Genomic Characterization of Laodelphax striatellus Permutotetra-like Virus and Self-Cleavage Function of Viral Capsid Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Populations and Virus
2.2. Cloning of Viral Genomic Sequences
2.3. Analysis of the Whole Genome of LsPLV
2.4. Gene Cloning, Prokaryotic Expression, and Purification of LsPLV Capsid Protein
2.5. Preparation of Polyclonal Antibody Against CP
2.6. Detection of Capsid Protein
3. Results
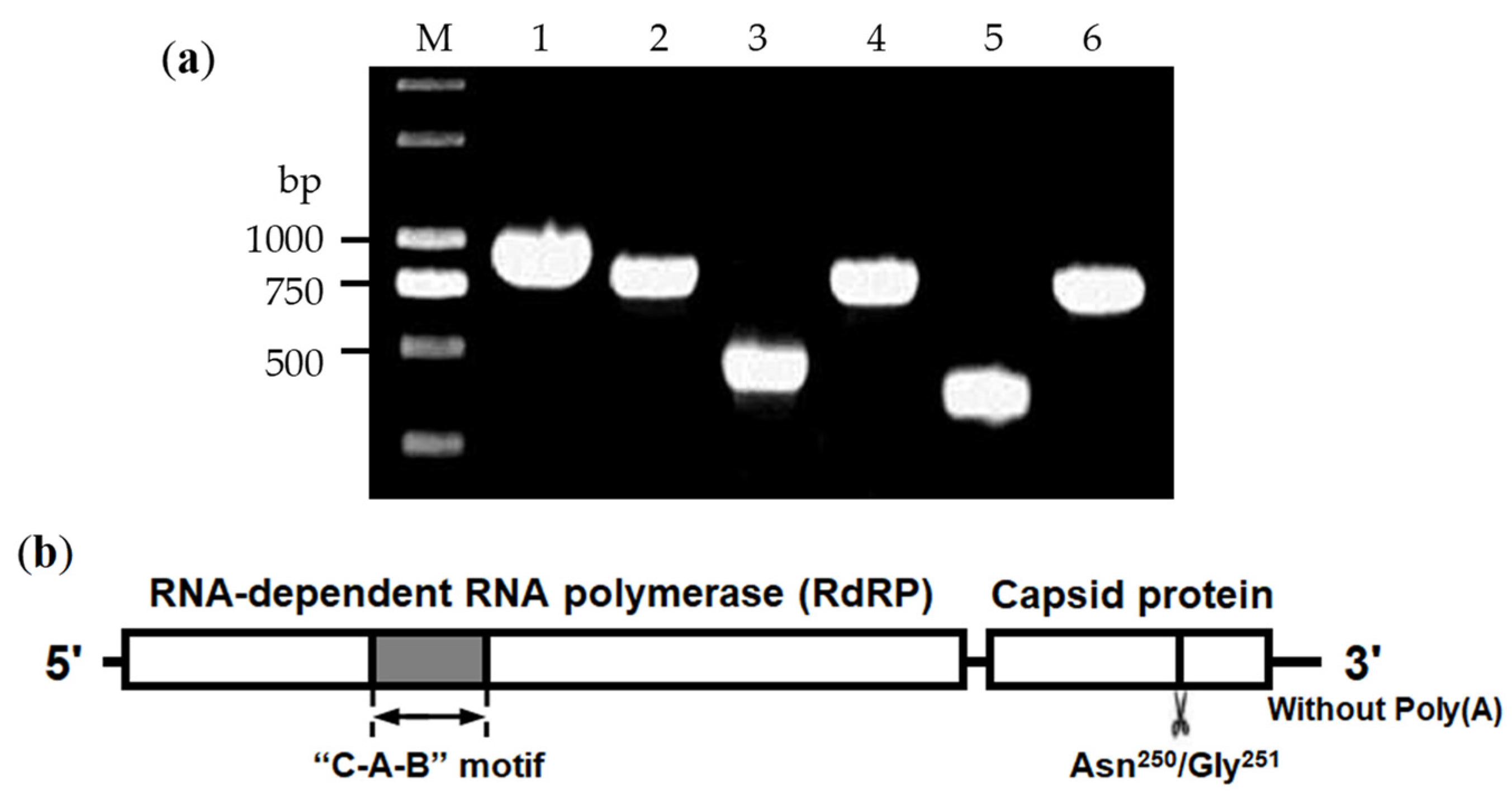
3.1. Cloning of LsPLV Genome
3.2. Analysis of LsPLV Genome Features
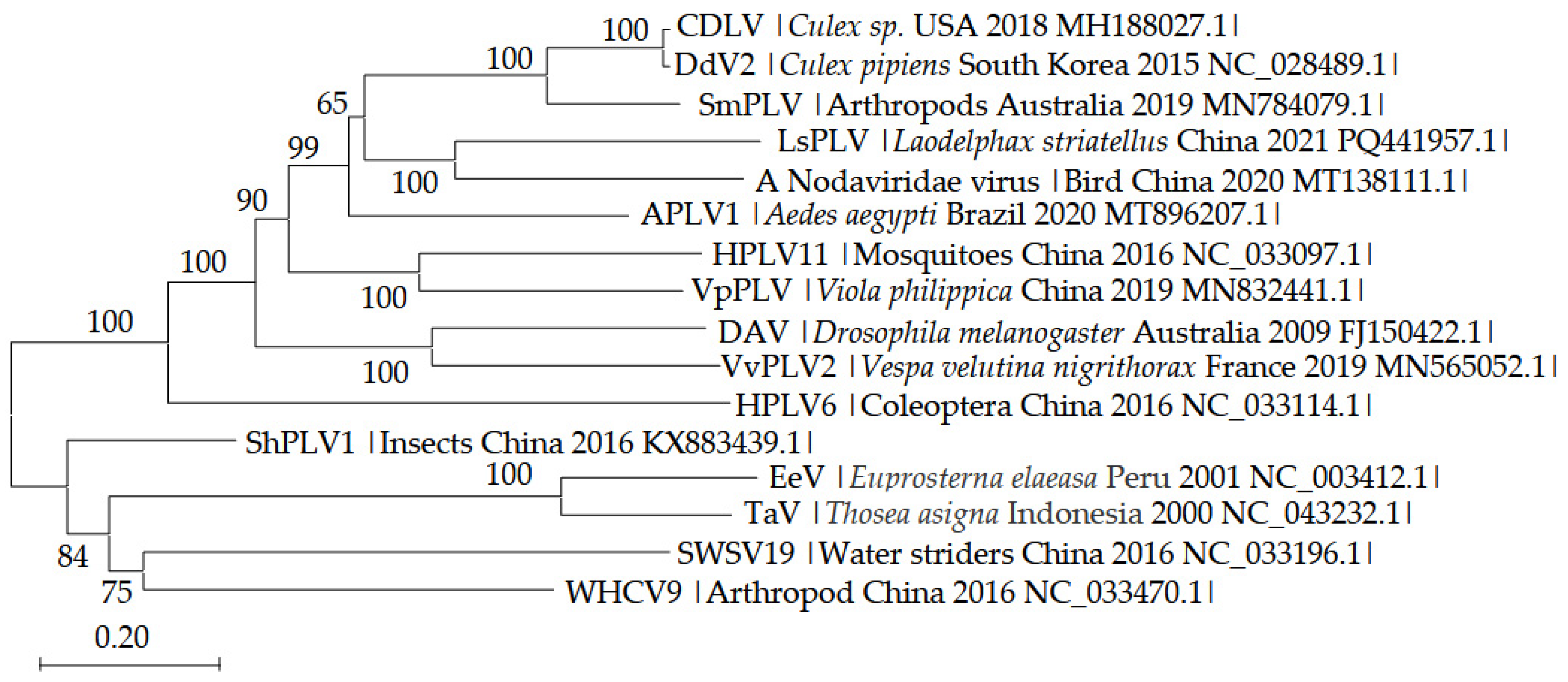
3.3. Phylogenetic Analysis of LsPLV Genome Sequence
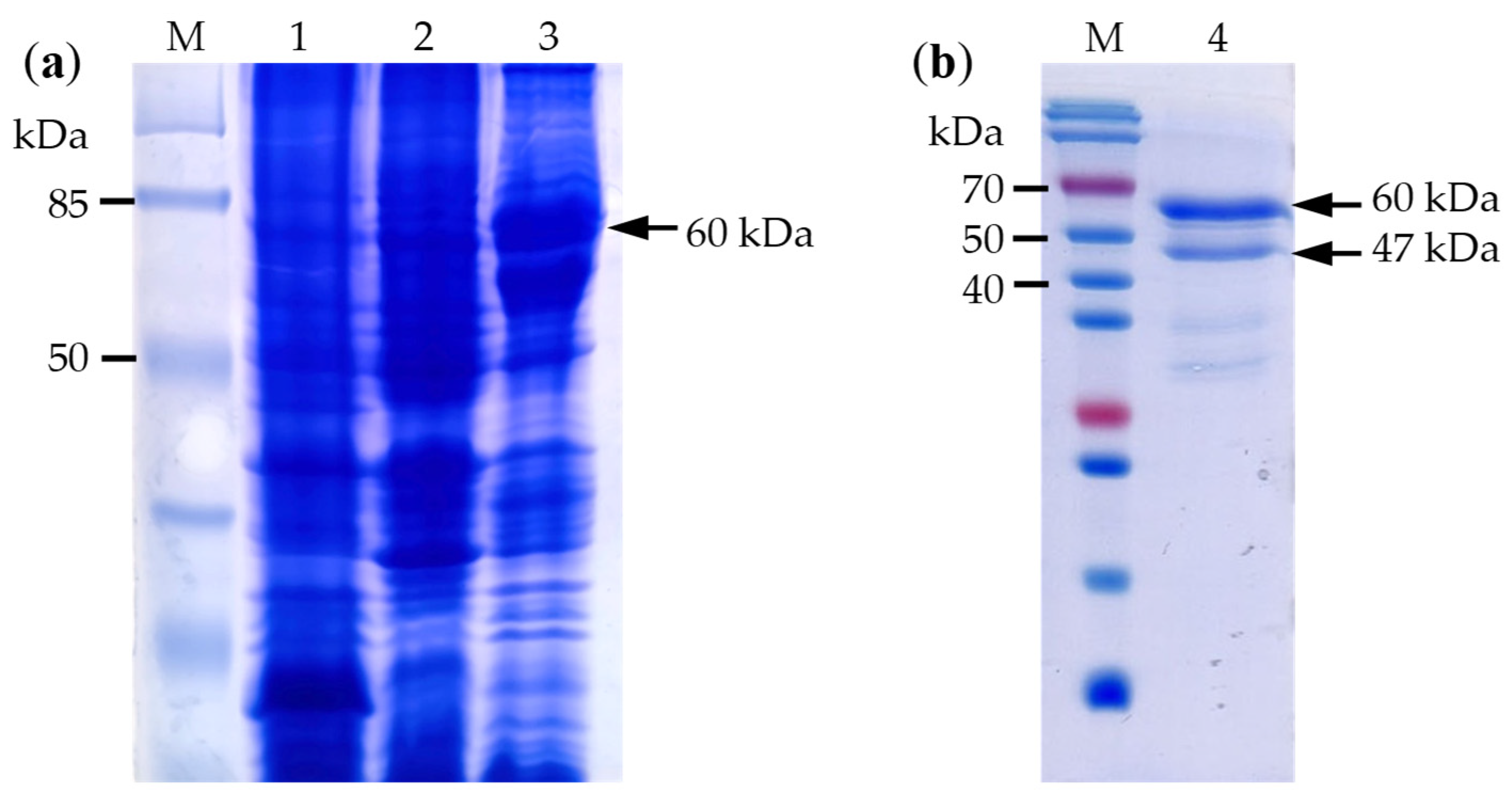
3.4. Cloning and Protein Expression of LsPLV CP Gene
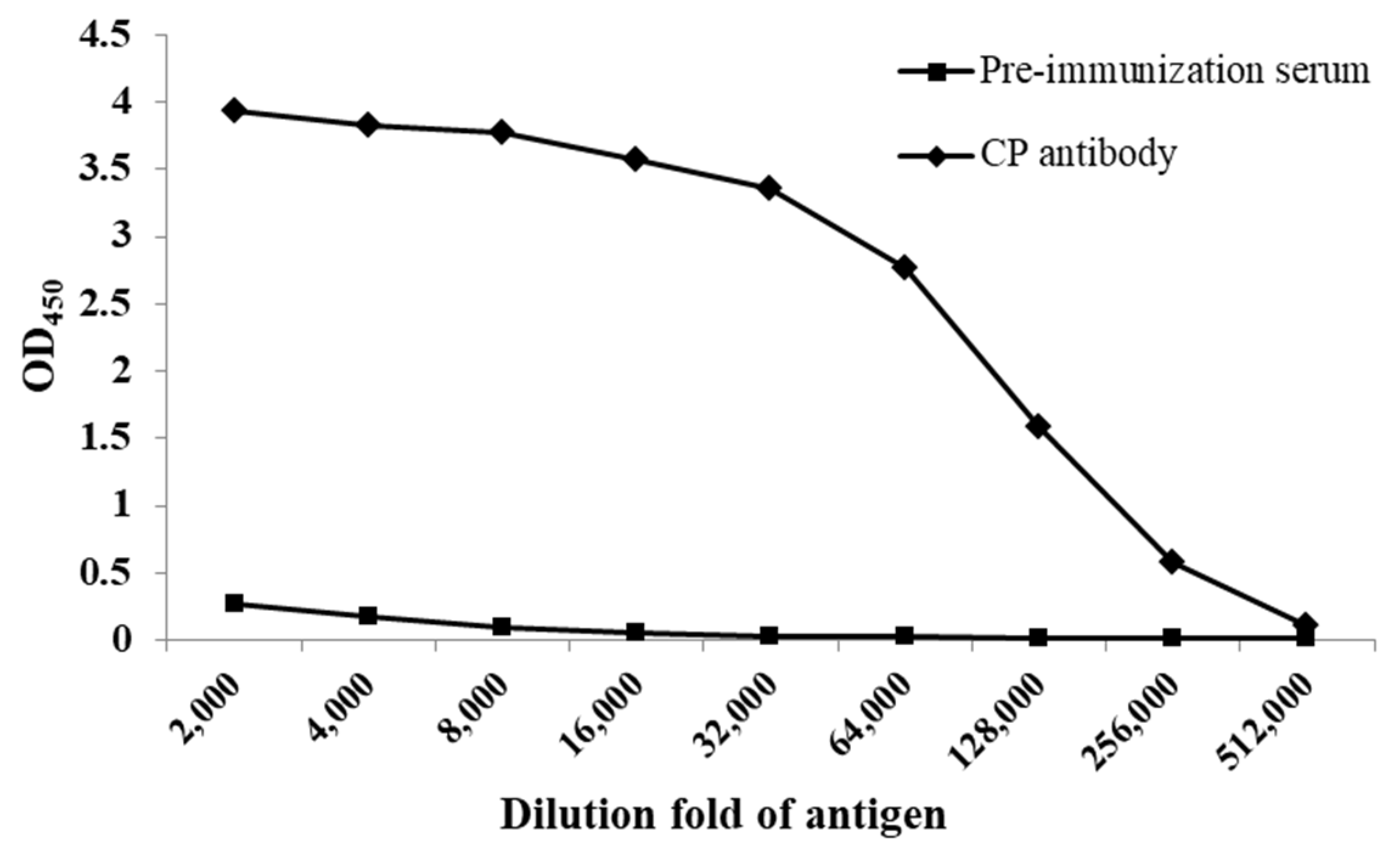
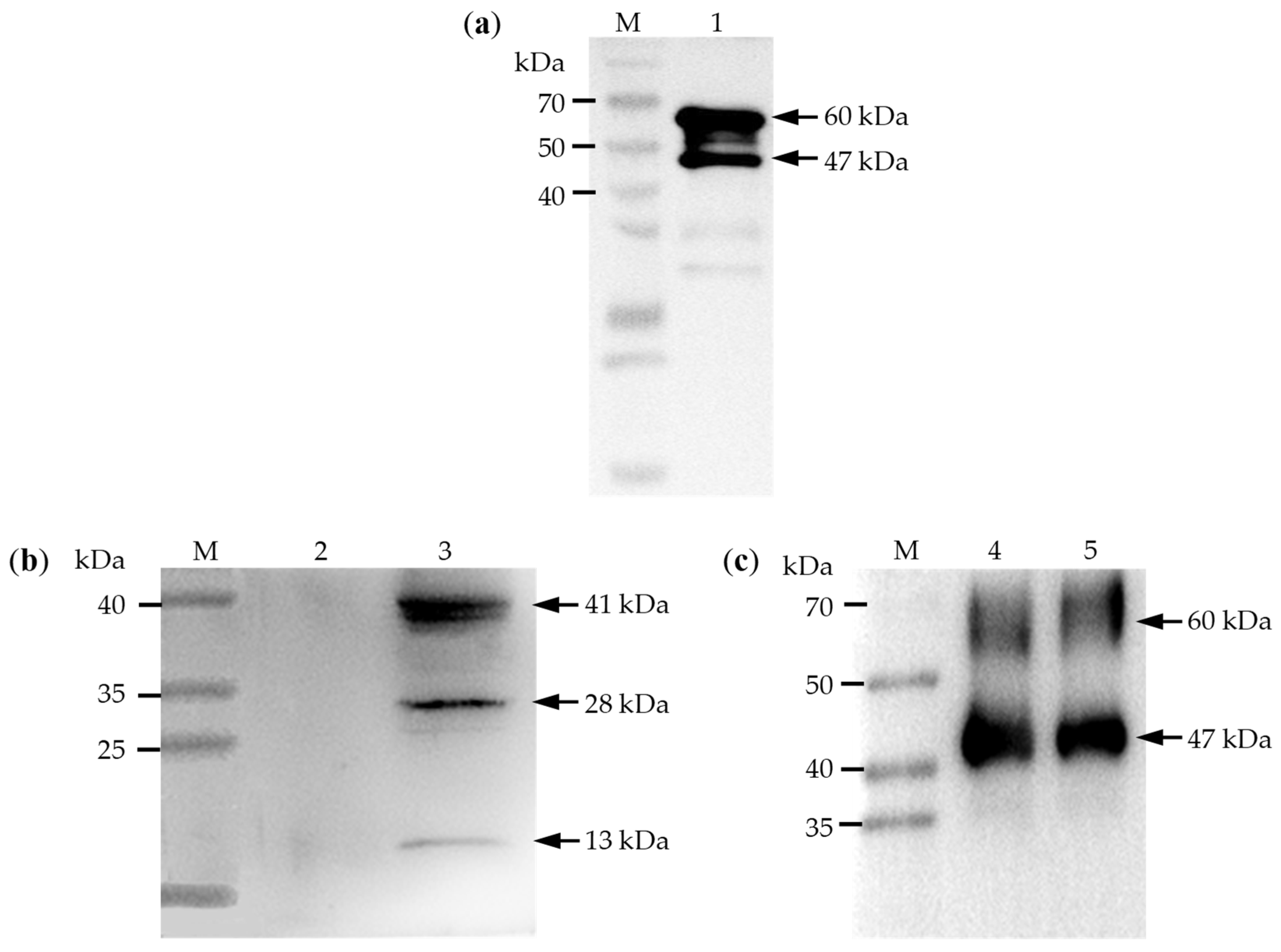
3.5. Potency and Specificity of CP Antibody
3.6. Detection of LsPLV in SBPH and Self-Cleavage Property of CP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tinsley, T.W. The potential of insect pathogenic viruses as pesticidal agents. Annu. Rev. Entomol. 1979, 24, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Rasgon, J.L. Densonucleosis viruses (‘densoviruses’) for mosquito and pathogen control. Curr. Opin. Insect Sci. 2018, 28, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the international committee on taxonomy of viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef]
- Yu, X.Z.; Jia, D.S.; Wang, Z.; Li, G.J.; Chen, M.N.; Liang, Q.F.; Zhou, Y.Y.; Liu, H.; Xiao, M.; Li, S.T.; et al. A plant reovirus hijacks endoplasmic reticulum-associated degradation machinery to promote efficient viral transmission by its planthopper vector under high temperature conditions. PLoS Pathog. 2021, 17, e1009347. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Huot, O.B.; Martin, K.M.; Kondo, H.; Dietzgen, R.G. Plant rhabdoviruses-their origins and vector interactions. Curr. Opin. Virol. 2019, 33, 198–207. [Google Scholar] [CrossRef]
- Ban, L.; Didon, A.; Jonsson, L.M.; Glinwood, R.; Delp, G. An improved detection method for the Rhopalosiphum padi virus (RhPV) allows monitoring of its presence in aphids and movement within plants. J. Virol. Methods 2007, 142, 136–142. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Keane, G.; Naish, N.; Evered, C.; Winstanley, D. Densovirus induces winged morphs in asexual clones of the rosy apple aphid, Dysaphis plantaginea. Proc. Natl. Acad. Sci. USA 2009, 106, 8465–8470. [Google Scholar] [CrossRef]
- Ban, L.P.; Ahmed, E.; Ninkovic, V.; Delp, G.; Glinwood, R. Infection with an insect virus affects olfactory behaviour and interactions with host plant and natural enemies in an aphid. Entomol. Exp. Appl. 2008, 127, 108–117. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.J.; Wang, X.; Li, X.L.; Zi, J.Y.; Ge, S.S.; Cheng, Z.B.; Zhou, T.; Ji, Y.H.; Deng, J.H.; et al. Rice stripe virus affects the viability of its vector offspring by changing developmental gene expression in embryos. Sci. Rep. 2015, 5, 7883. [Google Scholar] [CrossRef]
- Fang, S.; Yu, J.; Feng, J.; Han, C.; Li, D.; Liu, Y. Identification of rice black-streaked dwarf fijivirus in maize with rough dwarf disease in China. Arch. Virol. 2001, 146, 167–170. [Google Scholar] [CrossRef]
- Di, D.P.; Zhang, Y.L.; Zhang, A.H.; Yan, C.; Yang, F.; Lu, Y.G.; Tian, L.Z.; Wang, X.B.; Miao, H.Q. Identification of a virus transmitted by small brown planthopper in wheat. Acta Phytopathol. Sin. 2016, 46, 453–460. [Google Scholar] [CrossRef]
- Gong, Z.X.; Zheng, Q.X.; Peng, H.; Cao, T.Q.; Shikata, E. Study on relationship between WRSV in China and NCMV in Japan. Chin. J. Virol. 1985, 1, 257–261. [Google Scholar] [CrossRef]
- Piao, J.; Zhang, L.J.; Piao, J.A.; Zhou, Y.J.; Li, S. Discovery of viruses from small brown planthopper by small RNA deep sequencing. Biotechnol. Bull. 2022, 38, 281–288. [Google Scholar] [CrossRef]
- Toriyama, S.; Guy, P.L.; Fuji, S.; Takahashi, M. Characterization of a new picorna-like virus, himetobi P virus, in planthoppers. J. Gen. Virol. 1992, 73, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ge, S.S.; Wang, X.; Sun, L.J.; Liu, Z.W.; Zhou, Y.J. Facilitation of rice stripe virus accumulation in the insect vector by himetobi P virus VP1. Viruses 2015, 7, 1492–1504. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, P.P.; Liu, W.W.; Cao, M.J.; Massart, S.; Wang, X.F. Complete genome sequence and characterization of a new iflavirus from the small brown planthopper (Laodelphax striatellus). Virus Res. 2019, 272, 197651. [Google Scholar] [CrossRef]
- Yang, Q.K.; Zhang, Y.; Andika, I.B.; Liao, Z.F.; Kondo, H.; Lu, Y.H.; Cheng, Y.; Li, L.Y.; He, Y.Q.; He, Y.J. Horizontal transfer of a retrotransposon from the rice planthopper to the genome of an insect DNA virus. J. Virol. 2019, 93, e01516-18. [Google Scholar] [CrossRef]
- Hanzlik, T.N.; Gordon, K. The Tetraviridae. Ada. Virus Res. 1997, 48, 101–168. [Google Scholar] [CrossRef]
- Reinganum, C.; Robertson, J.S.; Tinsley, T.W. A new group of RNA viruses from insects. J. Gen. Virol. 1978, 40, 195–202. [Google Scholar] [CrossRef]
- Juckes, I.R.M. Comparison of some biophysical properties of the Nudaurelia β and ε viruses. J. Gen. Virol. 1979, 42, 89–94. [Google Scholar] [CrossRef]
- Ambrose, R.L.; Lander, G.C.; Maaty, W.S.; Bothner, B.; Johnson, J.E.; Johnson, K.N. Drosophila A virus is an unusual RNA virus with a T=3 icosahedral core and permuted RNA-dependent RNA polymerase. J. Gen. Virol. 2009, 90, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Pringle, F.M.; Zeddam, J.L.; Luke, B.T.; Ward, V.K. The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J. Mol. Biol. 2002, 324, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Poch, O.; Sauvaget, I.; Delarue, M.; Tordo, N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989, 8, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Piao, J.; Xu, C.L.; Piao, J.A.; Zhou, Y.J.; Li, S. Preparation of the polyclonal antibody of capsid protein VP1 of Himetobi P virus in the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae) and its application in the virus detection. Acta Entomol. Sin. 2019, 62, 823–829. [Google Scholar] [CrossRef]
- Jiang, J.X.; Chen, Z.X.; Zhou, X.P. Production of a monoclonal antibody to sugarcane mosaic virus and its application for virus detection in China. J. Phytopathol. 2010, 151, 361–364. [Google Scholar] [CrossRef]
- Mao, Q.Z.; Ye, Z.X.; Yuan, J.N.; Ning, C.; Chen, M.N.; Xu, Z.T.; Qi, Y.H.; Zhang, Y.; Li, T.; He, Y.J.; et al. Diversity and transmissibility of RNA viruses in the small brown planthopper, Laodelphax striatellus. J. Virol. 2024, 98, e0019124. [Google Scholar] [CrossRef]
- Yi, F.M.; Zhang, J.M.; Liu, C.F.; Hu, Y.Y. Advances in tetravirus research. Acta Entomol. Sin. 2005, 48, 792–798. [Google Scholar] [CrossRef]
- Yi, F.M.; Zhang, J.M.; Yu, H.Y.; Liu, C.F.; Wang, J.P.; Hu, Y.Y. Isolation and identification of a new tetravirus from Dendrolimus punctatus larvae collected from Yunnan Province, China. J. Gen. Virol. 2005, 86, 789–796. [Google Scholar] [CrossRef]
- Agrawal, D.; Johnson, J. Assembly of the T = 4 Nudaurelia capensis ω virus capsid protein, post-translational cleavage, and specific encapsidation of its mRNA in a baculovirus expression system. Virology 1995, 207, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Canady, M.A.; Tihova, M.; Hanzlik, T.N.; Johnson, J.E.; Yeager, M. Large conformational changes in the maturation of a simple RNA virus, Nudaurelia capensis ω virus (NωV). J. Mol. Biol. 2000, 299, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Pringle, F.M.; Kalmakoff, J.; Ward, V.K. Analysis of the capsid processing strategy of Thosea asigna virus using baculovirus expression of virus-like particles. J. Gen. Virol. 2001, 82, 259–266. [Google Scholar] [CrossRef]
- Taylor, D.J.; Krishna, N.K.; Canady, M.A.; Schneemann, A.; Johnson, J.E. Large-scale, pH-dependent quaternary structure changes in an RNA virus capsid are reversible in the absence of subunit autoproteolysis. J. Virol. 2002, 76, 9972–9980. [Google Scholar] [CrossRef]
- Hanzlik, T.N.; Dorrian, S.J.; Johnson, K.N.; Brooks, E.M.; Gordon, K.H.J. Sequence of RNA2 of the Helicoverpa armigera stunt virus (Tetraviridae) and bacterial expression of its genes. J. Gen. Virol. 1995, 76, 799–811. [Google Scholar] [CrossRef]
- Pringle, F.M.; Johnson, K.N.; Goodman, C.L.; McIntosh, A.H.; Ball, L.A. Providence virus: A new member of the Tetraviridae that infects cultured insect cells. Virology 2003, 306, 359–370. [Google Scholar] [CrossRef]
- Gordon, K.; Williams, M.R.; Hendry, D.A.; Hanzlik, T.N. Sequence of the genomic RNA of nudaurelia beta virus (Tetraviridae) defines a novel virus genome organization. Virology 1999, 258, 42–53. [Google Scholar] [CrossRef]
| Primer and Purpose | Sequence (5′→3′) | LsPLV Genome Location (nt) |
|---|---|---|
| Amplification of gene segments | ||
| LsPLV-F1 | ACAACGACAATGGACGCAAGCAACCC | 16-1182 |
| LsPLV-R1 | TGATCCGTCCATCTGTTTGAAATCTGG | |
| LsPLV-F2 | CCCAGATTTCAAACAGATGGACGG | 1155-1956 |
| LsPLV-R2 | GTAGAGGGCCAGGCAGAACTCC | |
| LsPLV-F3 | TAGACTACCCCGACTCCTCCGGAT | 1901-2495 |
| LsPLV-R3 | GTGGGGAGCGGGGTGACAAGGGG | |
| LsPLV-F4 | CCAACCCCCTGGACATAGTCTCCCTC | 2444-3305 |
| LsPLV-R4 | GCCTGGGCCAATCTTCTTTTCCGCCTCT | |
| LsPLV-F5 | AGGAAGCTGAACCGGAAGACGCT | 3253-3641 |
| LsPLV-R5 | CGTTGATGTTGAAGGACTGAAGAAGGG | |
| LsPLV-F6 | TCCCTTCTTCAGTCCTTCAACAT | 3614-4394 |
| LsPLV-R6 | GGTTTCCCTGACCCACGTACTTGC | |
| 5′RACE | ||
| 5′Adaptor primer | GCTGTCAACGATACGCTACGTAACGGCAT GACAGTGGGGGGGGGGGGGG | |
| 5′Race outer | GCTGTCAACGATACGCTACGTAAC | |
| LsPLV463-R | CCGTGTAGACCTTGGACACGCACAT | |
| LsPLV530-R | GCCCAGGACACTGTCGTTCTTCCCAT | |
| 3′RACE | ||
| 3′Race Adaptor | TACCGTCGTTCCACTAGTGATTTCACTATG ACGTTTTTTTTTTTTTTTT | |
| 3′Race outer | TACCGTCGTTCCACTAGTGATTTC | |
| 3′Race inner | AGTGATTTCACTATGACG | |
| LsPLV-CP-F1004 | GCAAGTACGTGGGTCAGGGAAACC | |
| LsPLV-CP-F931 | GGAGACACCATCAACTCCACGTCCTG | |
| Construction of expression vector | ||
| LsPLV-CP-F | GAGCTCATGAACAACCACACGTGTAAGAT | 3368-4480 |
| LsPLV-CP-R | AAGCTTCACGGACTGTGAAGAGAGTCG | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, J.; Zhang, J.; Zhang, L.; Piao, J.; Wang, H.; Xie, Y.; Li, S. Genomic Characterization of Laodelphax striatellus Permutotetra-like Virus and Self-Cleavage Function of Viral Capsid Protein. Microbiol. Res. 2025, 16, 9. https://doi.org/10.3390/microbiolres16010009
Piao J, Zhang J, Zhang L, Piao J, Wang H, Xie Y, Li S. Genomic Characterization of Laodelphax striatellus Permutotetra-like Virus and Self-Cleavage Function of Viral Capsid Protein. Microbiology Research. 2025; 16(1):9. https://doi.org/10.3390/microbiolres16010009
Chicago/Turabian StylePiao, Jun, Jiarui Zhang, Lujie Zhang, Jingai Piao, Haitao Wang, Yilin Xie, and Shuo Li. 2025. "Genomic Characterization of Laodelphax striatellus Permutotetra-like Virus and Self-Cleavage Function of Viral Capsid Protein" Microbiology Research 16, no. 1: 9. https://doi.org/10.3390/microbiolres16010009
APA StylePiao, J., Zhang, J., Zhang, L., Piao, J., Wang, H., Xie, Y., & Li, S. (2025). Genomic Characterization of Laodelphax striatellus Permutotetra-like Virus and Self-Cleavage Function of Viral Capsid Protein. Microbiology Research, 16(1), 9. https://doi.org/10.3390/microbiolres16010009






