First Seroepidemiological Study of Toxoplasma gondii Infection in Dromedaries (Camelus dromedarius) in Southern Tunisia
Abstract
1. Introduction
2. Materials and Methods
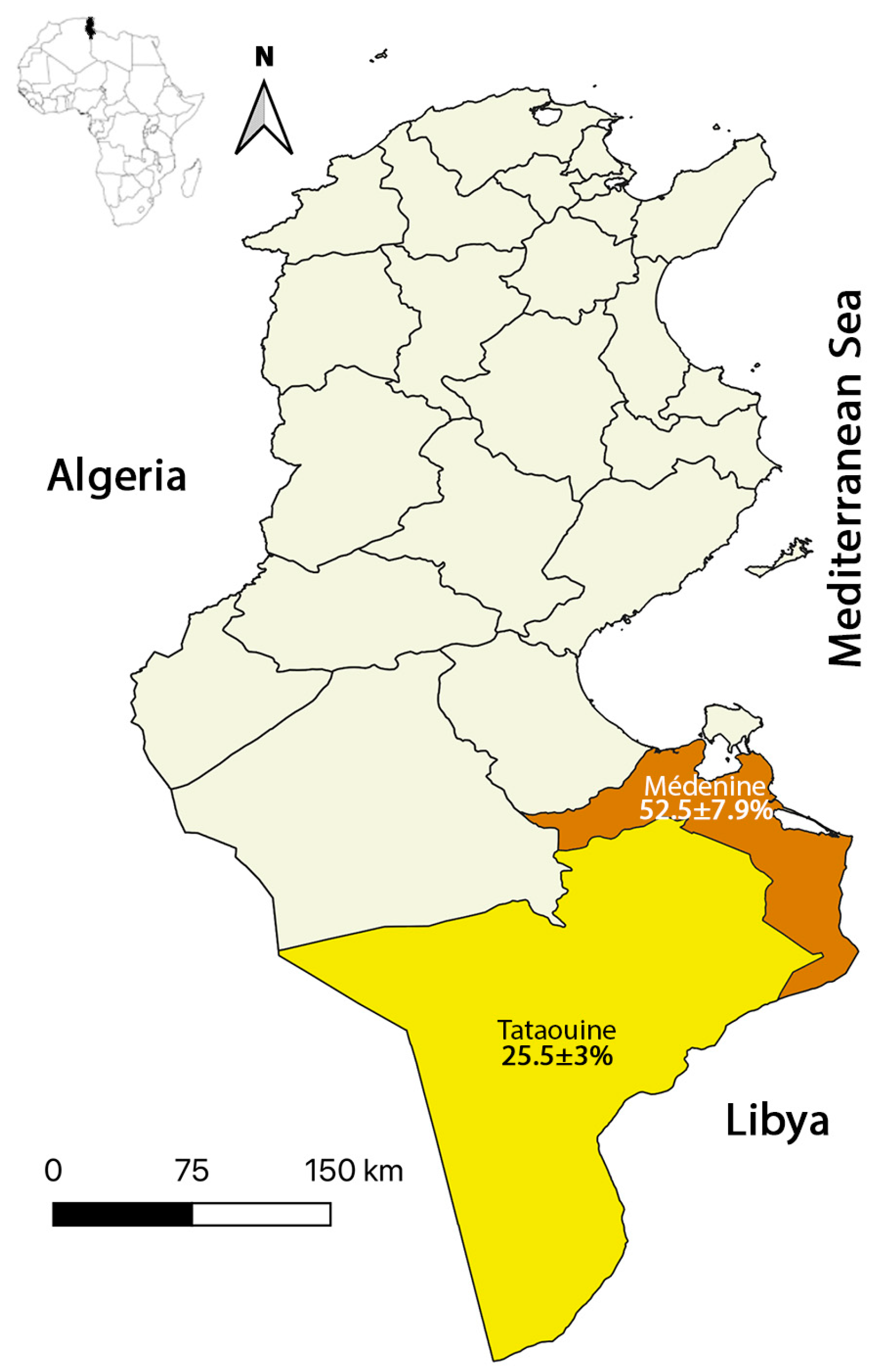
2.1. Study Region
2.2. Sample Size Determination
- N: the required sample size;
- Pexp: the expected prevalence;
- d: the desired absolute precision fixed to 5%.
2.3. Animal and Sample Collection
- NC: negative control;
- PC: positive control;
- S: sample;
- P: positive control.
2.4. Data Collection
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almeria, S.; Dubey, J.P. Foodborne transmission of Toxoplasma gondii infection in the last decade. An overview. Res. Vet. Sci. 2021, 135, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, J.K.; Dubey, J.P.; Miller, N.L. Toxoplasma gondii in cats: Fecal stages identified as coccidian oocysts. Science 1970, 167, 893–896. [Google Scholar] [CrossRef]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef]
- Tenter, A.M. Toxoplasma gondii in animals used for human consumption. Mem. Inst. Oswaldo Cruz. 2009, 104, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258, Erratum in Int. J. Parasitol. 2001, 31, 217–220. [Google Scholar] [CrossRef]
- Powell, C.C.; Brewer, M.; Lappin, M.R. Detection of Toxoplasma gondii in the milk of experimentally infected lactating cats. Vet. Parasitol. 2001, 102, 29–33. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S. Neosporosis, toxoplasmosis, and sarcocystosis in ruminants. Vet. Clin. North. Am. Food Anim. Pract. 2006, 22, 645–671. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M. Speculation on possible life cycles for the clonal lineages in the genus toxoplasma. Parasitol. Today. 1997, 13, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- Dubey, J.P. Re-examination of resistance of Toxoplasma gondii tachyzoites and bradyzoites to pepsin and trypsin digestion. Parasitology 1998, 116, 43–50. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Dardé, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Lachkhem, A.; Galal, L.; Lahmar, I.; Passebosc, K.; Riahi, H.; Plault, N.; Dardé, M.L.; Mercier, A.; Babba, H. First isolation and genotyping of Toxoplasma gondii strains from domestic animals in Tunisia. Sci. Rep. 2021, 11, 9328. [Google Scholar] [CrossRef] [PubMed]
- Amairia, S.; Rouatbi, M.; Rjeibi, M.R.; Nouasri, H.; Sassi, L.; Mhadhbi, M.; Gharbi, M. Molecular prevalence of Toxoplasma gondii DNA in goats’ milk and seroprevalence in Northwest Tunisia. Vet. Med. Sci. 2016, 2, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Boughattas, S.; Bergaoui, R.; Essid, R.; Aoun, K.; Bouratbine, A. Seroprevalence of Toxoplasma gondii infection among horses in Tunisia. Parasit. Vectors 2011, 4, 218. [Google Scholar] [CrossRef] [PubMed]
- Bouaicha-Zaafouri, F. La Fièvre Hémorragique de Crimée-Congo et la Toxoplasmose au Sud de la Tunisie: Perception des Eleveurs et Rôle des Rongeurs Sauvages. Master’s Thesis, Institut National de Agronomique de Tunisie, Tunis, Tunisia, 2021. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science Ltd.: London, UK, 2007; pp. 227–247. [Google Scholar]
- Abdallah, M.C.; Kamel, M.; Karima, B.; Samir, A.; Mohammed Hocine, B.; Djamel, K.; Rachid, K.; Khatima, A.O. First report of Toxoplasma gondii infection and associated risk factors in the dromedary camel (Camelus dromedarius) population in Southeast Algeria. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100475. [Google Scholar]
- Yektaseresht, A.; Ghane, M.; Atashbar, F.; Aliabadi, J. Serological survey of Toxoplasma gondii infection in the one-humped camels (Camelus dromedarius) in the south of Iran. Vet. Med. Sci. 2023, 9, 2386–2389. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, F.S.; Borujeni, M.R.; Rahimi, E.; Abdizadeh, R. Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Foodborne Pathog. Dis. 2013, 10, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, A.D. Antibodies in sera from camels (Camelus dromedarius) in western and southern regions of Central Province, Saudi Arabia. J. Egypt. Soc. Parasitol. 2012, 42, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, E.Z.; Dima, N.; Beyi, A.F.; Dawo, F.; Feyissa, N.; Jorga, E.; Di Marco, V.; Vitale, M. Toxoplasmosis in camels (Camelus dromedarius) of Borana zone, Oromia region, Ethiopia: Seroprevalence and risk factors. Trop. Anim. Health Prod. 2016, 48, 1599–1606. [Google Scholar] [CrossRef]
- Fatima, T.; Mehnaz, S.; Wang, M.; Yang, J.; Sajid, M.S.; Shen, B.; Zhao, J. Seroprevalence of Toxoplasma gondii in one-humped camels (Camelus dromedarius) of Thal and Cholistan deserts, Punjab, Pakistan. Parasitol. Res. 2019, 118, 307–316. [Google Scholar] [CrossRef]
- Gebremedhin, E.Z.; Yunus, H.A.; Tesfamaryam, G.; Tessema, T.S.; Dawo, F.; Terefe, G.; Di Marco, V.; Vitale, M. First report of Toxoplasma gondii in camels (Camelus dromedarius) in Ethiopia: Bioassay and seroepidemiological investigation. BMC Vet. Res. 2014, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Marawan, M.A.; Abdelhady, A.; Wakid, M.H. Seroprevalence and potential risk factors of Toxoplasma gondii in dromedary camels. Agriculture 2023, 13, 129. [Google Scholar] [CrossRef]
- Mohammed, O.B.; Amor, N.; Omer, S.A.; Alagaili, A.N. Seroprevalence of Toxoplasma gondii and Neospora caninum in Dromedary camels (Camelus dromedarius) from Saudi Arabia. Rev. Bras. Parasitol. Vet. 2020, 29, e019119. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Hamdi, S.; Hamdi, N.; Chandoul, W.; Smida, B.B.; Romdhane, S.B. Microscopic and serological survey of Trypanosoma evansi infection in Tunisian dromedary camels (Camelus dromedarius). Vet. Parasitol. Reg. Stud. Rep. 2022, 32, 100741. [Google Scholar] [CrossRef] [PubMed]
- Florant, C.; Pontanier, R. L’aridité en Tunisie Présaharienne: Climat, Sol, Végétation et Aménagement; Office de Recherche Scientifique et Technique Outre-Mer: Paris, France, 1982. [Google Scholar]
- Rouatbi, M.; Amairia, S.; Amdouni, Y.; Boussaadoun, M.A.; Ayadi, O.; Al-Hosary, A.A.T.; Rekik, M.; Ben Abdallah, R.; Aoun, K.; Darghouth, M.A.; et al. Toxoplasma gondii infection and toxoplasmosis in North Africa: A review. Parasite 2019, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, S.; Basso, W.; Benavides Silván, J.; Ortega-Mora, L.M.; Maksimov, P.; Gethmann, J.; Conraths, F.J.; Schares, G. Toxoplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol. 2019, 15, e00037. [Google Scholar] [CrossRef] [PubMed]
- Said Nouira: Table of Carnivores in Tunisia. Faculté des Sciences de Tunis: Tunis, Tunisia, Unpublished data. 2023.
- Tibary, A.; Fite, C.; Anouassi, A.; Sghiri, A. Infectious causes of reproductive loss in camelids. Theriogenology 2006, 66, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Komnenou, A.T.; Giadinis, N.D.; Kritsepi-Konstantinou, M.; Thomas, A.L.; Danika, S.; Terpsidis, K.; Petridou, E.; Papadopoulos, E. Abortion Related to Toxoplasma gondii Infection in a Bactrian Camel (Camelus bactrianus) in Greece: A Case Report. Iran. J. Parasitol. 2022, 17, 111–117. [Google Scholar] [CrossRef]
- Serrano-Martínez, E.; Collantes-Fernández, E.; Chávez-Velásquez, A.; Rodríguez-Bertos, A.; Casas-Astos, E.; Risco-Castillo, V.; Rosadio-Alcantara, R.; Ortega-Mora, L.M. Evaluation of Neospora caninum and Toxoplasma gondii infections in alpaca (Vicugna pacos) and llama (Lama glama) aborted foetuses from Peru. Vet. Parasitol. 2007, 150, 39–45. [Google Scholar] [CrossRef]
| Governorate | ||
|---|---|---|
| Médenine | Tataouine | |
| Surface area in km2 (% surface of Tunisia) | 9167 (5.6%) | 38,889 (23.77%) |
| Köppen classification | BWh | BWh |
| Bioclimatic zone | Sahara | Sahara |
| Mean altitude (m) | 103 | 237 |
| Mean annual precipitation (mm) | 131 | 116 |
| Range of mean annual temperature (°C) | 11.4–28.7 | 10.5–28.6 |
| Mean temperature in winter (°C) | 12.1 | 11.2 |
| Mean temperature in summer (°C) | 27.7 | 27.7 |
| Human population (% Tunisian population) | 522,000 (4.4%) | 152,500 (1.29%) |
| Annual meat production in tons (% Tunisian production) | ||
| Sheep | 2500 | 1800 |
| Goats | 1955 | 400 |
| Camels | 1800 | 230 |
| Cows | 1000 | 30 |
| Factor | Positive/Examined | % ±SE * | OR [95% CI] | |
|---|---|---|---|---|
| Locality | Médenine | 21/40 | 52.5 ± 7.9 | 3.232 [1.614; 6.474] # |
| Tataouine | 53/208 | 25.5 ± 3 | ||
| Age group (years) | <5 | 12/45 | 26.7 ± 6.6 | 0.827 [0.401; 1.708] |
| ≥5 | 62/203 | 30.5 ± 3.2 | ||
| Husbandry system | Extensive | 63/228 | 27.6 ± 3 | 0.312 [0.124; 0.789] # |
| Intensive | 11/20 | 55 ± 11.1 | ||
| Contact with cats | Yes | 20/30 | 66.7 ± 8.6 | 6.074 [2.678; 13.778] # |
| No | 54/218 | 24.8 ± 2.9 | ||
| Reproductive status | Pregnant | 17/102 | 16.7 ± 3.7 | 0.312 [0.168; 0.579] # |
| Non-pregnant | 57/146 | 39 ± 4 | ||
| History of abortion | Yes | 3/4 | N.A. | N.A. |
| No | 71/244 | 29.1 ± 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeljli, A.; Rekik, S.; Ben Smida, B.; Chandoul, W.; Sassi, L.; Gharbi, M. First Seroepidemiological Study of Toxoplasma gondii Infection in Dromedaries (Camelus dromedarius) in Southern Tunisia. Microbiol. Res. 2024, 15, 1091-1098. https://doi.org/10.3390/microbiolres15030072
Jeljli A, Rekik S, Ben Smida B, Chandoul W, Sassi L, Gharbi M. First Seroepidemiological Study of Toxoplasma gondii Infection in Dromedaries (Camelus dromedarius) in Southern Tunisia. Microbiology Research. 2024; 15(3):1091-1098. https://doi.org/10.3390/microbiolres15030072
Chicago/Turabian StyleJeljli, Afef, Syrine Rekik, Boubaker Ben Smida, Walid Chandoul, Limam Sassi, and Mohamed Gharbi. 2024. "First Seroepidemiological Study of Toxoplasma gondii Infection in Dromedaries (Camelus dromedarius) in Southern Tunisia" Microbiology Research 15, no. 3: 1091-1098. https://doi.org/10.3390/microbiolres15030072
APA StyleJeljli, A., Rekik, S., Ben Smida, B., Chandoul, W., Sassi, L., & Gharbi, M. (2024). First Seroepidemiological Study of Toxoplasma gondii Infection in Dromedaries (Camelus dromedarius) in Southern Tunisia. Microbiology Research, 15(3), 1091-1098. https://doi.org/10.3390/microbiolres15030072







