Blood Parasites (Haemosporida, Trypanosomatida) in Culex pipiens: A Study and Review of Hibernating and Active Mosquitoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Mosquitoes
2.2. Mosquito Identification Dissection and Microscopic Examination of Samples
2.3. DNA Extraction, PCR and Sequencing
2.4. Analysis of Sequences and Statistics
3. Results
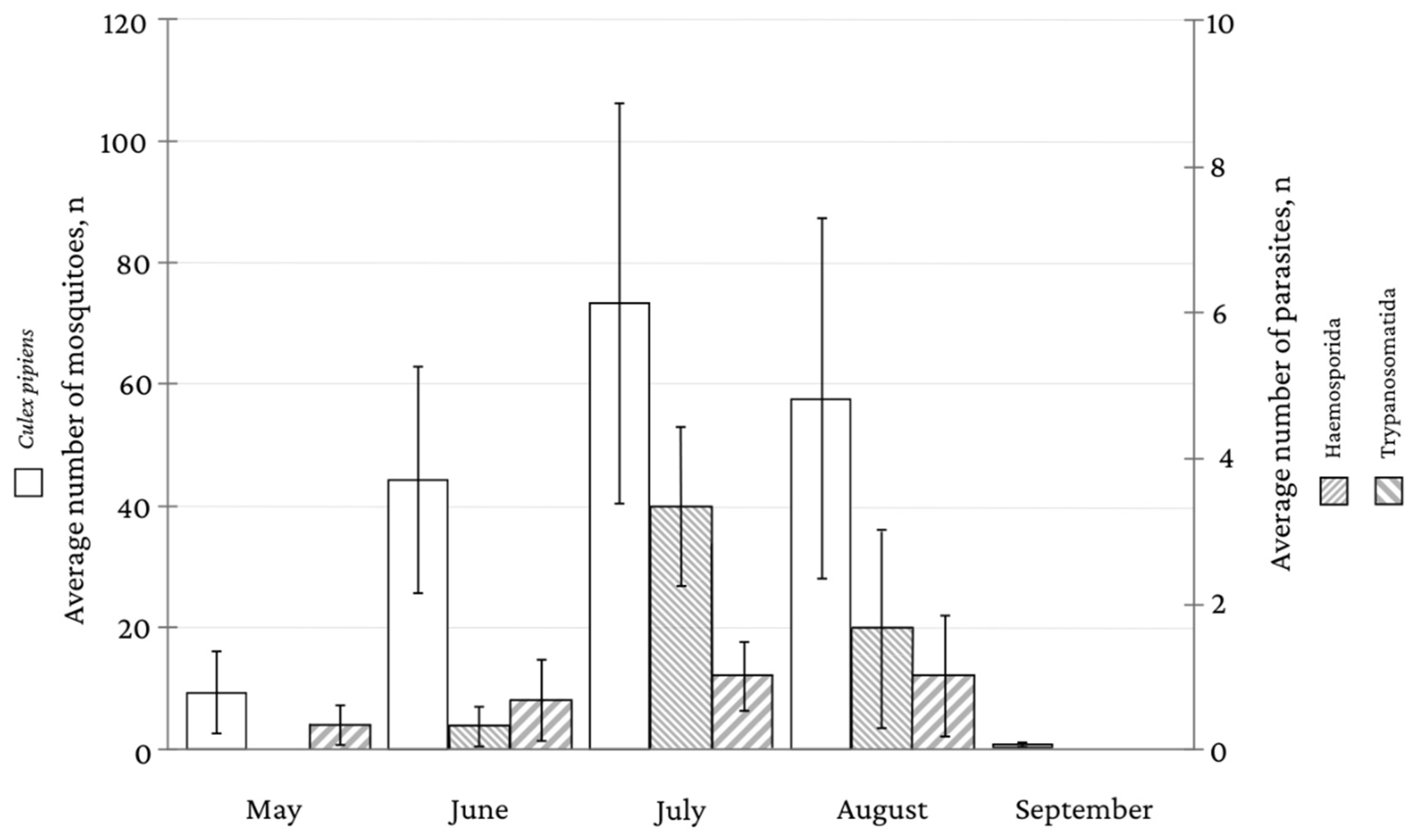
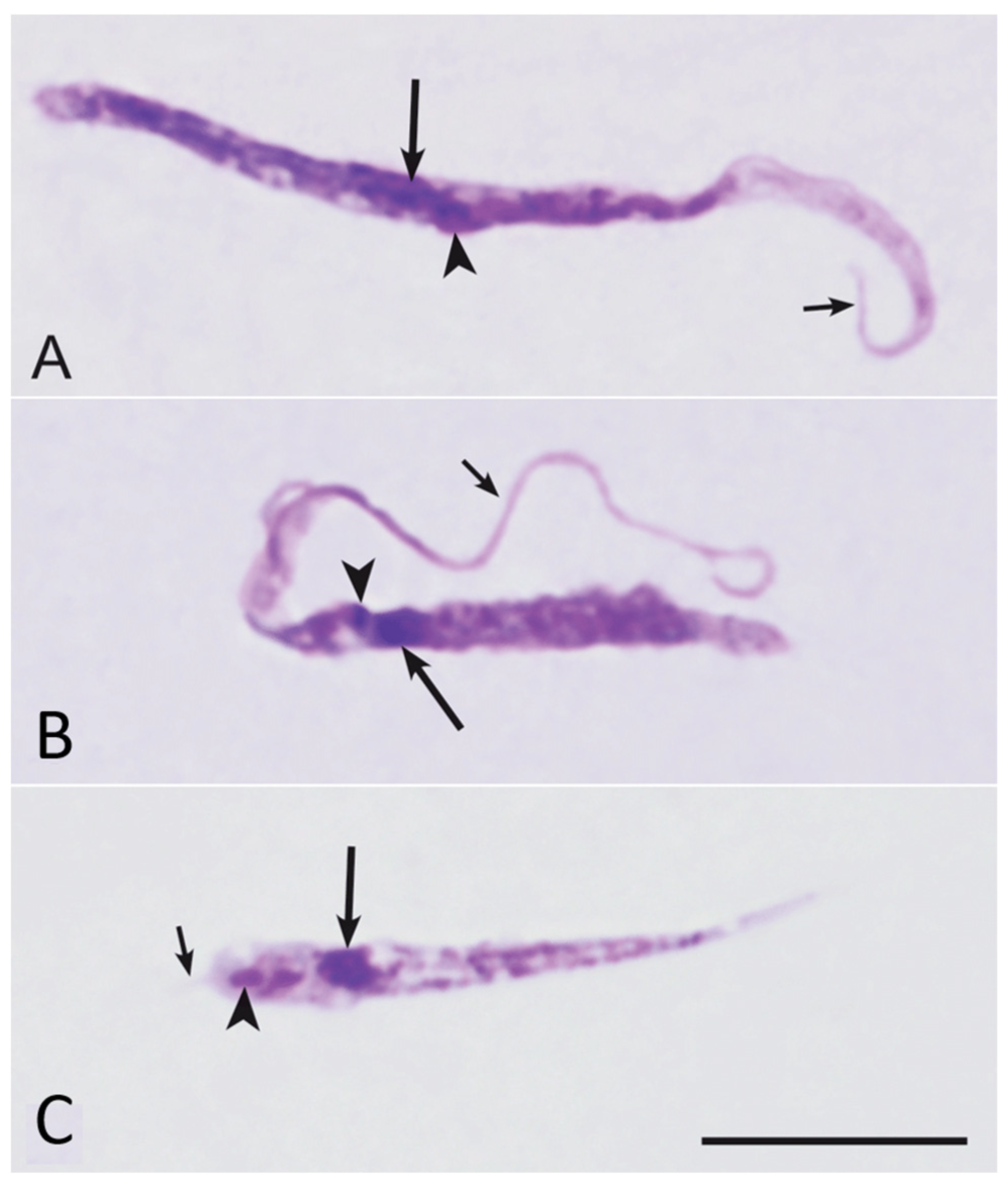
3.1. Infections with Trypanosomatida Parasites
3.2. Infections with Haemosporidian Parasites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manson, P. On the development of Filaria sanguinis hominis, and on the mosquito considered as a nurse. J. Linn. Soc. Lond. Zool. 1878, 14, 304–311. [Google Scholar] [CrossRef]
- Cox, F.E. History of the Discovery of the Malaria parasites and Their Vectors. Parasit. Vectors 2010, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Alarcon, D.; Palinauskas, V.; Schaefer, H.M. Diptera Vectors of Avian Haemosporidian Parasites: Untangling Parasite Life Cycles and Their Taxonomy. Biol. Rev. 2012, 87, 928–964. [Google Scholar] [CrossRef] [PubMed]
- Fialová, M.; Santolíková, A.; Brotánková, A.; Brzoňová, J.; Svobodová, M. Complete Life Cycle of Trypanosoma thomasbancrofti, an Avian Trypanosome Transmitted by Culicine Mosquitoes. Microorganisms 2021, 9, 2101. [Google Scholar] [CrossRef] [PubMed]
- Votýpka, J.; Szabová, J.; Rádrová, J.; Zídková, L.; Svobodová, M. Trypanosoma culicavium sp. nov., an Avian Trypanosome Transmitted by Culex Mosquitoes. Int. J. Syst. Evol. Microbiol. 2012, 62, 745–754. [Google Scholar] [CrossRef]
- Brugman, V.A.; Hernández-Triana, L.M.; Medlock, J.M.; Fooks, A.R.; Carpenter, S.; Johnson, N. The Role of Culex pipiens L. (Diptera: Culicidae) in Virus Transmission in Europe. Int. J. Environ. Res. Public Health 2018, 15, 389. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; PÉrez-Tris, J. MalAvi: A Public Database of Malaria Parasites and Related Haemosporidians in Avian Hosts Based on Mitochondrial Cytochrome b Lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Nicolescu, G.A. General Characterisation of the Mosquito Fauna (Diptera: Culicidae) in the Epidemic Area for West Nile Virus in the South of Romania. Eur. Mosq. Bull. 1998, 2, 13–18. [Google Scholar]
- Lundstrom, J.O. Vector Competence of Western European Mosquitoes for Arboviruses: A Review of Field and Experimental Studies. J. Vector. Ecol. 1994, 19, 23–36. [Google Scholar]
- Engel, D.; Jöst, H.; Wink, M.; Börstler, J.; Bosch, S.; Garigliany, M.M.; Jöst, A.; Czajka, C.; Lühken, R.; Ziegler, U. Reconstruction of the Evolutionary History and Dispersal of Usutu Virus, a Neglected Emerging Arbovirus in Europe and Africa. mBio 2016, 7, 10–1128. [Google Scholar] [CrossRef]
- Becker, N.; Petrić, D.; Boase, C.; Lane, J.; Zgomba, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Šlapeta, J.; Morin-Adeline, V.; Thompson, P.; McDonell, D.; Shiels, M.; Gilchrist, K.; Votýpka, J.; Vogelnest, L. Intercontinental Distribution of a New Trypanosome Species from Australian Endemic Regent Honeyeater (Anthochaera phrygia). Parasitology 2016, 143, 1012–1025. [Google Scholar] [CrossRef]
- Shaikevich, E.; Bogacheva, A.; Ganushkina, L. Dirofilaria and Wolbachia in Mosquitoes (Diptera: Culicidae) in Central European Russia and on the Black Sea Coast. Parasite 2019, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Čabanová, V.; Miterpáková, M.; Valentová, D.; Blažejová, H.; Rudolf, I.; Stloukal, E.; Hurníková, Z.; Dzidová, M. Urbanization Impact on Mosquito Community and the Transmission Potential of Filarial Infection in Central Europe. Parasit. Vectors 2018, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780874216561. [Google Scholar]
- Chagas, C.R.F.; Binkienė, R.; Ilgūnas, M.; Iezhova, T.; Valkiūnas, G. The Buffy Coat Method: A Tool for Detection of Blood Parasites without Staining Procedures. Parasit. Vectors 2020, 13, 104. [Google Scholar] [CrossRef]
- Becker, N.; Jöst, A.; Weitzel, T. The Culex pipiens Complex in Europe. J. Am. Mosq. Control Assoc. 2012, 28, 53–67. [Google Scholar] [CrossRef]
- Byrne, K.; Nichols, R.A. Culex pipiens in London Underground Tunnels: Differentiation between Surface and Subterranean Populations. Heredity 1999, 82, 7–15. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Briegel, H. Inability of Diapausing Culex pipiens (Diptera: Culicidae) to Use Blood for Producing Lipid Reserves for Overwinter Survival. J. Med. Entomol. 1989, 26, 318–326. [Google Scholar] [CrossRef]
- Eldridge, B.F.; Baileyl, C.L. Experimental Hibernation Studies in Culex pipiens (Diptera: Culicidae): Reactivation of Ovarian Development and Blood-Feeding in Prehibernating Females. J. Med. Entomol. 1979, 15, 462–467. [Google Scholar] [CrossRef]
- Bailey, C.L.; Faran, M.E.; Gargan, T.P.; Hayes, D.E. Winter Survival of Blood-Fed and Nonblood-Fed Culex pipiens L. Am. J. Trop. Med. Hyg. 1982, 31, 1054–1061. [Google Scholar] [CrossRef]
- Nelms, B.M.; MacEdo, P.A.; Kothera, L.; Savage, H.M.; Reisen, W.K. Overwintering Biology of Culex (Diptera: Culicidae) Mosquitoes in the Sacramento Valley of California. J. Med. Entomol. 2013, 50, 773–790. [Google Scholar] [CrossRef]
- Farajollahi, A.; Crans, W.J.; Bryant, P.; Wolf, B.; Burkhalter, K.L.; Godsey, M.S.; Aspen, S.E.; Nasci, R.S. Detection of West Nile Viral RNA from an Overwintering Pool of Culex pipens pipiens (Diptera: Culicidae) in New Jersey. J. Med. Entomol. 2003, 42, 490–494. [Google Scholar] [CrossRef]
- Valavičiūtė-Pocienė, K.; Kalinauskaitė, G.; Chagas, C.R.F.; Bernotienė, R. Avian Haemosporidian Parasites from Wild-Caught Mosquitoes with New Evidence on Vectors of Plasmodium matutinum. Acta. Trop. 2024, 256, 107260. [Google Scholar] [CrossRef]
- Gunay, F.; Picard, M.; Robert, V. MosKeyTool, an Interactive Identification Key for Mosquitoes of Euro-Mediterranean, Version 2.1; Last Update: 1 August 2018. 2018. Available online: https://www.medilabsecure.com/entomology-tools-0/moskeytool (accessed on 10 March 2024). (In English).
- Žiegytė, R.; Markovets, M.Y.; Bernotienė, R.; Mukhin, A.; Iezhova, T.A.; Valkiūnas, G.; Palinauskas, V. The Widespread Biting Midge Culicoides impunctatus (Ceratopogonidae) Is Susceptible to Infection with Numerous Haemoproteus (Haemoproteidae) Species. Parasit. Vectors 2017, 10, 397. [Google Scholar] [CrossRef]
- Hesson, J.C.; LundströM, J.O.; Halvarsson, P.; Erixon, P.; Collado, A. A Sensitive and Reliable Restriction Enzyme Assay to Distinguish between the Mosquitoes Culex torrentium and Culex pipiens. Med. Vet. Entomol. 2010, 24, 142–149. [Google Scholar] [CrossRef]
- Sambrook, J.; Green, M.R. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2012; Volume 1, ISBN 9781936113415. [Google Scholar]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Ostman, O.; Hansson, B.; Westerdahl, H.; Pinheiro, R.T. Host Specificity in Avian Blood Parasites: A Study of Plasmodium and Haemoproteus Mitochondrial DNA Amplified from Birds. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1583–1589. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New Pcr Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus From Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Iezhova, T.A.; Marzec, T.; Valkiūnas, G. Trypanosoma naviformis Sp. Nov. (Kinetoplastidae: Trypanosomatidae) from Widespread African Songbirds, the Olive Sunbird (Cyanomitra olivacea) and Yellow-Whiskered Greenbul (Andropadus latirostris). Zootaxa 2015, 4034, 342–350. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A.; Carlson, J.S.; Sehgal, R.N.M. Two New Trypanosoma Species from African Birds, with Notes on the Taxonomy of Avian Trypanosomes. J. Parasitol. 2011, 97, 924–930. [Google Scholar] [CrossRef]
- Zídková, L.; Cepicka, I.; Szabová, J.; Svobodová, M. Biodiversity of Avian Trypanosomes. Infect. Genet. Evol. 2012, 12, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Schoener, E.R.; Harl, J.; Himmel, T.; Fragner, K.; Weissenböck, H.; Fuehrer, H.P. Protozoan Parasites in Culex pipiens Mosquitoes in Vienna. Parasitol. Res. 2019, 118, 1261–1269. [Google Scholar] [CrossRef]
- Schoener, E.; Uebleis, S.S.; Cuk, C.; Nawratil, M.; Obwaller, A.G.; Zechmeister, T.; Lebl, K.; Rádrová, J.; Zittra, C.; Votýpka, J. Trypanosomatid Parasites in Austrian Mosquitoes. PLoS ONE 2018, 13, e0196052. [Google Scholar] [CrossRef]
- Svobodová, M.; Volf, P.; Votýpka, J. Trypanosomatids in Ornithophilic Bloodsucking Diptera. Med. Vet. Entomol. 2015, 29, 444–447. [Google Scholar] [CrossRef]
- Brotánková, A.; Fialová, M.; Čepička, I.; Brzoňová, J.; Svobodová, M. Trypanosomes of the Trypanosoma theileri Group: Phylogeny and New Potential Vectors. Microorganisms 2022, 10, 294. [Google Scholar] [CrossRef]
- Van Dyken, M.; Bolling, B.G.; Moore, C.G.; Blair, C.D.; Beaty, B.J.; Black IV, W.C.; Foy, B.D. Molecular Evidence for Trypanosomatids in Culex Mosquitoes Collected during a West Nile Virus Survey. Int. J. Parasitol. 2006, 36, 1015–1023. [Google Scholar] [CrossRef]
- Garcia, H.A.; Blanco, P.A.; Rodrigues, A.C.; Rodrigues, C.M.F.; Takata, C.S.A.; Campaner, M.; Camargo, E.P.; Teixeira, M.M.G. Pan-American Trypanosoma (Megatrypanum) trinaperronei n. sp. In the White-Tailed Deer Odocoileus virginianus Zimmermann and Its Deer Ked Lipoptena mazamae Rondani, 1878: Morphological, Developmental and Phylogeographical Characterisation. Parasit. Vectors 2020, 13, 308. [Google Scholar] [CrossRef]
- Böse, R.; Friedhoff, K.T.; Olbrich, S. Transmission of Megatrypanum Trypanosomes to Cervus dama by Tabanidae. J. Protozool. 1987, 34, 110–113. [Google Scholar] [CrossRef]
- Mazza, S.; Romana, C.; Fiora, A. Algunos Hemoparásitos de Mamíferos Del Norte. VII Reun. Soc. Argent. Patol. Reg. Norte 1932, 2, 990–997. [Google Scholar]
- Kingston, N.; Morton, J.K. Trypanosoma cervi sp. n. from Elk (Cervus canadensis) in Wyoming. J. Parasitol. 1975, 61, 17–23. [Google Scholar] [CrossRef]
- Kingston, N.; Bobek, B.; Perzanowski, K.; Wita, I.; Makis, L. Description of Trypanosoma (Megatrypanum) stefanskii sp. n. from Roe Deer (Capreolus capreolus) in Poland. J. Helminthol. Soc. Wash. 1992, 59, 89–95. [Google Scholar]
- Garcia, H.A.; Rodrigues, A.C.; Martinkovic, F.; Minervino, A.H.H.; Campaner, M.; Nunes, V.L.B.; Paiva, F.; Hamilton, P.B.; Teixeira, M.M.G. Multilocus Phylogeographical Analysis of Trypanosoma (Megatrypanum) Genotypes from Sympatric Cattle and Water Buffalo Populations Supports Evolutionary Host Constraint and Close Phylogenetic Relationships with Genotypes Found in Other Ruminants. Int. J. Parasitol. 2011, 41, 1385–1396. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Paiva, F.; Campaner, M.; Stevens, J.R.; Noyes, H.A.; Teixeira, M.M.G. Phylogeny of Trypanosoma (Megatrypanum) theileri and Related Trypanosomes Reveals Lineages of Isolates Associated with Artiodactyl Hosts Diverging on SSU and ITS Ribosomal Sequences. Parasitology 2006, 132, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.O.; Kostygov, A.Y.; Yurchenko, V. Development of Monoxenous Trypanosomatids and Phytomonads in Insects. Trends Parasitol. 2021, 37, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Votýpka, J.; Petrželková, K.J.; Brzoňová, J.; Jirků, M.; Modrý, D.; Lukeš, J. How Monoxenous Trypanosomatids Revealed Hidden Feeding Habits of Their Tsetse Fly Hosts. Folia Parasitol. 2021, 68, 1–6. [Google Scholar] [CrossRef]
- Boucinha, C.; Andrade-Neto, V.V.; Ennes-Vidal, V.; Branquinha, M.H.; dos Santos, A.L.S.; Torres-Santos, E.C.; d’Avila-Levy, C.M. A Stroll Through the History of Monoxenous Trypanosomatids Infection in Vertebrate Hosts. Front. Cell Infect. Microbiol. 2022, 12, 804707. [Google Scholar] [CrossRef] [PubMed]
- Truc, P.; Büscher, P.; Cuny, G.; Gonzatti, M.I.; Jannin, J.; Joshi, P.; Juyal, P.; Lun, Z.R.; Mattioli, R.; Pays, E.; et al. Atypical Human Infections by Animal Trypanosomes. PLoS Negl. Trop. Dis. 2013, 7, e2256. [Google Scholar] [CrossRef] [PubMed]
- Kostygov, A.Y.; Malysheva, M.N.; Ganyukova, A.I.; Razygraev, A.V.; Drachko, D.O.; Yurchenko, V.; Agasoi, V.V.; Frolov, A.O. The Roles of Mosquitoes in the Circulation of Monoxenous Trypanosomatids in Temperate Climates. Pathogens 2022, 11, 1326. [Google Scholar] [CrossRef]
- Jansen, S.; Heitmann, A.; Lühken, R.; Leggewie, M.; Helms, M.; Badusche, M.; Rossini, G.; Schmidt-Chanasit, J.; Tannich, E. Culex torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe. Viruses 2019, 11, 492. [Google Scholar] [CrossRef]
- Vanderplank, F.L. Seasonal and Annual Variation in the Incidence of Trypanosomiasis in Game. Ann. Trop. Med. Parasitol. 1947, 41, 365–374. [Google Scholar] [CrossRef]
- Pori, T.; Ndlovu, M.; Markus, M.B. Influence of Season and Other Factors on Avian Trypanosoma spp. and Microfilarial Prevalence in the Lowveld, South Africa. S. Afr. J. Sci. 2023, 119, 1–6. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Kazlauskienė, R.; Bernotienė, R.; Palinauskas, V.; Iezhova, T.A. Abortive Long-Lasting Sporogony of Two Haemoproteus Species (Haemosporida, Haemoproteidae) in the Mosquito Ochlerotatus cantans, with Perspectives on Haemosporidian Vector Research. Parasitol. Res. 2013, 112, 2159–2169. [Google Scholar] [CrossRef]
- Nourani, L.; Zakeri, S.; Dinparast Djadid, N. Dynamics of Prevalence and Distribution Pattern of Avian Plasmodium Species and Its Vectors in Diverse Zoogeographical Areas—A Review. Infect. Genet. Evol. 2020, 81, 104244. [Google Scholar] [CrossRef]
- Ejiri, H.; Sato, Y.; Sawai, R.; Sasaki, E.; Matsumoto, R.; Ueda, M.; Higa, Y.; Tsuda, Y.; Omori, S.; Murata, K.; et al. Prevalence of Avian Malaria Parasite in Mosquitoes Collected at a Zoological Garden in Japan. Parasitol. Res. 2009, 105, 629–633. [Google Scholar] [CrossRef]
- Shirotani, A.; Shibata, A.; Ejiri, H.; Sato, Y.; Tsuda, Y.; Hatakeyama, Y.; Iwano, H.; Murata, K.; Yukawa, M. Detection of Avian Malaria DNA from Mosquitoes in Kanagawa in Japan. J. Jpn. Vet. Med. Assoc. 2009, 62, 73–79. [Google Scholar] [CrossRef]
- Kim, K.; Tsuda, Y.; Yamada, A. Bloodmeal Identification and Detection of Avian Malaria Parasite from Mosquitoes (Diptera: Culicidae) Inhabiting Coastal Areas of Tokyo Bay, Japan. J. Med. Entomol. 2009, 46, 1230–1234. [Google Scholar] [CrossRef]
- Inumaru, M.; Yamada, A.; Shimizu, M.; Ono, A.; Horinouchi, M.; Shimamoto, T.; Tsuda, Y.; Murata, K.; Sato, Y. Vector Incrimination and Transmission of Avian Malaria at an Aquarium in Japan: Mismatch in Parasite Composition between Mosquitoes and Penguins. Malar. J. 2021, 20, 136. [Google Scholar] [CrossRef]
- Kim, K.S.; Tsuda, Y. Seasonal Changes in the Feeding Pattern of Culex pipiens pallens Govern the Transmission Dynamics of Multiple Lineages of Avian Malaria Parasites in Japanese Wild Bird Community. Mol. Ecol. 2010, 19, 5545–5554. [Google Scholar] [CrossRef]
- Ejiri, H.; Sato, Y.; Kim, K.S.; Hara, T.; Tsuda, Y.; Imura, T.; Murata, K.; Yukawa, M. Entomological Study on Transmission of Avian Malaria Parasites in a Zoological Garden in Japan: Bloodmeal Identification and Detection of Avian Malaria Parasite DNA from Blood-Fed Mosquitoes. J. Med. Entomol. 2011, 48, 600–607. [Google Scholar] [CrossRef]
- Odagawa, T.; Inumaru, M.; Sato, Y.; Murata, K.; Higa, Y.; Tsuda, Y.A. Long-Term Field Study on Mosquito Vectors of Avian Malaria Parasites in Japan. J. Vet. Med. Sci. 2022, 84, 1391–1398. [Google Scholar] [CrossRef]
- Kim, K.S.; Tsuda, Y. Avian Plasmodium Lineages Found in Spot Surveys of Mosquitoes from 2007 to 2010 at Sakata Wetland, Japan: Do Dominant Lineages Persist for Multiple Years? Mol. Ecol. 2012, 21, 5374–5385. [Google Scholar] [CrossRef]
- Glaizot, O.; Fumagalli, L.; Iritano, K.; Lalubin, F.; van Rooyen, J.; Christe, P. High Prevalence and Lineage Diversity of Avian Malaria in Wild Populations of Great Tits (Parus major) and Mosquitoes (Culex pipiens). PLoS ONE 2012, 7, e34964. [Google Scholar] [CrossRef]
- Lalubin, F.; Delédevant, A.; Glaizot, O.; Christe, P. Temporal Changes in Mosquito Abundance (Culex pipiens), Avian Malaria Prevalence and Lineage Composition. Parasit. Vectors 2013, 6, 307. [Google Scholar] [CrossRef]
- Kimura, M.; Darbro, J.M.; Harrington, L.C. Avian Malaria Parasites Share Congeneric Mosquito Vectors. J. Parasitol. 2010, 96, 144–151. [Google Scholar] [CrossRef]
- Huff, C.G. Studies on the Infectivity of Plasmodia of Birds for Mosquitoes, with Special Reference to the Problem of Immunity in the Mosquito. Am. J. Hyg. 1927, 7, 706–734. [Google Scholar] [CrossRef]
- Herman, G.M. Mosquito Transmission of Avian Malaria Parasites (Plasmodium circumflexum and P. cathemerium). Am. J. Hyg. 1938, 27, 345–350. [Google Scholar] [CrossRef]
- Zélé, F.; Vézilier, J.; L’Ambert, G.; Nicot, A.; Gandon, S.; Rivero, A.; Duron, O. Dynamics of Prevalence and Diversity of Avian Malaria Infections in Wild Culex pipiens Mosquitoes: The Effects of Wolbachia, Filarial Nematodes and Insecticide Resistance. Parasit. Vectors 2014, 7, 437. [Google Scholar] [CrossRef]
- Schoener, E.; Uebleis, S.S.; Butter, J.; Nawratil, M.; Cuk, C.; Flechl, E.; Kothmayer, M.; Obwaller, A.G.; Zechmeister, T.; Rubel, F.; et al. Avian Plasmodium in Eastern Austrian Mosquitoes. Malar. J. 2017, 16, 389. [Google Scholar] [CrossRef]
- Raffaele, G. Un Ceppo Italiano Di Plasmodium elongatum. Riv. Malariol. 1934, 13, 3–8. [Google Scholar]
- Micks, D.W. Investigations on the Mosquito Transmission of Plasmodium elongatum Huff, 1930. J. Natl. Malar. Soc. 1949, 8, 206–218. [Google Scholar]
- Garnham, P.C.C. Malaria Parasites and Other Haemosporidia; Blackwell Scientific Publications: Oxford, UK, 1966. [Google Scholar]
- Corradetti, A.; Garnham, P.C.C.; Laird, M. New Classification of the Avian Malaria Parasites. Parassitologia 1963, 5, 1–4. [Google Scholar]
- Corradetti, A.; Verolini, F.; Neri, L. Plasmodium (Haemamoeba) giovannolai n. Sp. Parassita Di Turdus merula. Parassitologia 1963, 5, 11–18. [Google Scholar]
- Akiba, K. Studies on Avian Malaria II. On the Species of Plasmodium from Chicken in Japan (P. japonicum Ishiguro, 1957), a Synonym for P. juxtanucleare Versiani and Gomes, 1941. Jpn. J. Vet. Sci. 1959, 21, 18. [Google Scholar]
- Christensen, B.M.; Barnes, H.J.; Rowley, W.A. Vertebrate Host Specificity and Experimental Vectors of Plasmodium (Novyella) kempi Sp. n. from the Eastern Wild Turkey in Iowa. J. Wildl. Dis. 1983, 19, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Coggeshall, L.T. The Infection of Anopheles quadrimaculatus with a Monkey Malaria Parasite, Plasmodium cynomolgi and with an Avian Parasite, Plasmodium lophurae. J. Parasitol. 1940, 26, S44–S45. [Google Scholar] [CrossRef]
- Martinez-de la Puente, J.; Muñoz, J.; Capelli, G.; Montarsi, F.; Soriguer, R.; Arnoldi, D.; Rizzoli, A.; Figuerola, J. Avian Malaria Parasites in the Last Supper: Identifying Encounters between Parasites and the Invasive Asian Mosquito Tiger and Native Mosquito Species in Italy. Malar. J. 2015, 14, 32. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Muñoz, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Avian Plasmodium in Culex and Ochlerotatus Mosquitoes from Southern Spain: Effects of Season and Host-Feeding Source on Parasite Dynamics. PLoS ONE 2013, 8, e66237. [Google Scholar] [CrossRef]
- Huff, C.G.A. New Variety of Plasmodium relictum from the Robin. J. Parasitol. 1937, 23, 400–404. [Google Scholar] [CrossRef]
- Manwell, R.D. Life-Cycle Of Plasmodium relictum var. matutinum. Am. J. Trop. Med. Hyg. 1940, 20, 859–866. [Google Scholar] [CrossRef]
- Manwell, R.D. Failure of Aedes aegypti and Culex pipiens to Transmit Plasmodium vaughani. J. Parasitol. 1947, 33, 167–169. [Google Scholar] [CrossRef]
- Kim, K.; Tsuda, Y. Sporogony and Sporozoite Rates of Avian Malaria Parasites in Wild Culex pipiens pallens and C. inatomii in Japan. Parasit. Vectors 2015, 8, 633. [Google Scholar] [CrossRef]
- Schmid, S.; Dinkel, A.; Mackenstedt, U.; Tantely, M.L.; Randrianambinintsoa, F.J.; Boyer, S.; Woog, F. Avian Malaria on Madagascar: Bird Hosts and Putative Vector Mosquitoes of Different Plasmodium Lineages. Parasit. Vectors 2017, 10, 6. [Google Scholar] [CrossRef]
- Kazlauskienė, R.; Bernotienė, R.; Palinauskas, V.; Iezhova, T.A.; Valkiūnas, G. Plasmodium relictum (Lineages pSGS1 and pGRW11): Complete Synchronous Sporogony in Mosquitoes Culex pipiens pipiens. Exp. Parasitol. 2013, 133, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Ionicǎ, A.M.; Zittra, C.; Wimmer, V.; Leitner, N.; Votýpka, J.; Modrý, D.; Mihalca, A.D.; Fuehrer, H.P. Mosquitoes in the Danube Delta: Searching for Vectors of Filarioid Helminths and Avian Malaria. Parasit. Vectors 2017, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Ventim, R.; Ramos, J.A.; Osório, H.; Lopes, R.J.; Pérez-Tris, J.; Mendes, L. Avian Malaria Infections in Western European Mosquitoes. Parasitol. Res. 2012, 111, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Inci, A.; Yildirim, A.; Njabo, K.Y.; Duzlu, O.; Biskin, Z.; Ciloglu, A. Detection and Molecular Characterization of Avian Plasmodium from Mosquitoes in Central Turkey. Vet. Parasitol. 2012, 188, 179–184. [Google Scholar] [CrossRef]
- Ruge, R. Untersuchungen über das Deutsche Proteosoma. Zentralbl. Bakteriol. Parasitenkd. Infekt. Hyg. 1901, 29, 187–191. [Google Scholar]
- Neumann, R.O. Die übertragung von Plasmodium praecox auf Kanarievögel Durch Stegmyia fasciata und die Entwicklung der Parasiten im Magen und der Speicheldr üsen Dieser Stechmucke. Arch. Protistenkd. 1908, 13, 23–69. [Google Scholar]
- Sergent, E.D.; Sergent, E.T. Etudes Sur Les Hematozoaires d’oiseaux. Ann. Inst. Pasteur 1907, 21, 251–280. [Google Scholar]
- Tate, P.; Vincent, M. The Susceptibility of Autogenous and Anautogenous Races of Culex pipiens to Infection with Avian Malaria (Plasmodium relictum). Parasitology 1934, 26, 512–522. [Google Scholar] [CrossRef]
- Hunninen, A.V. Comparative Susceptibility of Four Anopheline Mosquitoes to Plasmodium relictum. J. Natl. Malar. Soc. 1951, 10, 216–223. [Google Scholar]
- Hunninen, A.V. Comparative Development of Plasmodium relictum Oocysts in Anopheles quadrimaculatus, A. albimanus, and Culex pipiens. J. Parasitol. 1953, 39, 28–32. [Google Scholar] [CrossRef]
- Ejiri, H.; Sato, Y.; Kim, K.S.; Tsuda, Y.; Murata, K.; Saito, K.; Watanabe, Y.; Shimura, Y.; Yukawa, M. Blood Meal Identification and Prevalence of Avian Malaria Parasite in Mosquitoes Collected at Kushiro Wetland, A Subarctic Zone of Japan. J. Med. Entomol. 2011, 48, 904–908. [Google Scholar] [CrossRef]
- Raffaele, G. II Plasmodium Della Civetta (Athene noctua). Riv. Malariol. 1932, 9, 3–12. [Google Scholar]
- Synek, P.; Munclinger, P.; Albrecht, T.; Votýpka, J. Avian Haemosporidians in Haematophagous Insects in the Czech Republic. Parasitol. Res. 2013, 112, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, A.; Scanga, M. Plasmodium (Novyella) vaughani Subsp. merulae, n Subsp., Parassita Di Turdus merula, Con Descrizione Del Ciclo Pre-Eritrocitivo. Parassitologia 1972, 14, 85–93. [Google Scholar]
- Köchling, K.; Schaub, G.A.; Werner, D.; Kampen, H. Avian Plasmodium spp. and Haemoproteus spp. Parasites in Mosquitoes in Germany. Parasit. Vectors 2023, 16, 369. [Google Scholar] [CrossRef]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Marm Kilpatrick, A. “Bird Biting” Mosquitoes and Human Disease: A Review of the Role of Culex pipiens Complex Mosquitoes in Epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef]
- Hamer, G.L.; Kitron, U.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Walker, E.D. Culex pipiens (Diptera: Culicidae): A Bridge Vector of West Nile Virus to Humans. J. Med. Entomol. 2008, 45, 125–128. [Google Scholar] [CrossRef]

| Collection Date | Collection Site | Microscopy Positive | GenBank No | Trypanosomatid Species | Vertebrate Host |
|---|---|---|---|---|---|
| 25 May 2022. | Verkiai | − | PP946099 | Trypanosoma culicavium | Birds |
| 29 June 2022. | Kairėnai | + | PP946101 | T. culicavium | Birds |
| 29 June 2022. | Kairėnai | − | PP946100 | T. culicavium | Birds |
| 14 July 2022. | Kairėnai | − | PP946102 | T. theileri | Mammals |
| 3 August 2022. | Kairėnai | + | PP946104 | T. trinaperronei | Mammals |
| 3 August 2022. | Kairėnai | − | PP946105 | T. theileri | Mammals |
| 30 August 2022. | Kairėnai | + | PP946107 | T. culicavium | Birds |
| 18 July 2023. | Kairėnai | + | PP946103 | T. culicavium | Birds |
| 18 July 2023. | Kairėnai | − | PP946106 | T. theileri | Mammals |
| 7 December 2023. | Verkiai 1 | + | PP948731 | Monoxenous trypanosomatid | No vertebrate host |
| 8 December 2023. | Vilnius | − | PP948732 | Monoxenous trypanosomatid | No vertebrate host |
| Trypanosomatid Species | Isolate | Origin of Isolate | EI | WM | Country, Source |
|---|---|---|---|---|---|
| Trypanosoma thomasbancrofti | CUL15, CUL98 | Culex pipiens | + | Czech Republic [4,5] | |
| OF19 | Ornithomya fringilline * | + | Czech Republic [4] | ||
| PAS343 | Phylloscopus sibilatrix * | + | Czech Republic [4] | ||
| T. theileri | − | Cx. pipiens | + | Czech Republic [4,37] | |
| T. culicavium | CUL1, CUL6, CUL24, CUL28, CUL31 | Cx. pipiens | + | Czech Republic [5] | |
| − | Cx. pipiens s.l./torrentium, Cx. modestus, Cx. spp | + | Austria [35] | ||
| Trypanosoma sp. | CUL5, CUL2 | Cx. pipiens | + | Czech Republic [33] | |
| T. avium | Cx. pipiens s.l./torrentium | + | Austria [35] | ||
| Cx. pipiens, Cx. tarsalis | + | USA [38] |
| Plasmodium Species | Lineages | EI | WM | PCR | Country/References |
|---|---|---|---|---|---|
| P. cathemerium | pPADOM02 | + | Japan [56,57,58,59,60,61,62,63], Switzerland [64,65], USA [66] | ||
| unknown lineage | USA [67,68] | ||||
| P. circumflexum | pTURDUS1 | + | Switzerland [64] | ||
| P. durae | unknown lineage | Africa [15] | |||
| P. elongatum | pGRW06 | + | France [69] Austria [34,70] | ||
| unknown lineage | + | + | USA [67], Italy [71,72] | ||
| P. gallinaceum | pGALLUS01 | + | Japan [57,58,63] | ||
| P. garnhami | unknown lineage | + | Egypt [73] | ||
| P. giovannolai | unknown lineage | + | Italy [74,75] | ||
| P. homonucleophilum | pSW2 | + | Lithuania [24], Switzerland [64] | ||
| P. juxtanucleare | pGALLUS02 | + | Japan [63] | ||
| unknown lineage | + | Japan [76] | |||
| P. kempi | unknown lineage | + | USA [77] | ||
| P. lophurae | unknown lineage | USA [78] | |||
| P. matutinum | pLINN1 | + | France [69], USA [66], Italy [79], Spain [80], Austria [34] | ||
| + | + | Lithuania [24] | |||
| unknown lineage | USA [81,82,83] | ||||
| P. relictum | pGRW04 | + | Japan [62,63,84], Madagascar [85] | ||
| pGRW11 | + | Italy [79], Japan [58,60,84], Switzerland [64,65], France [69] | |||
| + | Lithuania[86] | ||||
| pSGS1 | + | Romania [87], Japan [58,60,61,62,84], Austria [34,70], France [69], Portugal [88], Italy [79], Switzerland [64,65], Spain [80], Turkey [89] | |||
| + | Lithuania [86] | ||||
| unknown lineage | + | Romania [87] | |||
| + | Germany [90,91], Algeria [92], USA [93], Columbia [94,95] | ||||
| P. rouxi | unknown lineage | + | USA [81,83] | ||
| Plasmodium sp. | pAFTRU5 | + | Switzerland [65], Italy [79] | ||
| pCOLL1 | + | France [69], Switzerland [65], Spain [80] | |||
| pCXPIP01, CXPIP02, pCXPIP03, pCXPIP04, pCXPIP05, pCXPIP06, pCXPIP07 | + | USA [66] | |||
| pCXPIP09 | + | Japan [56,57,58,59,60,61,62,63,84] | |||
| pCXPIP10, pCXPIP11, pCXPIP12, pCXPIP13, pCXPIP14 | + | Japan [58,60,63] | |||
| pCXPIP15 | + | Japan [61] | |||
| pCXPIP20, pCXPIP21, pCXPIP22, pCXPIP23 | + | Turkey [89] | |||
| pCXPIP24, pCXPIP25, pCXPIP26 | + | France [69] | |||
| pCXPIP30 | + | Madagascar [85] | |||
| pCXPIP31 | + | Japan [96] | |||
| pCXPIP32, pCXPIP33 | Italy (Iurescia et al., unpubl) | ||||
| pCXQUI01 | + | Japan [63] | |||
| pDELURB4 | + | Italy [79], France [69], Austria [34] | |||
| pDELURB5, pDONANA03, pDONANA05 | + | Austria [34], France [69] | |||
| pPADOM01 | + | France [69], Switzerland [65] | |||
| pSPHUM05 | + | Japan [59] | |||
| pSPMAG10, pZOCAP03, pSYCON02 | + | Japan [62] | |||
| P. subpraecox | unknown lineage | + | Italy [97] | ||
| P. unalis | pTUMIG03 | + | USA [66] | ||
| P. vaughani | pSYAT05 | + | France [69], USA [66], Lithuania [24], Italy [79], Japan [60], Switzerland [64,65], Turkey [89], Austria [34,70], Czech Republic [98] | ||
| unknown lineage | + | Italy [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valavičiūtė-Pocienė, K.; Kazak, M.; Iezhova, T.; Kalinauskaitė, G.; Bernotienė, R. Blood Parasites (Haemosporida, Trypanosomatida) in Culex pipiens: A Study and Review of Hibernating and Active Mosquitoes. Microbiol. Res. 2024, 15, 2184-2198. https://doi.org/10.3390/microbiolres15040146
Valavičiūtė-Pocienė K, Kazak M, Iezhova T, Kalinauskaitė G, Bernotienė R. Blood Parasites (Haemosporida, Trypanosomatida) in Culex pipiens: A Study and Review of Hibernating and Active Mosquitoes. Microbiology Research. 2024; 15(4):2184-2198. https://doi.org/10.3390/microbiolres15040146
Chicago/Turabian StyleValavičiūtė-Pocienė, Kristina, Margarita Kazak, Tatjana Iezhova, Gabrielė Kalinauskaitė, and Rasa Bernotienė. 2024. "Blood Parasites (Haemosporida, Trypanosomatida) in Culex pipiens: A Study and Review of Hibernating and Active Mosquitoes" Microbiology Research 15, no. 4: 2184-2198. https://doi.org/10.3390/microbiolres15040146
APA StyleValavičiūtė-Pocienė, K., Kazak, M., Iezhova, T., Kalinauskaitė, G., & Bernotienė, R. (2024). Blood Parasites (Haemosporida, Trypanosomatida) in Culex pipiens: A Study and Review of Hibernating and Active Mosquitoes. Microbiology Research, 15(4), 2184-2198. https://doi.org/10.3390/microbiolres15040146







