Abstract
Water scarcity is a major agricultural issue in most arid and semi-arid regions of the world. Alternative water supplies, such as the reuse of wastewater for agricultural irrigation, have been introduced. However, little is known about their impact on the soil and rhizosphere microbiomes that receive irrigation. Therefore, this work evaluates the impact of treated wastewater (TWW) irrigation on the soil and rhizosphere bacterial communities of date palms in Al Madinah, Saudi Arabia. In this study, metagenomic DNA from the rhizosphere of the date palm was sequenced using Illumina high-throughput sequencing. According to the observed OTUs, Chao1 richness estimations, and Shannon diversity values, soils from groundwater-irrigated date palms showed higher microbial diversity than did soils from TWW-irrigated date palms. A total of 569 OTUs were generated; most of them (97.3%) were assigned into 15 different phyla, whereas 2.7% were marked as unclassified. DNA sequence analysis of the WWT-irrigated rhizosphere showed that the most abundant phyla were Firmicutes (43.6%), Bacteroidetes (17.3%), Proteobacteria (15.2%), and Actinobacteria (14.6%), representing more than 90.7% of the total community, while the soil of the rhizosphere irrigated with GW was dominated by Actinobacteria (44.1%), Proteobacteria (23.4%), Firmicutes (15.5%), and Gemmatimonadetes (4.9%). The most frequently observed species in the two soils were also different. The dominant species in TWW-irrigated soil was Planococcus plakortidis, which is prevalent in saline and moderately saline habitats and can play an important ecological role. The GW-irrigated rhizosphere exhibited higher levels of biocontrol bacteria, particularly Nocardioides mesophilus. These results provide a comprehensive understanding and insights into the population dynamics and microbiome of date palm rhizosphere. The findings show that the irrigation water quality has a significant impact on the microbiome composition. Identifying the microbial diversity is the first step toward determining the best way to use TWW in irrigation.
1. Background
The scarcity of freshwater in arid and semi-arid regions, as well as rapid population growth and increased food demands, is an agricultural issue in most such areas, including the Middle East [1]. Alternative water supplies such as wastewater reuse have been adopted for irrigation in agriculture [2]. Irrigation with treated wastewater (TWW) is a sustainable solution to address water shortages by reducing the amount of water that should be removed from natural freshwater resources and reducing effluent dumping into water bodies, thereby preventing freshwater ecosystem degradation [3,4,5]. Despite its benefits, which include providing plants with organic compounds that help improve crop quality and plant growth [4,6], the use of sewage water for irrigation can also cause several human and environmental health issues since it can contaminate the soil [2]. This water contributes to the accumulation of chemicals, heavy metals [7,8], antibiotics, and human pathogens in the soil [9]. The accumulation of metals can have a deleterious impact on soil microbial diversity and activity by disrupting specific nutrient cycle pathways [10,11,12]. TWW irrigation may also alter soil physicochemical qualities by raising the salinity, electric conductivity (EC), and NO3 levels, as well as the levels of some elements such as boron, nitrogen, potassium, phosphorus, and iron, and by decreasing the soil pH, according to most research [13,14,15]. Wastewater irrigation is also related to health hazards; even as wastewater treatment reduces the number and types of microorganisms, pathogenic agents such as bacteria (e.g., Mycobacterium spp. and Legionella spp., vancomycin-resistant enterococci, and methicillin-resistant Staphylococcus aureus), viruses, protozoa, and helminths may persist, according to previous research [16,17,18,19,20]. Moreover, antibiotics and antibiotic resistance genes may remain in the TWW after treatment and can cause selection pressure for antibiotic-resistant microorganisms in the soil [2]. To protect human health and the environment, regulations and recommendations for water reuse have been developed. As a result, numerous countries have enacted laws and quality-of-water standards [21]. Some studies have focused on the impacts of wastewater irrigation on soil and rhizosphere microbial communities in the short and long term, including the influence of wastewater application on the biomass of microbes and enzyme activity at various soil depths and for several plant crops [9,22]. However, Krause et al. (2020) [23] found that irrigation with TWW resulted in a decline in bacterial populations due to soil pH changes. Other studies noticed an increase in the abundance of soil microbes after a long-term application of TWW irrigation [15,22]. The impact of irrigation with TWW on bacterial communities is mostly determined by the soil texture, since a higher clay content increases the buffering capacity of soils to respond to TWW [23]. According to Truu et al. (2009) [24], soil chemical and biochemical parameters were changed by irrigation with secondary processed wastewater, and the soil biological activity was positively modified by a willow coppice under short-term municipal wastewater irrigation. The negative impact of TWW irrigation on all microbial parameters, including enzymatic activities, compared to freshwater irrigation was also reported by Kayikcioglu (2012) [25]. Their study stated the role of heavy metals introduced by TWW irrigation and soil types in inhibiting the enzymatic activities of irrigated soil.
In the Kingdom of Saudi Arabia (KSA), characterized by an arid and semi-arid climate with minimal renewable water resources, groundwater and desalinated seawater are the main sources for drinking, irrigation, and industry [26]. Water usages from both sources of groundwater and desalinated water have increased annually by 2.5%, with the estimation that about 75% of the total water used then becomes generated wastewater [1]. The agricultural water demands accounted for 83–90% of the overall water demands from 1990 to 2009 [27]. Al-Madinah city, situated in the western part of the KSA, relies predominantly on groundwater, which accounts for 49–80% of the kingdom’s water resources, in order to meet its water demand, in particular the irrigation of date farms [26]. According to the data provided in 2013, the wastewater is treated in seven wastewater treatment plants in Al Madinah to produce about 2.2 MCMD-1 (2.2 million cubic meters per day), and 85% of the tertiary TWW is used in agriculture and industry; the remainder is discharged into Wadi Al Aquiq [27]. Date palm (Phoenix dactyllifera) is one of the most important and ancient crops grown in the Kingdom of Saudi Arabia. The kingdom is the second largest date palm producer, with a productivity of 1.07 million tons [28]. The city faces water shortages for many reasons, including over-pumping of groundwater, population growth, and the impact of arid and semi-arid conditions [26]. Therefore, the use of TWW as an additional water supply in Al-Madinah for the irrigation of some date palm fields is a practical option for reducing the pressure on aquifers with decreased groundwater levels. However, no research is given on its impact on the soil properties and the microbiomes of plant rhizospheres receiving irrigation. The rhizosphere, the area of soil directly affected by plant roots, is a vital habitat for interaction between plants and the microbial community. Plant roots release exudates and secondary metabolites that shape the structures of the microbial community and influence specific activities in the rhizosphere. Microorganisms support plants by providing necessary nutrients and protection against diseases [29]. These microbes contribute to several ecological and biological processes vital for plant health and growth, for instance, biogeochemical cycling, nitrogen fixation, phosphate solubilization, stimulating growth, decomposing organic matter, and assisting plants in coping with abiotic stress and diseases [14,30]. However, due to the estimate that more than 99 percent of the microbial population is non-culturable, our knowledge of the diversity of these microbial communities is still limited. Metagenomics is a culture-independent molecular technique recently applied to investigate the microbial communities associated with the rhizosphere of several plant crops using high-throughput 16S rRNA amplicon sequencing [31,32]. This technique, along with the high throughput of the DNA-sequencing platforms and data analysis software due to the development of bioinformatics, recently opened the opportunity to analyze the whole microbial communities of various environments [33]. Numerous endophytic bacterial species have been cultured from date palm roots and studied for their beneficial effects in previous studies. Additionally, bacterial rhizosphere communities in date palm have been studied under a variety of environmental conditions, including salinity [34] and drought conditions [35]. Using the high-throughput technique of next-generation sequencing, a recent study showed that environmental conditions such as salinity have a substantial impact on the date palm roots’ endophytic microbial community structures [36]. To our knowledge, this is the first metagenomics research on the date palm rhizosphere bacterial community composition following irrigation with treated wastewater in the Al Madinah municipality. The purpose of the present study was to investigate the role of irrigation water treatment on the bacterial population dynamics. A metagenomic analysis of the composition of the bacterial community in the rhizospheres of date palms that were irrigated with TWW is a first step toward understanding how watering plants with treated wastewater changes the structure and function of the bacterial community. This helps with managing soil and wastewater supplies in dry and semi-dry areas.
2. Materials and Methods
2.1. Site Selection and Soil Sampling
Soil samples from the rhizosphere of the date palms were obtained at 24°34′25.8″ N 39°34′56.8″ E (site 1) and 24°33′48.8″ N 39°35′21.8″ E (site 2) in Al Madinah province. The soil samples from the first field had been irrigated with treated wastewater for many years (more than 5 years), whereas samples from the second field had been irrigated with groundwater for more than 5 years. The wastewater used was tertiary treated water. In each field, four sub-samples of 100 g of soil were randomly sampled around the trees at a depth of 5–25 cm after visible vegetation was removed, which formed a composite sample. Samples were kept on ice and transported to the laboratory, where they were stored at −20 °C until DNA was extracted. Because this study does not involve human participants or endangered species, no ethical approval or authorization was required to gather soil samples. All experiments were performed in triplicate.
2.2. Soil Physicochemical Parameters
Physicochemical soil characteristics such as the soil pH, salinity, electric conductivity (EC), organic matter (OM) content, and total nitrogen (TN) (the Kjeldahl procedure) were measured at each site by the Laboratory of Soil Analysis (King Abdulaziz University) using standard procedures [37].
2.3. DNA Extraction, Illumina Library Preparation, and MiSeq DNA Sequencing
To construct DNA libraries, the hypervariable V3–V4 sections of the bacterial gene 16S rRNA were amplified using universal primers, including the Illumina adapter sequences, the index sequence, and the universal primers 341F: CCTACGGGNGGCWGCAG and 805R: GACTACHVGGGTATCTAATCC [38].
Microbial DNA (5 ng/µL) was amplified using 1 µM amplicon PCR Forward Primer, 1 µM amplicon PCR Reverse Primer, and Herculase II polymerase (Agilent, Santa Clara, CA, USA). The amplification was performed in a 25 μL reaction using the following program: 95 °C for 3 min; followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and a final elongation step at 72 °C for 5 min. Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) were used to purify the 16S V3 and V4 amplicon from free primers and primer dimer species. The attachment of dual indices and Illumina sequencing adapters was carried out using the Nextera XT Index Kit including Nextera XT Index Primer (N7xx), Nextera XT Index Primer (S5xx), and Herculase II polymerase (Agilent). PCR was performed using the following program: 95 °C for 3 min; followed by 8 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and a final elongation step at 72 °C for 5 min. AMPure XP beads were used to clean up the final library before quantification. The qualified libraries were paired-end sequencing using Illumina MiSeq technology at Macrogen, Inc. Seoul, South Korea.
2.4. Bioinformatics Analysis
Quality Analysis, Raw Data Trimming, and Filtering
The raw tags were obtained by merging the paired-end reads using the FLASH software tool (v1.2.11) (Fast Length Adjustment of Short Reads) [39]. Low-quality reads were filtered out, and extra-long tails were trimmed using CD-HIT-OTU/rDNATools (http://www.mothur.org/, accessed on 31 December 2020). Chimeric reads were identified and removed. Chimeras were also identified and eliminated using the UCHIME algorithm [40]. Secondary clusters were recruited into primary clusters. Noise sequences in clusters were removed. The remaining representative reads from non-chimeric clusters were clustered into OTUs at an OTU cutoff of 97% ID at the species level. The de novo CD-HIT algorithm was used in OTU picking. The taxonomic identity of each representative sequence was assigned to bacterial taxa using the BLASTN (https://www.ncbi.nlm.nih.gov/, accessed on 31 December 2020) system. For the taxonomic composition of each sample from the kingdom level to the species level, QIIME-UCLUST was used (Ribosomal Database Project (RDP) Classifier v.2.2). The diversity of bacterial communities was analyzed for each sample through the Shannon and Simpson indices. Also, the rarefaction curve and Chao1 values were obtained to estimate the alpha diversity by using alpha rarefraction.py within QIIME software (v1.8.0) (http://qiime.org/, accessed on 31 December 2020) [41,42]. The differences in the relative abundance of the different bacterial phyla, classes, orders, families, genera, and species were analyzed using Student’s t-test at p < 0.05. The 16S rRNA gene sequences obtained in this project were deposited in GenBank under the accession numbers OK235708–OK235997.
3. Results
3.1. Soil Physicochemical Parameters
Soil parameter values such as the pH, EC, TN, and OM content were significantly different between the two soil samples (Table 1).

Table 1.
Physicochemical soil characteristics.
3.2. Taxonomic Profiling and OTU Generation and Alignment
After quality filtering, a total of 24,885 high-quality reads were obtained, with an average of 12,442.5 clean tags for each sample. A statistical summary of the data is illustrated in Table 2. OTU representative sequences were taxonomically classified using UCLUST within QIIME using the Ribosomal Database Project (RDP) Classifier v.2.2. A total of 569 OTUs were generated after clustering at a 97% identity, with an average number of 284.5 sequences per sample. The OTU abundance of the groundwater-irrigated soil sample (GW) was higher than that of the treated-wastewater-irrigated soil (TWW) (327 and 242, respectively). This reflects the richness of the GW sample compared to the TWW sample (Table 2).

Table 2.
Summary of raw and quality-filtered reads per sample.
3.3. Community Richness and Diversity
The alpha rarefaction curves for each soil sample (Figure 1) indicated that the number of reads used in the analysis was adequate in identifying species/OTUs. Good’s coverage for the sequencing was >99% for both soil samples (Table 2). The indices of bacterial community richness (Chao 1, observed OTUs) and diversity (Shannon and Simpson) were calculated using QIIME (Table 3). The Chao1 index estimates showed a higher richness of bacterial taxa in the GW sample compared to the TWW sample (338 and 251, respectively). In addition, microbial diversity indices such as the Shannon and Simpson diversity indicated that the microbial community in the GW sample was more diverse than that in the TWW sample. According to the Shannon diversity estimations, the GW date palm rhizosphere had a higher value of 5.8 compared with that of the TWW rhizosphere, which was 4.9 (Table 3, Figure 1).
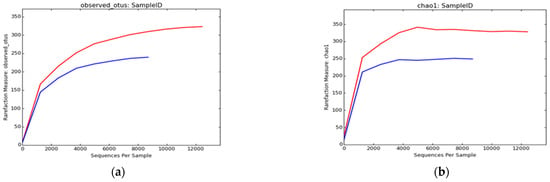

Figure 1.
Alpha diversity indices of bacterial communities from the two soil samples TWW (blue) and GW (red). Microbial richness was calculated using the observed OTUs (a), chao 1 estimator (b), and Shannon and Inverse Simpson indices (c).

Table 3.
Bacterial community diversity indices for the two soil samples.
3.4. Bacterial Diversity and Taxonomic Composition
The microbiome profile of the rhizosphere for the two samples was analyzed; the OTUs were assigned into 15 phyla, 41 classes, 79 orders, 119 families, 203 genera, and 261 species. The taxonomic composition for each sample at the phylum and class levels (OTU proportions) is shown in the charts in Figure 2 and Table 4. In total, 569 OTUs were generated; most of them (97.3%) were assigned into 15 different phyla, whereas 2.7% were marked as unclassified. The most abundant phyla were Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes (29.5%, 29.4%, 19.3%, and 9.4%, respectively), which represented 87.6% of the total OTUs, followed by Chloroflexi (2.7%), Gemmatimonadetes (3.0%), and Acidobacteria (2.0%) (Table 4). Notably, the top four phyla that dominated the rhizosphere soil irrigated with TWW were Firmicutes (43.6%), Bacteroidetes (17.3%), Proteobacteria (15.2%), and Actinobacteria (14.6%), representing more than 90.7% of the total community, while the rhizosphere soil irrigated with GW was dominated by Actinobacteria (44.1%), Proteobacteria (23.4%), Firmicutes (15.5%), and Gemmatimonadetes (4.9%), accounting for 87.6% of the total. Two phyla, Deinococcus-Thermus and Thermotogae, were only present in low abundance in the GW sample, while the phyla Verrucomicrobia and Rhodothermaeota were represented only in the TWW sample with a low percentage. The bacterial assignments at the genus and species levels were considered and compared between the samples. In total, there were 203 different genera, with 90 identified at some level of abundance in the two samples; 69 genera were unique to the GW sample, and 44 were unique to the TWW sample. A total of 261 different species were identified. Of these, 116 were identified at some level of abundance in the two samples; 92 species were unique to the GW sample, and 53 were unique to the TWW sample. The most frequently observed species in the two samples were also different. The most predominant species in the TWW sample was Planococcus plakortidis, with a relative abundance of 32.1%, followed by Paenisporosarcina quisquiliarum (8.6%), Pseudarthrobacter phenanthrenivorans (5.7%), Mariniflexile soesokkakense (4.2%), Flavitalea gansuensis (3.7%), and Fluviicola taffensis (3.1%). The latter four were not represented in the GW sample. Finally, the species Blastococcus saxobsidens accounted for 2.4% of the total. Meanwhile, the species Nocardioides mesophilus was the most abundant species in the GW sample (16.4%), followed by Luteimonas pelagia and Blastococcus saxobsidens with nearly equal abundance (6.4% and 6.3%, respectively). Next was Nocardioides panacisoli (4.5%), followed by Pelobacter carbinolicus (4.1%) and Aquihabitans daechungensis (3.3%); the two species L. pelagia and N. panacisoli were not present in the rhizosphere soil of the TWW sample.

Figure 2.
The relative abundance of the bacterial community at the phylum (a) and class (b) levels (calculated using QIIME). Each bacterial taxon is represented by a different color in the bar graphs. Legend: TWW, treated-wastewater-irrigated soil; GW, groundwater-irrigated soil. * Significantly abundant taxa.

Table 4.
The taxonomic composition for each sample at the phylum to species levels.
4. Discussion
Culture-independent metagenomic methods have advanced the study of microbial diversity and abundance in rhizosphere soils [43]. In this study, the diversity of microbial populations in the rhizosphere of date palms irrigated with TWW was assessed and compared to that in the rhizosphere of date palms irrigated with GW using metagenomic approaches. Groundwater-irrigated soils were taken as controls. While several studies have shown that the type of irrigation water influences the rhizosphere-associated microbiomes for many plants, no studies were available for date palms. Thus, the goal of this study was to look into how wastewater irrigation affects the bacterial community structure in the rhizosphere in comparison to non-wastewater-irrigated soils. Angin et al. (2005) [13] demonstrated that long-term wastewater irrigation changes the physical and chemical parameters of soils. Because of the high organic matter concentration of the irrigation water, wastewater-irrigated soils have an increased total nitrogen (TN) content and lower pH value than do non-wastewater-irrigated soils. Conversely, other researchers noted that after wastewater irrigation, the soil pH increased due to the accumulation of basic cations (especially Na) [22,44,45]. The results of the present study also showed a slight decrease in the pH value and an increase in the total nitrogen (TN) and organic matter contents. Many studies, including long- and short-term wastewater irrigation experiments, have been conducted. They observed that after wastewater application, the organic matter and nitrogen levels increased, which improved the soil fertility [25,45,46]. Liu et al. (2019) [47] also found that the TN input was higher in wastewater-treated soils than in groundwater-treated soils; however, the nitrogen utilization efficiency in wastewater-irrigated soils was much lower. The increase in the TN was proportional to the number of years of wastewater application. Moreover, the addition of organic compounds to the soil by wastewater irrigation can potentially alter the microbial composition by supplying nutrient resources for microbes [22]. In this work, the electric conductivity of the soil irrigated with wastewater was less than that of the soil irrigated with GW. Due to the addition of water-soluble nutrients, irrigation with TWW was expected to increase the electrical conductivity. Both soils’ EC values remained at the lower end of the EC values for saline soils, where values often exceed 4 dS m−1 (4000 μS/cm) [48]. Unpredictably, there was no increase in salt in the wastewater-irrigated soil, most likely due to the high quality of the TWW. The majority of studies that have investigated the impact of wastewater on the physiochemical parameters of irrigated soils observed an increase in salt content [13].
The bacterial diversity of the date palm rhizosphere has been studied using culture-dependent techniques, and limited research has been conducted using culture-independent methods [49]. In the current work, the rarefaction analyses suggest that the bacteria inhabiting the rhizospheres in TWW soil and GW soil around date palm trees are significantly different from those found in comparative studies of bacterial diversity in other wastewater-irrigated rhizospheres and non-wastewater-irrigated soils for other plants. The richness of the TWW-irrigated rhizosphere was less than that of the GW sample. This may be attributed to the decrease in pH as a result of TWW irrigation. However, other research found that TWW irrigation enhanced the microbial abundance [15,22,50]. In concordance with this study, Liu et al. (2019) [47] reported a higher number of OTUs with substantially higher abundance in groundwater-irrigated soil compared to wastewater-irrigated soil. The same study observed that Acidobacteria (23.5 percent of the total OTUs) or Gemmatimonadetes (22.6 percent) dominated the groundwater-irrigated soil. This is in contrast to a previous study by Krause et al. (2020) [23], which found that the same phyla, Proteobacteria, Acidobacteria, Actinobacteria, and Bacteroidetes, dominated the bacterial communities of the cucumber rhizosphere in TWW- and potable water (PW)-irrigated soil, accounting for more than 60% of the total community. The present study showed that the bacterial communities of the date palm rhizosphere in TWW-irrigated soil and GW-irrigated soil were dominated by different phyla. Actinobacteria (44.1%), Proteobacteria (23.4%), Firmicutes (15.5%), and Gemmatimonadetes (4.9%) were the top four most common phyla in groundwater-irrigated soil. In contrast, Firmicutes (43.6%), Bacteroidetes (17.3%), Proteobacteria (15.2%), and Actinobacteria (14.6%) were the top four bacterial phyla in wastewater-irrigated soil. The results of this study were consistent with those of Bastida et al. (2017) [51], who found that reclaimed water irrigation enhanced the abundance of Bacteroidetes while decreasing the abundance of Acidobacteria.
In this work, sequence analyses of 16S rRNA genes in soil irrigated with TWW revealed an increase in the phyla Firmicutes (43.6%) and Bacteroidetes (17.3%) as compared to soil irrigated with GW, in which they represented 15.5% and 1.6%, respectively. Firmicutes contains spore-forming bacteria such as Bacilli, which accounted for 42.6% of the total classes in this study. Spores can ensure bacterial survival during periods of environmental stress. It is extremely likely that the palm trees in this study selected for a restricted group that can live in harsh environments for long periods. The proportions of Bacteroidetes were also higher in TWW soil compared to the GW sample (17.3% and 1.6%, respectively). These bacteria are engaged in the decomposition of plant material and organic compounds such as starch, proteins, and cellulose, and their abundance increases with increasing soil pH and nitrogen and phosphorus availability [52]. The increasing prevalence of these phyla is attributed to their fast rate of growth in the presence of the organic nutrients and water present in wastewater; they are classified as copiotrophs [53]. Bacteroidetes, Firmicutes, and Proteobacteria are commonly termed copiotrophs [54,55]. This is in line with prior research, which found that irrigation with reclaimed water and piggery effluent dramatically increased the abundance of Bacteroidetes in rhizosphere soil [47]. Bougnom et al. (2020) [56] also reported a high abundance of Bacteroidetes and Firmicutes in wastewater-irrigated fields; their relative abundance levels were 65% and 15.7% greater, respectively, in irrigated fields compared to non-irrigated fields. Members of Firmicutes can produce spores in adverse conditions that sporulate in response to increased nutrients. This can explain why they are becoming more common in irrigated fields that contain a high amount of organic compounds. Firmicutes and Actinobacteria were found in abundance in Antarctic soil because these two phyla are composed mainly of spore-forming groups that assist in the survival of bacteria during periods of environmental stress [57].
Bacterial genera related to Bacteroidetes (Mariniflexile, Flavitalea, Fluviicola, Flavobacterium, and Algoriphagus) were identified in the TWW rhizosphere but not detected in the GW rhizosphere. Some of these genera have also been identified in previous studies [58]. In contrast to the current findings, the most frequent bacterial phyla in the soil were Proteobacteria, Acidobacteria, and Actinobacteria, with Bacteroidetes, Firmicutes, and Planctomycetes being less common. In fact, the abundance of the major phyla differs between soils [59]. A previous study by Mosqueira et al. (2019) [49] indicated that the Gamma-proteobacteria class dominated the Tunisian date palm rhizosphere (67%) at all the examined oases, and Alpha-proteobacteria was the second most dominant class in the rhizosphere (20%). Actinobacteria were also abundant, but the Firmicutes class was not commonly observed. In this study, Proteobacteria sequences were represented in a substantial proportion (15.2% in TWW soil, compared to 23.4 percent in GW soil), but this was not the dominant phylum. Liu et al. (2019) [47] found that Bacteroidetes and Alpha- and Gamma-Proteobacteria, which were more prevalent in wastewater-irrigated soils, accounted for just 7.0, 7.0, and 8.7 percent, respectively, in groundwater-irrigated soils. This is consistent with previous studies in which members of four major phyla (Proteobacteria, Actinobacteria, Acidobacteria, and Verrucomicrobia) were common to nearly all rhizospheres, according to phylogenetic analysis of the DNA sequences. These four groups of bacteria account for more than 75% of the total 16S rDNA gene clone libraries studied [60]. In this study, Actinobacteria were also abundant in the rhizosphere in TWW soil (14.6%) but were not the dominant phylum. Actinobacteria was the dominant phylum in the GW-irrigated rhizosphere, with a frequency of 44.1%. Members of this phylum have been reported to produce antimicrobial and nematocidal chemicals, as well as preventing cyst nematode infections in the soybean plant [61]. The results of other studies were different; 16S ribosomal DNA amplicon pyrosequencing revealed an increase in the relative abundance of Gamma-proteobacteria and a decline in the relative abundance of Actinobacteria in TWW-irrigated soils [48]. Cui et al. (2019) [62] also observed that Proteobacteria was the dominant phylum in maize rhizosphere soil after a treatment with a marigold flower fermentation wastewater neutralization solution. A study by Ferjani et al. (2015) [34] that assessed the bacterial communities associated with the date palm rhizosphere soil in seven Tunisian oases showed a high proportion of Proteobacteria (65%) (mostly Gammaproteobacteria 87.69%) and Actinobacteria (26%) and a low abundance of Firmicutes (7%) and Bacteroidetes (2%), which is not consistent with the current results. They observed that Gammaproteobacteria are mostly represented in the date palm root system by the orders Enterobacterales and Pseudomonadales. The Enterobacteriales group contains many species that originated from intestinal environments. They are the result of the use of natural fertilizers. Pseudomonadales includes organisms that serve as bioprotectors for date palms against environmental stress [49]. In concordance with this work, Gemmatimonadetes was not one of the most common phyla in wastewater-irrigated crops according to Bougnom et al. (2020) [56]. This phylum was more dominant in the GW-irrigated rhizosphere (4.9%) than the TWW-irrigated soil (1.0%). The phylum Acidobacteria was also found in a minor percentage in the TWW rhizosphere, as its members are known to be more abundant in environments with low carbon availability [31,54]. In this study, the top nine genera, which represent the core rhizosphere microbiome, were Planococcus, Paenisporosarcina, Blastococcus, Pseudarthrobacter, Mariniflexile, Flavitalea, Fluviicola, Brevundimonas, and Flavobacterium. Among these, the latter six were not represented in the GW-irrigated rhizosphere soil. The genera Planococcus, Paenisporosarcina, and Blastococcus were represented in minor percentages. These results confirm the different patterns at the genus level in a TWW-irrigated rhizosphere, with Planococcus, a member of the class Bacilli within the phylum Firmicutes, being the most prevalent genus, with a relative frequency of 32.12%. Members of this genus are dominant in saline and moderately saline habitats and play an important ecological role due to their Gram-positive cell walls and their ability to produce spores [58]. The dominant species in TWW-irrigated soil was Planococcus plakortidis, which was first isolated from the marine sponge Plakortis simplex [63]. However, the groundwater-irrigated soil was dominated by Nocardioides, a genus of the order Actinomycetales. Nocardioides species are widely distributed in nature [64]. In contrast to these findings, several studies employing high-throughput 16S rRNA amplicon sequencing found that irrigation with wastewater reduced the percentage of potential plant-growth-promoting bacteria in the rhizosphere while increasing the abundance of pathogenic microorganisms in the rhizosphere root endosphere of maize and bulk soil. The increasing abundance of potentially pathogenic microorganisms and antibiotic resistance genes in wastewater-irrigated soils may put farm workers and consumers of wastewater-irrigated crops at risk [18,53,62].
The genus distribution in the bacterial communities associated with the rhizospheres of the date palm irrigated with TWW was different not only from that of other plant species but also from other soil rhizospheres of the date palm reported by other researchers in other regions. In trying to understand the microbial diversity tendencies, some studies proposed that the soil characteristics could be the key factors that determine rhizosphere microbial populations, while others suggested that other factors, such as environmental conditions, plant species, agricultural practices, and complex interactions among these variables, could influence and shape the rhizosphere’s microbial diversity [9]. In this context, the unique microbiological profile of TWW-irrigated soils in this study may be related to the irrigation with wastewater, which changes both the soil’s microbial structure and functions by altering the soil’s physiochemical properties, which can stimulate or inhibit microbial cells. Indeed, the selection process in the rhizosphere is also based on functional traits rather than taxonomic relatedness [65].
5. Conclusions
This study investigated the taxonomical diversity of the bacterial communities found in the rhizospheres of plants watered with TWW and those watered with GW. The use of TWW in the irrigation of date palms may change the physicochemical properties of the soil and restructure the rhizosphere microbiome. However, other factors, such as the soil texture, porosity, agricultural techniques, etc., can also be responsible for this difference. In the current study, TWW-irrigated soil and GW-irrigated soil were dominated by different phyla. Firmicutes dominated the bacterial community in TWW-irrigated soil, while Actinobacteria and Proteobacteria dominated at the GW-irrigated site. This study improves our understanding of the bacterial community in TWW-irrigated soil, which will help in understanding the impact of TWW irrigation on the bacterial community in the date palm rhizosphere and how it shapes the microbiome to address water scarcity in arid and semi-arid regions. However, further metagenomic studies with a larger number of samples are needed to determine how irrigation with TWW influences the rhizosphere microbiome’s structure and function. In addition, further work on the determination of all the chemical and physical parameters of soil, such as heavy metal, antibiotic, phosphorus, potassium, and Fe contents, is required.
Funding
No funding was received for conducting this study.
Institutional Review Board Statement
This article does not contain any studies with human participants or animals performed by the author.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed in this study are included in this published article. All 16S rRNA sequences generated from this study have been deposited in GenBank under accession numbers OK235708–OK235997.
Conflicts of Interest
The author declares no conflict of interest.
References
- Aburizaiza, O.S.; Mahar, G.A. Degree of Wastewater Treatment versus Types of Reuses: Case Study, Saudi Arabia. Glob. Nest J. 2016, 18, 569–581. [Google Scholar] [CrossRef]
- Chopyk, J.; Nasko, D.J.; Allard, S.; Bui, A.; Treangen, T.; Pop, M.; Mongodin, E.F.; Sapkota, A.R. Comparative Metagenomic Analysis of Microbial Taxonomic and Functional Variations in Untreated Surface and Reclaimed Waters Used in Irrigation Applications. Water Res. 2020, 169, 115250. [Google Scholar] [CrossRef] [PubMed]
- Toze, S. Reuse of Effluent Water-Benefits and Risks. Agric. Water Manag. 2006, 80, 140–159. [Google Scholar] [CrossRef]
- Mañas, P.; Castro, E.; De Las Heras, J. Irrigation with Treated Wastewater: Effects on Soil, Lettuce (Lactuca sativa L.) Crop and Dynamics of Microorganisms. J. Environ. Sci. Health—Part A Toxic/Hazardous Subst. Environ. Eng. 2009, 44, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, Q.; Li, C.; Wei, Y.; Zhao, Y.; Feng, Y.; Zheng, H.; Li, F.; Li, H. Impacts of Aquaculture Wastewater Irrigation on Soil Microbial Functional Diversity and Community Structure in Arid Regions. Sci. Rep. 2017, 7, 11193. [Google Scholar] [CrossRef] [PubMed]
- Jueschke, E.; Marschner, B.; Tarchitzky, J.; Chen, Y. Effects of Treated Wastewater Irrigation on the Dissolved and Soil Organic Carbon in Israeli Soils. Water Sci. Technol. 2008, 57, 727–733. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health Risks of Heavy Metals in Contaminated Soils and Food Crops Irrigated with Wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Satpati, S.; Nayek, S.; Garai, D. Effect of Wastewater Irrigation on Vegetables in Relation to Bioaccumulation of Heavy Metals and Biochemical Changes. Environ. Monit. Assess. 2010, 165, 169–177. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater Reuse in Irrigation: A Microbiological Perspective on Implications in Soil Fertility and Human and Environmental Health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, L.; Frankenberger, W.T.; Chang, A.C. Soil Enzyme Activities of Long-Term Reclaimed Wastewater-Irrigated Soils. J. Environ. Qual. 2008, 37, S-36–S-42. [Google Scholar] [CrossRef]
- Bourgeois, E.; Dequiedt, S.; Lelièvre, M.; van Oort, F.; Lamy, I.; Maron, P.A.; Ranjard, L. Positive Effect of the Miscanthus Bioenergy Crop on Microbial Diversity in Wastewater-Contaminated Soil. Environ. Chem. Lett. 2015, 13, 495–501. [Google Scholar] [CrossRef]
- Kandeler, F.; Kampichler, C.; Horak, O. Influence of Heavy Metals on the Functional Diversity of Soil Microbial Communities. Biol. Fertil. Soils 1996, 23, 299–306. [Google Scholar] [CrossRef]
- Angin, I.; Yaganoglu, A.V.; Turan, M. Effects of Long-Term Wastewater Irrigation on Soil Properties. J. Sustain. Agric. 2005, 26, 31–42. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Mazahreh, N. Changes in Soil Fertility Parameters in Response to Irrigation of Forage Crops with Secondary Treated Wastewater. Commun. Soil Sci. Plant Anal. 2003, 34, 1281–1294. [Google Scholar] [CrossRef]
- Wafula, D.; White, J.R.; Canion, A.; Jagoe, C.; Pathak, A.; Chauhan, A. Impacts of Long-Term Irrigation of Domestic Treated Wastewater on Soil Biogeochemistry and Bacterial Community Structure. Appl. Environ. Microbiol. 2015, 81, 7143–7158. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Olson, N.D.; Raspanti, G.A.; Goldstein, R.E.R.; Gibbs, S.G.; Sapkota, A.; Sapkota, A.R. Antibiotic Concentrations Decrease during Wastewater Treatment but Persist at Low Levels in Reclaimed Water. Int. J. Environ. Res. Public Health 2017, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Akponikpè, P.B.I.; Wima, K.; Yacouba, H.; Mermoud, A. Reuse of Domestic Wastewater Treated in Macrophyte Ponds to Irrigate Tomato and Eggplant in Semi-Arid West-Africa: Benefits and Risks. Agric. Water Manag. 2011, 98, 834–840. [Google Scholar] [CrossRef]
- Cui, B.; Liang, S. Monitoring Opportunistic Pathogens in Domestic Wastewater from a Pilot-Scale Anaerobic Biofilm Reactor to Reuse in Agricultural Irrigation. Water 2019, 11, 1283. [Google Scholar] [CrossRef]
- Kulkarni, P.; Olson, N.D.; Paulson, J.N.; Pop, M.; Maddox, C.; Claye, E.; Rosenberg, R.E.; Sharma, M.; Gibbs, S.G.; Mongodin, E.F.; et al. Science of the Total Environment Conventional Wastewater Treatment and Reuse Site Practices Modify Bacterial Community Structure but Do Not Eliminate Some Opportunistic Pathogens in Reclaimed Water. Sci. Total Environ. 2018, 639, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Makowska, N.; Philips, A.; Nowis, K.; Trzebny, A.; Koczura, R.; Mokracka, J. Metagenomic Analysis of b -Lactamase and Carbapenemase Genes in the Wastewater Resistome. Water Res. 2020, 170, 115277. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.P.T.; Castle, J.W.; Rodgers, J.H. Application of Water Quality Guidelines and Water Quantity Calculations to Decisions for Beneficial Use of Treated Water. Appl. Water Sci. 2011, 1, 85–101. [Google Scholar] [CrossRef][Green Version]
- Hidri, Y.; Bouziri, L.; Maron, P.A.; Anane, M.; Jedidi, N.; Hassan, A.; Ranjard, L. Soil DNA Evidence for Altered Microbial Diversity after Long-Term Application of Municipal Wastewater. Agron. Sustain. Dev. 2010, 30, 423–431. [Google Scholar] [CrossRef]
- Krause SM, B.; Dohrmann, A.B.; Gillor, O.; Christensen, B.T.; Merbach, I.; Tebbe, C.C. Soil Properties and Habitats Determine the Response of Bacterial Communities to Agricultural Wastewater Irrigation. Pedosphere 2020, 30, 146–158. [Google Scholar] [CrossRef]
- Truu, M.; Truu, J.; Heinsoo, K. Changes in Soil Microbial Community under Willow Coppice: The Effect of Irrigation with Secondary-Treated Municipal Wastewater. Ecol. Eng. 2009, 35, 1011–1020. [Google Scholar] [CrossRef]
- Kayikcioglu, H. Short-Term Effects of Irrigation with Treated Domestic Wastewater on Microbiological Activity of a Vertic Xerofluvent Soil under Mediterranean Conditions. J. Environ. Manag. 2012, 15, 108–114. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Aly, A.A.; Aldhumri, S.A.; Al-Barakaha, F.N. Hydrochemical and Quality Assessment of Groundwater Resources in Al-Madinah City, Western Saudi Arabia. Sustainability 2020, 12, 3106. [Google Scholar] [CrossRef]
- Chowdhury, S.; Al-Zahrani, M. Characterizing Water Resources and Trends of Sector Wise Water Consumptions in Saudi Arabia. J. King Saud Univ.-Eng. Sci. 2015, 27, 68–82. [Google Scholar] [CrossRef]
- Salama, K.F.; Randhawa, M.A.; Al Mulla, A.A.; Labib, O.A. Heavy Metals in Some Date Palm Fruit Cultivars in Saudi Arabia and Their Health Risk Assessment. Int. J. Food Prop. 2019, 22, 1684–1692. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of Plant Domestication on Rhizosphere Microbiome Assembly and Functions. Plant Mol. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef]
- Ambardar, S.; Sangwan, N.; Manjula, A.; Rajendhran, J.; Gunasekaran, P.; Lal, R.; Vakhlu, J. Identification of Bacteria Associated with Underground Parts of Crocus Sativus by 16S RRNA Gene Targeted Metagenomic Approach. World J. Microbiol. Biotechnol. 2014, 30, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Kazeeroni, E.A.; Al-sadi, A.M. 454-Pyrosequencing Reveals Variable Fungal Diversity Across Farming Systems. Front. Plant Sci. 2016, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Sudarikov, K.; Tyakht, A.; Alexeev, D. Methods for The Metagenomic Data Visualization and Analysis. Curr. Issues Mol. Biol. 2017, 24, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Ferjani, R.; Marasco, R.; Rolli, E.; Cherif, H.; Cherif, A.; Gtari, M.; Boudabous, A.; Daffonchio, D.; Ouzari, H.I. The Date Palm Tree Rhizosphere Is a Niche for Plant Growth Promoting Bacteria in the Oasis Ecosystem. Biomed Res. Int. 2015, 2015, 153851. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W. Proline Accumulation Is a General Response to Abiotic Stress in the Date Palm Tree (Phoenix dactylifera L.). Genet. Mol. Res. 2015, 14, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; Al-Harrasi, I.; Alansari, A.S.; Al-Yahyai, R.; Glick, B.R. The Use of High Throughput DNA Sequence Analysis to Assess the Endophytic Microbiome of Date Palm Roots Grown under Different Levels of Salt Stress. Int. Microbiol. 2016, 19, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Black, C.A.; Evans, D.D.; White, J.L.; Ensminger, L.E.; Clark, F.E.; Dinauer, R.C. Methods of Soil Analysis: Part 1 Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Wiley: New York, NY, USA, 2015. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Magoc, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. 2011, 27, 2194–2200. [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; Desantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A Flexible Tool for Aligning Sequences to a Template Alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using QIIME to Analyze 16S rRNA Gene Sequences from Microbial Communities. Curr. Protoc. 2011, 36, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.; De Hollander, M.; Pijl, A.; Liu, B.; Kuramae, E.E. Cultivation-Independent and Cultivation-Dependent Metagenomes Reveal Genetic and Enzymatic Potential of Microbial Community Involved in the Degradation of a Complex Microbial Polymer. Microbiome 2020, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.L.; Mecham, B. On Golf Course Fairways. Agron. J. 2005, 97, 717–721. [Google Scholar] [CrossRef]
- Adrover, M.; Farrus, E.; Moya, G.; Vadell, J. Chemical Properties and Biological Activity in Soils of Mallorca Following Twenty Years of Treated Wastewater Irrigation. J. Environ. Manag. 2012, 95, S188–S192. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, L.; Chang, A.; Zhang, Y. No Title Impact of Long-Term Reclaimed Wastewater Irrigation on Agricultural Soils: A Preliminary Assessment. J. Hazard. Mater. 2010, 183, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Neal, A.L.; Zhang, X.; Cui, E.; Gao, F.; Fan, X.; Hu, C. Increasing Livestock Wastewater Application in Alternate-Furrow Irrigation Reduces Nitrification Gene Abundance but Not Nitrification Rate in Rhizosphere. Biol. Fertil. Soils 2019, 55, 439–455. [Google Scholar] [CrossRef]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo Papa, G.; Dazzi, C.; Benedetti, A. Salinity and Bacterial Diversity: To What Extent Does the Concentration of Salt Affect the Bacterial Community in a Saline Soil? PLoS ONE 2014, 9, e106662. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, M.J.; Marasco, R.; Fusi, M.; Michoud, G.; Merlino, G.; Cherif, A.; Daffonchio, D. Consistent Bacterial Selection by Date Palm Root System across Heterogeneous Desert Oasis Agroecosystems. Sci. Rep. 2019, 9, 4033. [Google Scholar] [CrossRef] [PubMed]
- Frenk, S.; Hadar, Y.; Minz, D. Resilience of Soil Bacterial Community to Irrigation with Water of Different Qualities under Mediterranean Climate. Environ. Microbiol 2014, 16, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Torres, I.F.; Romero-Trigueros, C.; Baldrian, P.; Větrovský, T.; Bayona, J.M.; Alarcón, J.J.; Hernández, T.; García, C.; Nicolás, E. Combined Effects of Reduced Irrigation and Water Quality on the Soil Microbial Community of a Citrus Orchard under Semi-Arid Conditions. Soil Biol. Biochem 2017, 104, 226–237. [Google Scholar] [CrossRef]
- Aislabie, J.; Deslippe, J.R.; Dymond, J. Soil Microbes and Their Contribution to Soil Services. In Ecosystem Services in New Zealand: Conditions and Trends; Dymond, J., Ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. [Google Scholar]
- Broszat, M.; Nacke, H.; Blasi, R.; Siebe, C.; Huebner, J.; Daniel, R. Wastewater Irrigation Increases the Abundance of Potentially Harmful Gammaproteobacteria in Soils in Mezquital Valley, Mexico. Appl. Environ. Microbiol. 2014, 80, 5282–5291. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-Biome Metagenomic Analyses of Soil Microbial Communities and Their Functional Attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Bougnom, B.P.; Thiele-Bruhn, S.; Ricci, V.; Zongo, C.; Piddock, L.J.V. Raw Wastewater Irrigation for Urban Agriculture in Three African Cities Increases the Abundance of Transferable Antibiotic Resistance Genes in Soil, Including Those Encoding Extended Spectrum β-Lactamases (ESBLs). Sci. Total Environ. 2020, 698, 134201. [Google Scholar] [CrossRef]
- Teixeira, L.C.R.S.; Peixoto, R.S.; Cury, J.C.; Sul, W.J.; Pellizari, V.H.; Tiedje, J.; Rosado, A.S. Bacterial Diversity in Rhizosphere Soil from Antarctic Vascular Plants of Admiralty Bay, Maritime Antarctica. ISME J. 2010, 4, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Mirza, B.S.; Mehnaz, S.; Mirza, M.S.; Mclean, J.; Malik, K.A. Impact of Soil Salinity on the Microbial Structure of Halophyte Rhizosphere Microbiome. World J. Microbiol. Biotechnol. 2018, 34, 136. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S RRNA and 16S RRNA Genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tian, J.; Shi, F.; Su, L.; Liu, K.; Xiang, M. Rhizosphere Bacterial Communities Associated with Healthy and Heterodera Glycines -Infected Soybean Roots. Eur. J. Soil Biol. 2013, 58, 32–37. [Google Scholar] [CrossRef]
- Nour, S.M.; Lawrence, J.R.; Zhu, H.; Swerhone GD, W.; Welsh, M.; Welacky, T.W.; Topp, E. Bacteria Associated with Cysts of the Soybean Cyst Nematode (Heterodera glycines). Appl. Environ. Microbiol. 2003, 69, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Fan, X.; Li, Z.; Liu, Y.; Neal, A.L.; Hu, C.; Gao, F. Variations in Soil and Plant-Microbiome Composition with Different Quality Irrigation Waters and Biochar Supplementation. Appl. Soil Ecol. 2019, 142, 99–109. [Google Scholar] [CrossRef]
- Kaur, I.; Das, A.P.; Acharya, M.; Klenk, H.P.; Sree, A.; Mayilraj, S. Planococcus plakortidis Sp. Nov., Isolated from the Marine Sponge Plakortis simplex (Schulze). Int. J. Syst. Evol. Microbiol. 2012, 62, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Prauser, H. Nocardioides, a New Genus of the Order Actinomycetales. Int. J. Syst. Evol. Microbiol. 1976, 26, 58–65. [Google Scholar] [CrossRef]
- Yan, Y.; Kuramae, E.E.; De Hollander, M.; Klinkhamer, P.G.L.; Van Veen, J.A. Functional Traits Dominate the Diversity-Related Selection of Bacterial Communities in the Rhizosphere. ISME J. 2017, 11, 56–66. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).