage it causes is generally limited under the country’s typically hot and dry climatic conditions. However, when rainfall occurs shortly before harvest and is accompanied by moderate temperatures, significant outbreaks of gray mold can lead to considerable losses in yield and fruit quality. This pattern mirrors observations in other Mediterranean regions, where the timing of rainfall is a critical factor in disease severity. Moroccan vineyards may thus experience sporadic but sometimes severe B. cinerea epidemics, underlining the importance of targeted monitoring and management during critical periods of the growing season.
Botrytis cinerea is a major pathogen of grapes, causing significant impacts throughout the production chain. In the vineyard, infection by B. cinerea, particularly under wet and humid conditions, can lead to substantial yield losses by destroying grape bunches and causing premature berry drop [40]. Even low infection levels (often less than 5% of berries) can negatively impact the overall quality of grape harvests, as B. cinerea produces volatile organic compounds that alter the aroma and flavor profile of both table grapes and wine [41]. Some of these compounds, such as 1-octen-3-ol and phenylethyl alcohol, impart undesirable earthy or mushroom notes that are perceptible even at low concentrations [42] Beyond the vineyard, B. cinerea may continue to cause losses during storage, transport, and marketing, as the fungus can spread rapidly under favorable post-harvest conditions, affecting not only wine grapes but also fresh table grapes and other high-value crops. For these reasons, monitoring and controlling B. cinerea infection levels is essential throughout the production and post-harvest chain to ensure both yield and product quality.
Populations of B. cinerea collected from seven distinct geographical regions exhibited a morphological phenotype consistent with the species, as previously reported by Mirzaei et al. [43], and were confirmed as B. cinerea through molecular identification [8]. In the present study, isolates obtained from Moroccan vineyards during the same growing season demonstrated substantial morphological variability. This diversity was particularly evident in sclerotia production and form, conidial size, and colony growth rate on PDA medium.
Historically, Paul [6], as cited by Lorbeer [44], was the first to classify B. cinerea into three morphological types: sclerotial (Sc), sporulating (Sp), and mycelial (M). This classification was later supported by Lorbeer [44], who observed that the prevalence of each morphotype varied depending on the host plant. For instance, mycelial types were predominant on flax, whereas sclerotial types were rare and sporulating forms were absent.
In our study, colony morphology on PDA allowed the classification of isolates into eight morphotypes, following the criteria established by Martinez et al. [7]. Among them, morphotype SIII—characterized by large, irregularly distributed sclerotia—was the most frequently observed. Conversely, mycelial morphotypes MI and MIV, defined, respectively, by flat or dense woolly mycelial growth, were the least represented. These findings are in agreement with those of Achleitner, D [45], who observed a predominance of the sclerotial morphotype on grapevine in Austria, as well as with Acosta Morel et al. [46], who reported a high frequency of sclerotial isolates on grapevine in Spain. The mycelial growth rate of B. cinerea isolates was significantly influenced by both temperature and isolate identity. The highest growth rate was observed at 21 °C, followed by 25 °C and 15 °C, in decreasing order. These findings are consistent with those reported by Martinez et al. [7], who confirmed the crucial role of temperature in regulating the growth rate of B. cinerea. Similarly, Mirzaei et al. [43] also observed that B. cinerea isolates exhibited significantly faster growth at 20 °C, reinforcing the existence of a thermal optimum around this range for the species.
In this study, varying levels of aggressiveness were observed among B. cinerea isolates when tested on two differential grapevine genotypes, allowing for a clearer distinction of their virulence. All isolates were able to induce lesions on detached leaves, though the severity of symptoms differed significantly. Such variation in aggressiveness is often attributed to differences in the activity of cell wall-degrading enzymes and the secretion of oxalic acid, a potential pathogenicity factor [35]. Indeed, higher oxalic acid accumulation is generally correlated with increased pathogen virulence, while lower production tends to be associated with reduced aggressiveness [47].
Transposable elements (TEs), known for their ability to move within the genome, constitute a substantial portion of eukaryotic genomes and play a critical role in gene regulation and organismal evolution. In B. cinerea, genetic diversity has been widely assessed based on the presence or absence of two major transposable elements, Boty and Flipper, which define four molecular genotypes: transposa, Boty-only, Flipper-only, and vacuma. The analysis of TE genotypes has become a key tool for evaluating the genetic diversity within B. cinerea populations, with several studies reporting significant variation in their distribution frequencies [48,49].
In the present study, the analysis of 24 isolates collected from Moroccan vineyards, as shown in Table 2, revealed a predominance of the transposa genotype (41.7%), followed by vacuma (29.2%), Flipper-only (16.7%), and Boty-only (12.5%). These findings are consistent with previous reports on grapevine isolates in Italy [50], New Zealand [19], and California [51], all of which also identified transposa as the dominant genotype. This repeated observation across distinct geographic and climatic regions suggests that the transposa genotype may confer a selective advantage, potentially contributing to its prevalence in vineyard-associated fungal populations. Moreover, this supports the hypothesis that high TE-associated genetic diversity may underlie the marked phenotypic variability observed in B. cinerea. Additionally, a temporal shift in TE genotype distribution has been reported by Martinez et al. [52], who observed that vacuma isolates were more frequent during the flowering stage, whereas transposa isolates dominated at harvest. Such seasonal variation may reflect differential adaptation of TE genotypes to environmental conditions and phenological stages of the host plant.

Table 2.
Summary of the main characteristics of representative Botrytis isolates analyzed in this study. For each isolate, the table indicates the sampling locality, grapevine cultivar of origin, aggressivity level, morphotype, mean spore area (µm2), radial growth rate (mm d−1), mating type, and detected transposable elements. Dashes indicate missing or undetermined data.
Analyses of the distribution and frequency of the MAT1-1 and MAT1-2 alleles were conducted in order to assess the potential for recombination and evolutionary capacity of B. cinerea populations originating from vineyards. A better understanding of the MAT1 locus and the fundamental principles of the mating process may pave the way for novel strategies to control B. cinerea. Most studies have demonstrated that heterothallic isolates represent the predominant fraction of natural populations, with a balanced distribution of MAT1-1 and MAT1-2 types in a 1:1 ratio. In contrast, homothallic isolates are rarely observed [50,53]. Until now, the distribution of mating types within Moroccan populations of B. cinerea had not been investigated. The results of this study revealed that the majority of the isolates were heterothallic.
However, the χ2 test indicated a significant deviation of the MAT1-1/MAT1-2 ratio from the expected 1:1 distribution. This finding suggests the occurrence of asexual reproduction, or alternatively, a mixed reproductive mode combining both sexual and asexual reproduction. Similar results were reported by Qiao et al. [54], who observed that among 183 B. cinerea isolates obtained from tomato crops across several regions, all were heterothallic, yet the distribution of mating types did not conform to the expected 1:1 ratio.
The results of this study highlight a considerable phenotypic, physiological, and genetic diversity within B. cinerea populations associated with grapes in Moroccan vineyards. The observed variations in transposon distribution, reproductive mode, and aggressiveness provide valuable insights into the dynamics of the pathogen. This high genetic variability has direct implications for disease management, as it facilitates the emergence and spread of fungicide-resistant or highly virulent strains. Such complexity challenges the efficacy of chemical, biological, and cultural control measures, emphasizing the need to characterize population structure as a foundation for effective and sustainable control strategies. In this context, integrated management approaches, combining cultural practices (e.g., canopy management and sanitation), strategic fungicide rotation, and the application of biological control agents, are strongly recommended. When adapted to local disease pressure and guided by ongoing population monitoring, these complementary strategies offer the most robust and long-term control of gray mold in vineyards. We hope that this research will contribute to a better understanding of the biological mechanisms underlying this notorious fungal pathogen and support the development of more targeted and durable management practices.
5. Conclusions
In summary, this study provides an integrative description of the morphological, physiological, genetic, and phylogenetic diversity of Botrytis cinerea populations collected from vineyards in Morocco. By combining data on lesion development, growth rate, spore morphology, mating type, transposable elements, and isolate origin, together with phylogenetic analyses, we revealed a high degree of variability within natural populations. This variability illustrates the adaptive capacity of B. cinerea and highlights the complex interplay between its genetic background and biological traits. Such knowledge is essential to better understand the epidemiology of gray mold in vineyards and to anticipate its behavior in relation to host interactions and environmental conditions. Furthermore, the characterization of natural variants and their phylogenetic relationships will provide a valuable framework for future studies on the genetic dissection of B. cinerea–host interactions and for the development of sustainable management strategies in viticulture.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microbiolres16100219/s1, Figure S1: Representative photos of grape bunches and berries exhibiting gray mold symptoms used for isolation of B. cinerea. The left panel shows the cultivar ‘Redglobe’, and the right panel shows ‘Aledo’; Table S1: List of Botrytis cinerea isolates with their respective localities, grapevine cultivars of origin, years of isolation, and morphotypes; Figure S2: Gel electrophoresis profile showing PCR amplification with the specific primer pair C729+/−, yielding a 700 bp fragment. Numbers above the lanes indicate isolate codes; M: OXGEN™ 500–10 kb DNA ladder; Table S2: Adjusted means (emmean) and statistical groupings for spore surface area of each isolate; Table S3: Primers used in the experiments; Table S4: Composition of PCR mixtures and cycling conditions for each locus analyzed in this study; Table S5: Botrytis sequences from GenBank used in the phylogenetic analysis.
Author Contributions
Conceptualization, F.A., K.H. and M.H.; methodology, F.A., K.H., M.H. and H.Y.; software, F.A. and I.H.; validation, K.H., M.H. and A.A.; formal analysis, F.A. and C.E.G.; investigation, F.A., C.E.G. and I.H.; resources, A.A.; data curation, F.A. and K.H.; writing—original draft preparation, F.A. and K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PRIMA-MiDiVine project 1564 coordinated by Aziz Aziz, 2021/2025 (https://www.univ-reims.fr/MiDiVine/) (Innovative Approaches Promoting Functional Microbial Diversity for Sustainable Grapevine Health and Productivity in Vineyard Systems of Mediterranean Areas), supported by the European Union with co-funding by MESRSI (Morocco), and ANR (France).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alfonso, C.; Raposo, R.; Melgarejo, P. Genetic Diversity in Botrytis cinerea Populations on Vegetable Crops in Greenhouses in South-Eastern Spain. Plant Pathol. 2000, 49, 243–251. [Google Scholar] [CrossRef]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis: Biology, Pathology and Control; Springer Netherlands: Dordrech, 2007; ISBN 978-1-4020-2626-3. [Google Scholar]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The Cause of Grey Mould Disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Jarvis, W. Botrytinia and Botrytis Species: Taxonomy, Physiology, and Pathogenicity, A Guide to the Literature; Canada Department of Agriculture: Ottawa, ON, Canada, 1977; Volume Monograph, ISBN D-662-00794-8. [Google Scholar]
- Paul, W.R.C. A Comparative Morphological and Physiological Study of a Number of Strains of Botrytis cinerea Pers. with Special Reference to Their Virulence. Trans. Br. Mycol. Soc. 1929, 14, 118–135. [Google Scholar] [CrossRef]
- Martinez, F.; Blancard, D.; Lecomte, P.; Levis, C.; Dubos, B.; Fermaud, M. Phenotypic Differences Between Vacuma and Transposa Subpopulations of Botrytis cinerea. Eur. J. Plant Pathol. 2003, 109, 479–488. [Google Scholar] [CrossRef]
- Rigotti, S.; Gindro, K.; Richter, H.; Viret, O. Characterization of Molecular Markers for Specific and Sensitive Detection of Botrytis cinerea Pers.: Fr. in Strawberry (Fragaria × Ananassa Duch.) Using PCR. FEMS Microbiol. Lett. 2002, 209, 169–174. [Google Scholar] [CrossRef]
- Fournier, E.; Giraud, T.; Albertini, C.; Brygoo, Y. Partition of the Botrytis cinerea Complex in France Using Multiple Gene Genealogies. Mycologia 2005, 97, 1251–1267. [Google Scholar] [CrossRef]
- Fournier, E.; Levis, C.; Fortini, D.; Leroux, P.; Giraud, T.; Brygoo, Y. Characterization of Bc-Hch, the Botrytis cinerea Homolog of the Neurospora crassahet-c Vegetative Incompatibility Locus, and Its Use as a Population Marker. Mycologia 2003, 95, 251–261. [Google Scholar] [CrossRef]
- Walker, A.-S.; Gautier, A.; Confais, J.; Martinho, D.; Viaud, M.; Le Pêcheur, P.; Dupont, J.; Fournier, E. Botrytis pseudocinerea, a New Cryptic Species Causing Gray Mold in French Vineyards in Sympatry with Botrytis cinerea. Phytopathology 2011, 101, 1433–1445. [Google Scholar] [CrossRef]
- Plesken, C.; Weber, R.W.S.; Rupp, S.; Leroch, M.; Hahn, M. Botrytis pseudocinerea Is a Significant Pathogen of Several Crop Plants but Susceptible to Displacement by Fungicide-Resistant B. cinerea Strains. Appl. Env. Microbiol. 2015, 81, 7048–7056. [Google Scholar] [CrossRef]
- Leroch, M.; Plesken, C.; Weber, R.W.S.; Kauff, F.; Scalliet, G.; Hahn, M. Gray Mold Populations in German Strawberry Fields Are Resistant to Multiple Fungicides and Dominated by a Novel Clade Closely Related to Botrytis cinerea. Appl. Env. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef]
- Fournier, E.; Giraud, T.; Loiseau, A.; Vautrin, D.; Estoup, A.; Solignac, M.; Cornuet, J.M.; Brygoo, Y. Characterization of Nine Polymorphic Microsatellite Loci in the Fungus Botrytis cinerea (Ascomycota). Mol. Ecol. Notes 2002, 2, 253–255. [Google Scholar] [CrossRef]
- Giraud, T.; Fortini, D.; Levis, C.; Leroux, P.; Brygoo, Y. RFLP Markers Show Genetic Recombination in Botryotinia Fuckeliana (Botrytis cinerea) and Transposable Elements Reveal Two Sympatric Species. Mol. Biol. Evol. 1997, 14, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Paplomatas, E.J.; Pappas, A.C.; Antoniadis, D. A Relationship among Fungicide-resistant Phenotypes of Botrytis cinerea Based on RAPD Analysis. J. Phytopathol. 2004, 152, 503–508. [Google Scholar] [CrossRef]
- Staats, M.; Van Baarlen, P.; Van Kan, J.A.L. AFLP Analysis of Genetic Diversity in Populations of Botrytis Elliptica and Botrytis tulipae from the Netherlands. Eur. J. Plant Pathol. 2007, 117, 219–235. [Google Scholar] [CrossRef]
- Thompson, J.R.; Latorre, B.A. Characterization of Botrytis cinerea from Table Grapes in Chile Using RAPD-PCR. Plant Dis. 1999, 83, 1090–1094. [Google Scholar] [CrossRef]
- Johnston, P.R.; Hoksbergen, K.; Park, D.; Beever, R.E. Genetic Diversity of Botrytis in New Zealand Vineyards and the Significance of Its Seasonal and Regional Variation. Plant Pathol. 2014, 63, 888–898. [Google Scholar] [CrossRef]
- Diolez, A.; Marches, F.; Fortini, D.; Brygoo, Y. Boty, a Long-Terminal-Repeat Retroelement in the Phytopathogenic Fungus Botrytis cinerea. Appl. Env. Microbiol. 1995, 61, 103–108. [Google Scholar] [CrossRef]
- Levis, C.; Fortini, D.; Brygoo, Y. Flipper, a Mobile Fot1-like Transposable Element in Botrytis cinerea. Mol. Gen. Genet. 1997, 254, 674–680. [Google Scholar] [CrossRef]
- Giraud, T.; Fortini, D.; Levis, C.; Lamarque, C.; Leroux, P.; LoBuglio, K.; Brygoo, Y. Two Sibling Species of the Botrytis cinerea Complex, transposa and vacuma, Are Found in Sympatry on Numerous Host Plants. Phytopathology 1999, 89, 967–973. [Google Scholar] [CrossRef]
- Bergquist, R.R.; Lorbeer, J.W. Apothecial Production, Compatibility and Sex in Botryotinia squamosa. Mycologia 1972, 64, 1270–1281. [Google Scholar] [CrossRef]
- Faretra, F.; Antonacci, E.; Pollastro, S. Sexual Behaviour and Mating System of Botryotinia Fuckeliana, Teleomorph of Botrytis cinerea. Microbiology 1988, 134, 2543–2550. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Rotolo, C.; Pollastro, S.; Faretra, F. Molecular Analysis of the Mating Type (MAT 1) Locus in Strains of the Heterothallic Ascomycete Botrytis cinerea. Plant Pathol. 2016, 65, 1321–1332. [Google Scholar] [CrossRef]
- FAO FAOSTAT–Cultures et Produits Animaux. Available online: https://www.fao.org/faostat/fr/#data/QCL (accessed on 18 August 2025).
- OIV Statistiques Par Pays-Production et Superficie de Raisin Au Maroc. Available online: https://www.oiv.int/fr/what-we-do/data-discovery-report?oiv (accessed on 18 August 2025).
- Aoujil, F.; Litskas, V.; Yahyaoui, H.; El Allaoui, N.; Benbouazza, A.; Aziz, A.; Hafidi, M.; Habbadi, K. Sustainability Indicators for the Environmental Impact Assessment of Plant Protection Products Use in Moroccan Vineyards. Horticulturae 2024, 10, 473. [Google Scholar] [CrossRef]
- Conlon, B.H.; Schmidt, S.; Poulsen, M.; Shik, J.Z. Orthogonal Protocols for DNA Extraction from Filamentous Fungi. STAR Protoc. 2022, 3, 101126. [Google Scholar] [CrossRef]
- Muñoz, C.; Gómez Talquenca, S.; Oriolani, E.; Combina, M. Genetic Characterization of Grapevine-Infecting Botrytis cinerea Isolates from Argentina. Rev. Iberoam. Micol. 2010, 27, 66–70. [Google Scholar] [CrossRef] [PubMed]
- van Kan, J.; Duarte, J.; Dekkers, E.; Dyer, P.; Kohn, L.M. The Botrytis cinerea Mating Type Loci. In Proceedings of the 15th International Botrytis Symposium, Cadiz, Spain, 30 May–4 June 2010; p. 39. [Google Scholar]
- NM ISO 20837:2008; Microbiology of food and animal feeding stuffs — Polymerase chain reaction (PCR) for the detection of food-borne pathogens — General requirements and definitions. Service de Normalisation Industrielle Marocaine (SNIMA): Rabat, Morocco, 2008.
- Jakobija, I.; Bankina, B.; Klūga, A. Morphological Variability of Botrytis cinerea—Causal Agent of Japanese Quince Grey Mould. Agron. Res. 2020, 18, 433. [Google Scholar] [CrossRef]
- Pei, Y.; Tao, Q.; Zheng, X.; Li, Y.; Sun, X.; Li, Z.; Qi, X.; Xu, J.; Zhang, M.; Chen, H.; et al. Phenotypic and Genetic Characterization of Botrytis cinerea Population from Kiwifruit in Sichuan Province, China. Plant Dis. 2019, 103, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.A.M.; Ali, A.M.; Elsherbiny, E.A.; Atwa, A.A. Morphological, Genetic and Pathogenic Variability among Botrytis cinerea Species Complex Causing Gray Mold of Strawberry. Physiol. Mol. Plant Pathol. 2024, 134, 102395. [Google Scholar] [CrossRef]
- Aouzal, S.; Krimi Bencheqroun, S.; Zelmat, L.; Mbasani-Mansi, J.; Zakari, Z.; Haggoud, A.; Gaboun, F.; Khayi, S.; Houmairi, H.; Mentag, R. Genetic Diversity and Population Structure of Moroccan Botrytis spp. Strains, Causing Chocolate Spot Disease in Faba Bean. Arch. Phytopathol. Plant Prot. 2022, 55, 761–772. [Google Scholar] [CrossRef]
- Ourras, S.; Elouark, M.; El Aymani, I.; Msairi, S.; Chliyeh, M.; Selmaoui, K.; Abdelaziz El Alaoui, M.; Mouden, N.; Touhami, A.O.; Douira, A. First Report of Botrytis cinerea on Saffron Corms in Morocco. In Advances in Bioinformatics and Biomedical Engineering; Mabrouki, J., Ed.; IGI Global: Hershey, PA, USA, 2025; pp. 165–180. ISBN 9798369394502. [Google Scholar]
- Sellal, Z.; Dahmani, J.; Benkirane, R.; Ouazzani Touhami, A.; Douira, A. Pathogenic Capacity of Botrytis cinerea on Leaves of Pyrus Mamorensis, an Endemic Tree of Mamora Forest in Morocco. AJB 2017, 2, 125–129. [Google Scholar] [CrossRef]
- Hammoumi, S.; Bentata, F.; Khalifi, H.; Karim, S.; Maafa, I.; Elwahab, F.; Gaboun, F.; Brhadda, N.; Ziri, R.; Labhilili, M. Molecular Identification and Characterization of Botrytis spp. from Strawberry in Morocco. from Strawberry in Morocco. 2025, 23, 494. [Google Scholar] [CrossRef]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine Bunch Rots: Impacts on Wine Composition, Quality, and Potential Procedures for the Removal of Wine Faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar] [CrossRef]
- Steel, C.C.; Schwarz, L.J.; Qiu, Y.; Schueuermann, C.; Blackman, J.W.; Clark, A.C.; Schmidtke, L.M. Thresholds for Botrytis Bunch Rot Contamination of Chardonnay Grapes Based on the Measurement of the Fungal Sterol, Ergosterol. Aust. J. Grape Wine Res. 2020, 26, 79–89. [Google Scholar] [CrossRef]
- Lopez Pinar, A.; Rauhut, D.; Ruehl, E.; Buettner, A. Effects of Bunch Rot (Botrytis cinerea) and Powdery Mildew (Erysiphe necator) Fungal Diseases on Wine Aroma. Front. Chem. 2017, 5, 20. [Google Scholar] [CrossRef]
- Mirzaei, S.; Mohammadi Goltapeh, E.; Shams-Bakhsh, M.; Safaie, N.; Chaichi, M. Genetic and Phenotypic Diversity among Botrytis cinerea Isolates in Iran. J. Phytopathol. 2009, 157, 474–482. [Google Scholar] [CrossRef]
- Lorbeer, J.W. Variation in Botrytis and Botryotinia. Biol. Botrytis 1980, 19–40. [Google Scholar]
- Achleitner, D. Investigations of Latent Infection of Grape Bunches with Botrytis cinerea. Ph.D. Thesis, Universität für Bodenkultur, Wien, Austria, 2008. [Google Scholar]
- Acosta Morel, W.; Marques-Costa, T.M.; Santander-Gordón, D.; Anta Fernández, F.; Zabalgogeazcoa, I.; Vázquez De Aldana, B.R.; Sukno, S.A.; Díaz-Mínguez, J.M.; Benito, E.P. Physiological and Population Genetic Analysis of Botrytis Field Isolates from Vineyards in Castilla y León, Spain. Plant Pathol. 2019, 68, 523–536. [Google Scholar] [CrossRef]
- Fernández Acero, F.J.; Carbú, M.; El-Akhal, M.R.; Garrido, C.; González-Rodríguez, V.E.; Cantoral, J.M. Development of Proteomics-Based Fungicides: New Strategies for Environmentally Friendly Control of Fungal Plant Diseases. IJMS 2011, 12, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Tayal, P.; Sharma, E.; Kapoor, R. Analyses of Genetic and Pathogenic Variability among Botrytis cinerea Isolates. Microbiol. Res. 2014, 169, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Shen, F.; Xu, H.; Li, Y.; Liu, D. Characterization of Botrytis cinerea Isolates From Grape Vineyards in China. Plant Dis. 2018, 102, 40–48. [Google Scholar] [CrossRef]
- Campia, P.; Venturini, G.; Moreno-Sanz, P.; Casati, P.; Toffolatti, S.L. Genetic Structure and Fungicide Sensitivity of Botrytis cinerea Populations Isolated from Grapevine in Northern Italy. Plant Pathol. 2017, 66, 890–899. [Google Scholar] [CrossRef]
- DeLong, J.A.; Saito, S.; Xiao, C.-L.; Naegele, R.P. Population Genetics and Fungicide Resistance of Botrytis cinerea on Vitis and Prunus spp. in California. Phytopathology 2020, 110, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; Dubos, B.; Fermaud, M. The Role of Saprotrophy and Virulence in the Population Dynamics of Botrytis cinerea in Vineyards. Phytopathology 2005, 95, 692–700. [Google Scholar] [CrossRef]
- Wessels, B.A.; Linde, C.C.; Fourie, P.H.; Mostert, L. Genetic Population Structure and Fungicide Resistance of Botrytis cinerea in Pear Orchards in the Western Cape of South Africa. Plant Pathol. 2016, 65, 1473–1483. [Google Scholar] [CrossRef]
- Qiao, G.; Li, X.; Huang, J.; Lin, X.; Zhou, Y. Analysis and Molecular Detection of Botrytis cinerea Mating Type Genes. Mycosystema 2015, 34, 108–116. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).