Abstract
Zika virus (ZIKV) is an emerging flavivirus that represents significant public health challenges, particularly in the Americas, and is a substantial risk to other parts of the world due to its rapid expansion and its established association with neurological disorders, including Guillain–Barré syndrome and an intrauterine fetal infection that can cause microcephaly, blindness, and other congenital neurological complications. To date, no vaccine to prevent ZIKV infections has been approved. Therefore, developing a safe and effective vaccine against this virus is a global health priority. This review analyzes the ZIKV outbreaks, as well as associated neurological complications, its genome, and immunological responses. The current vaccines in development have reported results from preclinical and clinical trials about novel approaches to obtain safer and more effective vaccines and the challenges faced by ZIKV vaccine development.
1. Introduction
Zika virus (ZIKV) is an emerging flavivirus that was first isolated in 1947 from the blood of Rhesus monkey 766 in Uganda [1]. The first evidence of human infection by this virus was detected in 1952 when neutralizing antibodies were observed in sera of patients from East Africa. In the years following, it became evident that ZIKV was confined mainly to areas in Africa [2,3,4,5]. However, in 2007, isolated outbreaks were reported on the island of Yap, resulting in a major epidemic that infected approximately three-quarters of Yap residents, with about 5000 of the 6700 residents affected [6]. Subsequently, in 2014, another significant outbreak occurred in French Polynesia [7]. At the end of 2015, the virus was detected for the first time in Brazil [8]; since then, it has attracted global attention due to its rapid expansion, particularly in tropical and subtropical regions. As of January 2018, the Centers for Disease Control and Prevention (CDC) had reported 223,477 confirmed cases in 87 countries and territories worldwide, of which 48 are from Latin America [9,10]. It has been reported that ZIKV transmission to humans can occur through sexual transmission, bodily fluids, blood transfusion, and vertical transmission from mother to fetus during pregnancy [11,12,13,14,15]. However, the most frequent form of transmission is through the puncturing of the skin during the feeding process of female mosquitoes of the genus Aedes (A. aegypti, A. furcifer, A. taylori, A. luteocephalus, and A. africanus) [16,17]. The incubation period of the virus is 3 to 12 days, and the most frequent clinical manifestations seen in infected patients are high fever, skin rash, conjunctivitis, headache, malaise, and muscle-and-joint pain that lasts from 2 to 7 days and automatically resolves over time [10]. It should be noted that only 18% of ZIKV infection cases are symptomatic, and many people may never recognize that they have been infected [6]. Despite the enormous efforts of researchers, there is still no clinically approved drug to treat the disease; however, several drug candidate molecules have been discovered that have shown activity against ZIKV in vitro [18,19].
2. Neurological Complications
On 1 February 2016, the World Health Organization (WHO) declared ZIKV an international public health emergency [20] because the Zika outbreaks that have occurred in the Americas in recent years have been associated with Congenital Zika Syndrome (CZS). CZS is an intrauterine fetal infection that can cause congenital neurological complications such as microcephaly, ventriculomegaly, intracranial calcification, ocular abnormalities, and hearing loss [21,22]. CZS can also cause fetal development disorders such as growth restriction or fetal death when women are infected with the virus during the first trimester of pregnancy [21,23,24,25,26,27]. Cellular tropism and transmission mechanisms of ZIKV from mother to fetus during early pregnancy are still unknown in many aspects [28]. However, ZIKV has been shown to effectively target neural progenitor cells (NPCs), drastically shrinking them. In addition, it can cross the placenta and infect the amniotic fluid and fetal brain tissues, causing a significant impact on brain development [23,24,25]. ZIKV infection leads to cell cycle arrest, apoptosis, and inhibition of neural precursor cell differentiation, leading to cortical thinning and microcephaly [24]. In the context of ZIKV circulation, between 2015 and January 2018, more than 3720 cases of children born with congenital disabilities associated with previous ZIKV infections were reported in the Americas [28]. Likewise, during the epidemics in French Polynesia (2014) and the Americas (2015–2016), the incidence of Guillain–Barré syndrome (GBS) increased, and an association between GBS and ZIKV was established in a small proportion of those infected through epidemiological studies [29,30]. GBS is the most common cause of acute flaccid paralysis worldwide, with an incidence rate of approximately 1 for every 100,000 person-years [31]. GBS is an acute inflammatory polyradiculoneuropathy mediated by the immune system that classically presents progressive weakness, sensory changes, and hyporeflexia and is usually triggered by previous infection or other antigenic stimuli [32,33]. To date, 14 countries have reported an increased incidence of GBS associated with ZIKV [34]. In Brazil in 2015, 1708 cases of GBS with laboratory confirmation of prior ZIKV infection were reported [35]. In Colombia, among 381 cases of patients with neurological syndromes, 42 were confirmed as GBS associated with the virus [36]. In Mexico, there have been 95 cases in which the association between ZIKV and GBS was evaluated, and it was concluded that GBS occurs among patients with clinical symptoms [37,38].
3. ZIKV Genome
The ZIKV genome consists of a single chain of positive-sense RNA of approximately 11 kb delimited by two non-coding regions (5′- and 3′-NCR) and a single open reading frame encoding a polyprotein, which is processed into three structural proteins (capsid protein (C), precursor membrane (prM), and envelope protein (E)) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [39,40,41,42]. The latter are involved in viral genome replication, virion assembly, polyprotein processing, and evasion from the host’s antiviral responses [43,44].Results obtained by cryogenic electron microscopy (cryo-EM) have shown that the mature structure of ZIKV contains a capsid surrounded by a lipid bilayer that contains 180 copies of the E and M proteins in an icosahedral arrangement of 90 antiparallel heterodimers of E:M proteins, completely covering the viral surface (Figure 1A), like other flaviviruses [45,46,47]. The E protein consists of the following three domains: domain I (EDI), domain II (EDII), and domain III (EDIII) (Figure 1B) [40,41,46]. EDI acts as a bridge between EDII and EDIII, playing a crucial role in protein folding and the recognition of cell receptors [41,45]. EDII is responsible for the dimerization of the protein and contains the fusion peptide, which helps in the fusion of viral and cell membranes during the entry of viruses into the cell and is highly conserved in flaviviruses [40,42]. EDIII is an immunoglobulin-like domain that binds the virus to cell receptors [47,48]. The E protein exhibits only one Asn154 glycosylation site (in the glycan loop), identified as relevant for neurovirulence (Figure 1C) [47,48,49].
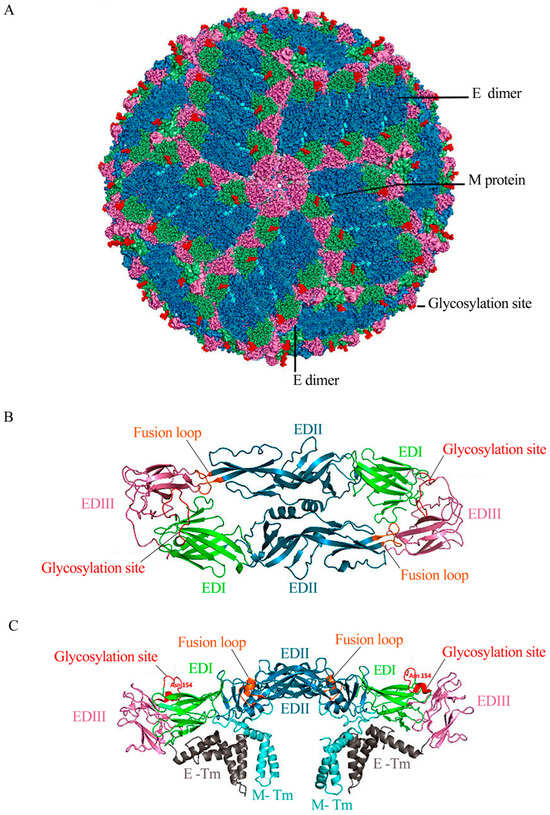
Figure 1.
Structure of ZIKV. (A) Bioinformatics modeling of the mature structure of ZIKV (Based on Protein Data Bank [PDB] 5IRE, 3.8 Å resolution) [45]. E proteins are organized as dimers, and three parallel dimers form the raft configuration. Thirty of these herringbone-like rafts completely cover the viral surface. In the dimers of the E protein, EDI, EDII, and EDIII are shown in green, blue, and pink, respectively. The M protein, hardly seen in the lower layer (cyan), fills the space between the E-protein rafts. The conserved glycosylation site in Asn154 is highlighted in red. (B) Top view of the E-protein dimer. EDI, EDII, and EDIII are shown in green, blue, and pink, respectively. The fusion loop located between residues 98–109 is shown in orange. (C) A side view of the E-protein dimer shows the transmembrane domains of E protein (E-TM) in gray and the transmembrane stem of M (M-Tm) in cyan.
Phylogenetic trees based on the entire coding region or NS5 region revealed the following three distinct genetic lineages of ZIKV reflecting the geographic origin: East Africa (Uganda prototype strain), West Africa (Senegal strains), and Asia (Yap Island strain). This study concluded that the Asian lineage diverged from a common ancestor and spread throughout Southeast Asia and the Pacific [50]. Congenital disabilities have only been reported in cases of infection by ZIKV strains belonging to the Asian lineage, including those identified during a 2016 outbreak in Angola. Therefore, it is crucial to note that the strains circulating in the Americas have been shown to belong to this lineage [9,24,27].
Similarly, a recent study demonstrated that these strains have a serine–asparagine substitution at prM position 139, leading to a more severe microcephalic phenotype and a thinner cortex than the ancestral strain in mice [50,51,52]. The occurrence of the S139N substitution is associated with increased reports of children born with congenital microcephaly and other serious neurological abnormalities, including GBS. This study suggested a possible explanation for the unexpected causal link between ZIKV and microcephaly and a probable understanding of how ZIKV evolved from a harmless mosquito-borne virus to a congenital pathogen with global impact [52,53].
4. Immune Response
Regarding the innate immune response, it has been shown that the activation and secretion of type I interferons (IFN-I) are critical because they can inhibit ZIKV replication in human cell lines and model mice [43,44,54,55,56]. In response to these mechanisms, ZIKV can antagonize IFN-I signaling during infection and disrupt the transcription of hundreds of IFN-stimulated genes (ISG), which encode the proteins that inhibit virus replication and spread [44,55,57,58]. Non-structural proteins NS1, NS3, NS4A, and NS5 have been identified as critical suppressors of IFN-I production [43,57,59]. The NS5 protein inhibits IFN-I signaling in human cells through the degradation of STAT2 in the ZIKV-infected host [54,56,57,58]. Therefore, knowing this mechanism of evasion of the immune response by the virus is essential to provide new venues for the development of antiviral drugs against ZIKV [59].
The adaptive immune response to ZIKV is protective and mediated by antibody-producing B cells in humoral immunity and T cells in cellular immunity [43,44,54,60]. In humoral immunity, viral proteins E, prM, and NS1 are the main targets of the neutralizing antibody response induced by ZIKV; these antibodies are essential for the generation of protection against virus infection [60,61,62]. Several isolated human monoclonal antibodies (mAbs) have demonstrated broad specificity against all ZIKV strains and potent protective activity both in vitro [62,63] and in vivo [64,65]. One of the most potent neutralizing antibodies, ZIKV-117, has demonstrated in vivo protective efficacy in mice against strains from Africa, Asia, and America. In addition, it decreased placental and fetal infection and reduced mortality in mice [65,66,67]. Likewise, it was found that there are highly specific neutralizing antibodies to ZIKV that recognize EDIII from E protein and have been reported to be the most potent neutralizers [65,68,69,70]. Another group of antibodies that recognize EDI and EDII has also been identified. They cross-reacted with DENV in vitro, which could cause antibody-dependent enhancement (ADE) and worsen the disease [71].
On the other hand, studies carried out in humans, non-human primates (NHPs), and mice infected by the virus have shown that the immunodominant response of ZIKV-specific CD8+ T cells is directed mainly towards structural proteins E, prM, and C, while CD4+ T response is directed toward proteins E, NS1, and NS5 [44,71,72,73,74]. Studies in mice demonstrated that CD8+ T has a protective role during infection, since the adoptive transfer of CD8+ T to Ifnar1−/− mice prevented central nervous system (CNS) infection and reduced weight loss and viral load [74,75,76]. Another study revealed that CD8+ T responses in pregnant mice decreased compared to non-pregnant mice. These results suggest that lowered T-cell-mediated immunity during pregnancy may increase the spread of the virus to the fetus [77]. Finally, although the adaptive immune response to the virus has been attributed to CD8+ T cells, the participation of CD4+ T cells is not fully understood [78]. A study revealed that an immune response involving CD4+ T cells and IFNγ signaling promoted the generation of B cells and anti-ZIKV neutralizing antibodies. This response was correlated with reduced viral load in the CNS and survival [73,78]. Likewise, it showed that the adoptive transfer of purified CD4+ T cells prevented weight loss associated with the infection and protected mice from a lethal challenge with ZIKV. Therefore, it was concluded that CD4+ T cells could play a crucial role in the protective immune response to ZIKV [21,78,79].
4.1. Antibody-Dependent Enhancement (ADE) of ZIKV Infection
There is a complex serological interaction between ZIKV and other human pathogenic flaviviruses, mainly with DENV [80]. The consequence of this interaction is the production of cross-reactive antibodies generated by previous infections with heterologous viruses, which can cause disease-enhancing activity through ADE [63,66,71,80,81]. The cell biology of ADE is still unknown [81]. However, it is believed to increase the efficacy of infection within the host after secondary infection (especially after reinfection by DENV) [82]. This occurs due to the presence of high concentrations of non-neutralizing antibodies that cross-react against the structural proteins of the secondary virus, facilitating the uptake of the virus by cells that express FcγR receptors and, therefore, the stimulation of higher viral loads [81,83,84,85]. Different in vitro studies have demonstrated the phenomenon of ADE among certain flaviviruses because specific antibodies against DENV [83,84,86] and WNV [87,88] increased the infectivity of ZIKV in cell culture. Likewise, in vivo studies have shown that DENV disease is lethally potentiated in mice [68,71,87,89] and NHPs when previously exposed to ZIKV [90,91]. On the other hand, analyses of mAbs generated from sera from donors infected with ZIKV and DENV also demonstrated cross-reactivity between these flaviviruses [71,86,88,92,93].
Antibodies prone to ADE were found to target epitopes of the prM and E protein fusion loop (FLE), a highly cross-reactive antigenic site [93,94]. These antibodies had little neutralizing activity. However, they potently promoted ADE [86,94,95].
It is unclear whether ZIKV antibodies could cause ADE in reinfection with DENV in humans. However, the first known patient with a fatal case of DENV strongly associated with prior exposure to ZIKV was recently reported in the United States [96]. This finding suggests that pre-existing immunity to ZIKV likely played a role in triggering ADE and intensified the DENV1 infection, leading to a fatal case of dengue hemorrhagic fever/Down syndrome consistent with ADE [95,96]. Therefore, given that the ZIKV outbreaks in recent years have occurred in DENV-endemic areas, the possibility that pre-existing antibodies may cause ADE or that an increased risk of fetal transmission of a ZIKV infection exists are among the most critical concerns in flavivirus vaccine design. The most recent research suggests that the mutation or deletion of prM and FLE in the design of new vaccines against ZIKV should help to reduce the risk of the induction of antibodies prone to ADE [95,96].
4.2. Evaluation of ZIKV Vaccines
ZIKV has challenged the scientific community to address a relatively poorly characterized pathogen that represents a substantial threat to global public health due to its rapid expansion throughout the Americas and a close association with neurological complications such as Guillain–Barré syndrome and congenital microcephaly in neonates. Research is currently underway to develop a prophylactic vaccine against the virus. Strategies under study span multiple vaccine platforms, including live attenuated and inactivated whole-virus vaccines, nucleic acid vaccines (DNA and RNA vaccines), viral vector-based vaccines, peptide subunit vaccines, recombinant proteins, and virus-like particles (Figure 2). Although no licensed vaccines or antivirals are yet available to prevent and control ZIKV, several vaccine candidates are undergoing clinical trials. For this reason, the rapid development of safe and effective vaccines against the virus is a public health priority. In the following section, we will offer an in-depth overview of the current ZIKV vaccine candidates and platforms undergoing clinical trials.
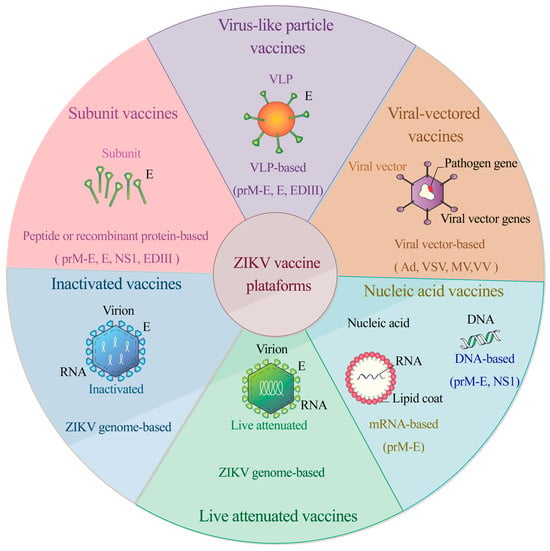
Figure 2.
Vaccine platforms under development against ZIKV. Strategies span multiple vaccine platforms, including live attenuated and inactivated whole-virus vaccines, nucleic acid vaccines (DNA and RNA), viral vector-based vaccines, peptide subunit vaccines, recombinant proteins, and virus-like particles.
4.3. Inactivated Vaccines
Since the outbreaks of 2015 in Brazil, the development of inactivated vaccines has been the primary strategy used by various research groups. ZPIV is a whole-virus, formalin-inactivated, alum-adjuvanted vaccine derived from the Puerto Rican strain PRVABC59 developed by the Walter Reed Army Institute of Research [97]. Preclinical studies in BALB/c mice showed that after a single immunization administered intramuscularly with alum, high levels of neutralizing antibodies were generated, conferring complete protection against viremia [97]. In a subsequent study, protective efficacy was also demonstrated in Rhesus macaques. After administration of two doses of vaccine in a month, specific binding antibodies to ZIKV-Env and neutralizing specific antibodies against ZIKV produced protection, which was subsequently confirmed with a second challenge to the monkeys one year later [64]. The safety and immunogenicity of ZPIV in humans were tested in three placebo-controlled clinical trials of two doses of 5 μg 28 days apart (NCT02963909, NCT02952833, and NCT02937233), and a fourth dose reduction study examining safety and immunogenicity was completed in August 2021 (NCT03008122) [60,98]. The results showed that the ZPIV vaccine is safe and well tolerated in a two-dose regimen at either weeks 0 and 4 or weeks 0 and 2. The ZPIV candidate generated potent neutralizing antibody titers in healthy adults, thus demonstrating its ability to induce a sufficient immune response that could offer potential clinical benefit [98,99]. Alternatively, Takeda Pharmaceuticals is developing a similar candidate that relies upon a purified inactivated virus vaccine called TAK-426. A Phase 1 clinical trial (NCT03343626) was completed in November 2020. The trial demonstrated that TAK-426 was well tolerated, had an acceptable safety profile, and was immunogenic in both flavivirus-naive and flavivirus-primed adults aged 18–49. Because of the limitations of the trial, there was not enough information on the durability of the response. The results showed that the 10 μg dose had the best immunogenicity and safety profile, justifying its selection for use in the Phase 2 clinical trial that was scheduled for June2023 (NCT05469802) to describe the side effects and the immunogenicity of TAK-426 in two different age groups [100]. VLA-1601, another inactivated vaccine, is also being evaluated. Although the Phase 1 clinical trial (NCT03425149) concluded in November 2018, the official final report has not been published yet. BBV121, developed by Bharat Biotech, Telangana, India, is another purified inactivated viral vaccine candidate. This vaccine formulation, containing the African prototype strain MR766 [101], has successfully completed a Phase 1 clinical trial (NCT04478656). The trial focused on assessing the safety, tolerability, and immunogenicity of two doses across three escalating cohorts of BBV121, compared to a placebo. It concluded in November 2018, but the report has not been published yet.
4.4. Live Attenuated Vaccines
The attenuated chimeric vaccine rZIKV/D4Δ30-713 uses ZIKV prM-E in a DENV-4 backbone, with a 30-nucleotide deletion in the 3 ‘UTR to attenuate viral replication. The Phase 1 clinical trial (NCT03611946) evaluated safety and immunogenicity in 56 adults with no previous flavivirus infection. This trial finished in March 2022 and concluded that the vaccine was safe and well tolerated, but the seroconversion was poor because chimerization can render highly attenuated viruses. Therefore, these results demonstrate that rZIKV/D4Δ30-713 is over-attenuated and will not continue being a vaccine candidate [102,103,104,105]. One of the drawbacks of live attenuated vaccines is that over-attenuation can compromise vaccine efficacy.
4.5. DNA Vaccines
Another popular strategy is the use of DNA vaccines. In 2016, three studies entered clinical trials for VRC5283 and VRC5288 vaccines, which are derived from French Polynesian strain H/PF/2013 of Asian lineage that encodes the prM and E proteins developed by the Vaccine Research Center (VRC) and the National Institute of Allergies and Infectious Diseases (NIAID) [106]. The design consisted of inserting the prM and E protein sequences into a vector containing the cytomegalovirus promoter (VRC8400), which has been clinically evaluated in previous studies, showing an excellent safety profile [107,108]. For VRC5283, prM was exchanged for prM from JEV, and for VRC5288, the final 98 amino acids of the E protein were substituted by the JEV analog sequences [106,107]. Preclinical studies in BALB/c mice and Rhesus macaques demonstrated that the two constructs could induce strong neutralizing specific antibody responses against ZIKV after two administrations four weeks apart [106]. In a separate trial, vaccines also reduced and eliminated viremia in some animals after a viral challenge, providing protection against the virus [60,106]. Based on these findings, both vaccines advanced to phase 1 clinical trials to evaluate safety, tolerability, and immunogenicity in humans (NCT02996461 and NCT02840487, respectively) [109]. The two evaluated DNA vaccines were safe and well-tolerated, and most adverse events were mild. Both vaccines were immunogenic, but the most remarkable effects were seen with VRC5283. This result is promising because 100% (14 of 14) of the participants who received it by needle-free injection in divided doses had detectable antibody responses and greater neutralizing and T-cell antibody responses. Therefore, VRC5283 advanced to the next phase of clinical trials (NCT03110770). The Phase 2 clinical trial concluded in October 2019. It aimed to assess safety, immunogenicity, and efficacy in populations in South and Central America, the Caribbean, and the USA endemic zone for ZIKV, using a vaccination regime at 0, 4, and 8 weeks with needle-free delivery using a Stratis device. The conclusions have yet to be published, but the results have already been uploaded to the NCT03110770 case record online. It was observed that after the 28-day analysis, the titers of Zika antigen-specific neutralizing antibodies were high. The best regime was four injections of 4 mg of vaccine administrated IM by a needle-free injection device, producing 187.629 geometric mean titers (GMT) of Zika antigen-specific neutralizing antibodies, which suggests a robust immune response. No severe adverse events were reported, and the most important reaction was at the injection site, causing pain; malaise; and, in some cases, headache and myalgia [110]. The fourth study, GLS-5700, used a vaccine constructed from the consensus sequence of prM genes and ZIKV E protein, using several isolates of the infection-causing virus in humans [111]. Preclinical studies demonstrated that this synthetic DNA vaccine administered by the CELLECTRA-3P electroporation device generated cellular and humoral immune responses, including the production of neutralizing antibodies in mice and NHPs, providing complete prevention against viremia [111]. The GLS-5700 DNA vaccine was evaluated in two clinical trials involving different study subjects. One was conducted in a healthy population (NCT02809443); in contrast, the other evaluated a population of adults seropositive for dengue virus (NCT02887482). Both trials have finished already, but only the results of the first trial have been published (2021). In the first trial, it was reported that the GLS-5700 vaccine did not produce any severe adverse events, and local minor reactions at the injection site included pain, redness, swelling, and itching. Remarkably, 62% of participants developed neutralizing antibodies, as demonstrated in a Vero-cell assay; a neuronal cell assay also showed that 70% and 95% of participant sera blocked infection by 90% and 50%, respectively. All these results suggest that vaccine-induced humoral responses are protective [112]. This candidate will be entering future trials to corroborate its efficiency.
4.6. RNA Vaccines
mRNA-1325 and mRNA-1893 are modified mRNA vaccines encapsulated in lipid nanoparticles (NPLs) encoding prM and E proteins derived from a 2007 Micronesia strain developed by Moderna Therapeutics, a biotechnology company in Cambridge, MA, USA. [113]. They were designed by replacing prM sequences with human IgE signal or JEV sequences [113,114]. Preclinical studies showed that after two administrations, both induced high levels of protecting neutralizing antibodies against ZIKV in multiple mouse models, including some in immunocompetent and immunosuppressed pregnant mice [113]. The protective responses elicited by mRNA-NPL vaccines were durable, even 14 weeks after the booster dose, and exposed mice showed no morbidity or mortality [113]. Given these results, both vaccines advanced to Phase 1 clinical trials for human testing (NCT03014089 and NCT04064905) [113]. By 2021, both clinical trials had already finished, and a complete report was published in 2023, remarking on the effectiveness and safety of both candidates. The data showed that mRNA-1325 did not elicit good titers of ZIKV-specific neutralizing Ab (NAb) responses, so it was discarded to participate in future clinical trials. In contrast, mRNA-1893 was well tolerated and only produced grade 1 and 2 adverse reactions at higher doses. No severe adverse events were reported. A 100 μg dose induced the best ZIKV-specific NAb response in flavivirus-seronegative participants and a 10 μg dose in flavivirus-seropositive participants. A two-dose schedule elicited a Nab response against ZIKV that persisted for at least 12 months after the last dose. One limitation of the study was that the antibody-dependent enhancement with dengue virus was not measurable. However, the mRNA-1893 Phase 1 clinical trial provided good results and will advance to a Phase 2 study in approximately 800 flavivirus-seropositive and -seronegative adults from the USA and Puerto Rico (NCT04917861); this study has an estimated completion date of April 2024 [115].
A similar vaccine has been developed by another research group, synthesized with a modified mRNA, nucleoside 1-methylpseudouridineand encapsulated in NPLs [61,116]. Their results showed that the vaccine elicited robust and long-lasting neutralizing antibody responses in mice and NHPs. In C57BL/6 mice, a single intradermal 30 μg immunization protected against immune challenges at two weeks and five months after vaccination, and a single dose of 50 μg was enough to protect NHPs against a challenge five weeks after vaccination [116]. These data demonstrated that nucleoside-modified mRNA-NPL produces rapid and long-lasting protective immunity; therefore, it is a promising new vaccine candidate against ZIKV [116].
4.7. Viral-Vectored Vaccines
Another vaccine approach involves expressing ZIKV genes along various viral vectors, including adenovirus, vesicular stomatitis virus (VSV), and live attenuated measles virus (MV). This strategy allows for the production of heterologous antigens after immunization [117,118,119]. MV-ZIKA is a vector of the Schwarz MV vaccine expressing the soluble prM and E proteins (MV-Zika-sE) developed by Themis Bioscience GmbH, Wien, Austria [120].
Preclinical studies in an allogeneic mouse pregnancy model showed that vaccination with this candidate induces robust humoral and cellular immune responses directed against the E protein of ZIKV [116,120]. In addition, it reduced the virus load in different organs and prevented fetal infection. Therefore, this vaccine has promising immunogenicity and protective capacity against infection and was evaluated in a Phase 1 clinical trial completed in 2018; the results have not been published yet (NCT02996890) [120]. A second, similar candidate vaccine (MV-ZIKV-RSP) against ZIKV was also evaluated in a Phase 1 clinical trial (NCT04033068) completed in June 2020. An official report has yet to be published, but the results are available online on the case record page (NCT04033068). Although no serious adverse effects were observed, there was no sign of significant induction of anti-ZIKA-RSP antibodies respecting the placebo groups [114,119].
Recombinant chimpanzee adenoviruses are currently being explored as vaccine vectors for multiple pathogens. ChAdOx1 is a replication-deficient chimpanzee adenoviral vaccine against ZIKV developed by the University of Oxford [121]. The vaccine design consisted of an expression cassette for prM and E proteins (without the transmembrane domain) [121]. In preclinical trials, a single dose without adjuvant elicited adequate levels of protective responses in mice exposed to ZIKV, as well as a specific, long-lasting immune response against ZIKV-Env without in vitro evidence of an ADE against DENV [121]. Due to its safety and immunogenicity profiles in humans, paired with its suitability for large-scale production under Good Manufacturing Practices (GMP), this vaccine was evaluated in two clinical trials—one of them in Oxford, England (NCT04015648), and the other in Monterrey, Mexico (NCT04440774). Both cases evaluated healthy volunteers aged 18–50. Also, both Phase 1 clinical trials are already completed, but to this date, no one has published the results. A similar ChAdOx1 vaccine against dengue virus (ChAdOx1 Chik) is being developed. A Phase 1 clinical trial (NCT03590392) showed that a single dose induced IgG and T-cell responses against CHIKV structural antigens. In addition, it showed excellent safety, tolerability, and 100% PRNT50 seroconversion after a single dose [121,122]. We need to await the results for ChAdOx1 Zika, expecting similar results. There is another adenoviral vector-based vaccine called Ad26.ZIKV.001. This vaccine took its design from a non-replicating adenoviral vector, type 26 (Ad26), that encodes the ZIKV M-Env antigens and was evaluated in preclinical stages in mice and NHPs; in these assays, the vaccine produced complete protection against viremia from ZIKV challenge [123]. Later, the Ad26.ZIKV.001 vaccine advanced to a Phase I clinical trial (NCT03356561) concluded in 2019 with favorable results. This clinical trial showed that all regimens were well tolerated, and in both regimes, ZIKV neutralizing titers peaked 14 days after the second vaccination. Neutralizing antibodies persisted for at least 1 year. A one-dose regimen of 1 × 1011 vp Ad26.ZIKV.001 induced seroconversion in all participants 56 days after vaccination. In addition, Env-specific cellular responses were induced. All these results show Ad26.ZIKV.001 as a promising candidate for further development [124]; however, no Phase 2 clinical trial has been scheduled yet.
In contrast, a promising example of a chimeric vaccine utilizing insect viruses is the ARPV/ZIKV vaccine, which incorporates genetic material encoding the prM and envelope E proteins of ZIKV onto an arbovirus (ARPV) backbone. This construct relies upon the idea that insect-specific viruses are incapable of replication within vertebrate hosts, so these vaccines are reliably safe. However, the immunogenicity is not good enough yet, and it will be necessary to improve this vaccine to elicit durable and protective immune responses against ZIKV infection [125].
Table 1 shows a summary of ZIKV vaccine candidates in ongoing clinical trials. Furthermore, in addition to the vaccine candidates being tested in clinical trials, there are also vaccine designs that use novel strategies for vaccine construction. These proposals will be described next.

Table 1.
ZIKV vaccine candidates in clinical development.
4.8. Recombinant and Subunit Vaccines
Recombinant and subunit-based vaccines have been developed as an alternative platform due to their notable safety, relative ease of antigen production, and modest cost [126]. Two research groups have proposed constructions of recombinant subunit vaccines using 80% and 90% of the gene encoding the ZIKV E protein’s N-terminal region in Escherichia coli and Drosophila, respectively [126,127]. The vaccines demonstrated immunogenicity and protective efficacy in immunocompetent mice by eliciting both humoral and cellular immune responses and protecting them against ZIKV in an in vivo challenge [126,127]. Another study described the production of EDIII in E. coli by inclusion bodies [70]. The vaccine was evaluated through subcutaneous immunization of mice with 25 and 50 μg of EDIII for 11 weeks. It stimulated virus-specific neutralizing antibody responses with titers above the threshold and correlated with protective immunity against ZIKV. An E-protein dimer was constructed in a recent study without prM or the immunodominant fusion loop epitope [128]. Mice immunization induced dimer-specific antibodies that protected them against ZIKV during pregnancy, with no evidence of ADE or antibody cross-reaction with those of DENV [128]. Recently, one of the most novel approaches was developing an intranasal vaccine that uses EDIII fused to the FLIPr protein, a formyl I receptor inhibitor, and an FcγR antagonist. The vaccine was tested in immunodeficient AG129 mice and induced protective immune responses against ZIKV. These results promise safer vaccines for high-risk populations, such as pregnant women, immunosuppressed infants, immunocompromised individuals, and the elderly [129]. Another attractive vaccine candidate is zDIII-F, which consists of a bacterial ferritin-based nanoparticle fused in frame with ZIKA envelope protein domain III at the amino terminus of ferritin. Immunization of mice with a single dose of zDIII-F resulted in the robust induction of neutralizing antibodies, protecting them from the lethal viral challenge. The vaccine induced the production of CD4 T cells and CD8 T cells, suggesting a complete immune response. The passive transfer of neutralizing antibodies from vaccinated subjects to naïve animals protected them against the lethal viral challenge [130].
4.9. Virus-Like Particle (VLP) Vaccines
Among the platforms currently under study, virus-like particles (VLPs) are a promising alternative for vaccine development because they are nanostructures that mimic the organization and conformation of viruses but lack the viral genome that allow their production and subsequent antigen presentation. They can trigger a strong humoral and cellular immune response due to their repetitive structures [131]. In the last four years, several studies have described strategies to assemble and produce VLPs of ZIKV using various virus proteins and testing their efficacy in animals. In 2017, a research group described the production in the Expi293 cell line of the joint expression of structural (CprME) and non-structural (NS2B/NS3) virus proteins. Immunization of BALB/c mice with the VLP vaccine stimulated significantly higher neutralizing antibody titers than an inactivated vaccine [132]. Another study proposed ZIKV-VLPs utilizing the hepatitis B core antigen (HBcAg) fused to EDIII, which proved capable of rapid production in large, readily purifiable quantities in Nicotiana benthamiana [133]. HBcAg-zDIII VLPs are highly immunogenic, as two doses elicited potent humoral and cellular responses in C57BL/6 mice and did not show evidence of ADE against DENV [133]. Immunologically optimized VLPs generated from the cucumber mosaic virus with a Th-cell epitope of tetanus toxin (CuMVtt) were also produced using EDIII and tested in BALB/c mice [134]. The vaccine induced high levels of specific IgG after a single injection, and such antibodies neutralized ZIKV without potentiating DENV infection in vitro [134].
In recent studies, the production of ZIKV-VLP in recombinant immune complexes (RICs) derived from plants and cloned in Pichia pastoris GS115 has been described using the EDIII fused to the hepatitis B surface antigen (HBcAg) protein [135]. This construct was shown to be immunogenic in BALB/c mice [136]. Furthermore, VLPs have been produced in the baculovirus expression system using prM and E proteins. They have demonstrated good immunogenicity in immunized mice, as they stimulate high levels of virus-neutralizing antibody titers, ZIKV-specific IgG titers, and potent memory T-cell responses [137]. A novel vaccine strategy has been reported using a phage display-produced mimetic envelope dimer epitope (EDE) docked on adeno-associated (AAV) capsids (VLP), which induces neutralizing antibodies and does not elicit ADE. This mimetic protein, called mimotope, is designated to be a dual-vaccine candidate for ZIKV and DENV. After immunization with one of these mimotopes, antibodies that recognized both viruses and were protective against the infection were induced, in addition to not eliciting ADE [138].
4.10. Epitope-Based Peptide Vaccines: An Immunoinformatics Approach
Currently, computational methods are an essential tool in developing new-generation vaccines. Due to the advances in computational immunoinformatics and immunogenomics, isolated epitopes that may stimulate specific humoral or cellular immune responses can be predicted [139]. Epitope-based vaccines are considered a cost-effective approach in developing vaccines meant to avoid whole-pathogen use. Some tools allow researchers to identify B-cell and T-cell epitopes using protein sequences or sequence alignments [139,140]. The prediction of B-cell epitopes is based on artificial neural networks that identify epitopes using mathematical algorithms and classify them according to the obtained score. The higher the peptide score, the greater the probability that it becomes an epitope [140]. The prediction of T-cell epitopes determines the binding affinity of a peptide to alleles of the major histocompatibility complex (MHC and HLA in humans), as they would be presented on the surface of the cell through the cross-presentation pathway, as well as the binding affinity of epitopes that interact with the transporter associated with antigen processing (TAP) [140,141,142].
In recent years, epitope-based peptide vaccines have been advertised as a new approach to obtaining more effective or safer vaccines against ZIKV because of the associated neurological disorders and autoimmunity syndromes [140]. The most recent designs of vaccines are based on peptides [143,144,145,146], T-cell epitopes [147,148], epitopes that bind to MHC-I and MHC-II [144,145], B-cell epitopes [147,148], and multi-pathogenic vaccines (that can simultaneously protect against CHIKV) [146]. In addition, vaccines based on phage VLPs that carry potential B-cell epitopes have been explored [149]. Satisfactory results have been reported with this type of vaccine in mice and cell lines. Several studies adopting immunoinformatics approaches are underway to find a candidate vaccine against ZIKV soon.
As an example of epitope-based vaccines, in 2020, Shahid et al. published an in silico design of a multi-epitope-based peptide vaccine, which was constructed by exploring the ZIKV proteome using immunoinformatic tools [147]. They used the ZIKV proteome to predict B-cell, T-cell, and IFN-γ epitopes and constructed a final sequence linked by AAY and GPGPG linkers. The final construct was of 435 amino acids and was evaluated in simulations of toxicity and immunogenicity. This strategy’s effectiveness relies on producing a complete immune response because the selected epitopes should trigger humoral and cellular immune responses. Even though there was no in vitro construct, the researchers concluded it could be a suitable candidate if validated experimentally [147].
In Table 2, we present a descriptive summary of promising strategies used to develop new ZIKV vaccines.

Table 2.
Promising strategies used to develop new ZIKV vaccines.
In summary, the development of vaccines against ZIKV has been the subject of intense research, encompassing various vaccine platforms in phases of development and evaluation. Next, a comparative evaluation of the main vaccine candidates will be presented, considering the results obtained in preclinical and clinical trials.
Inactivated vaccines: Clinical trials show that ZPIV has a tolerable safety profile and has been demonstrated to generate a sufficient immune response that could provide a potential clinical benefit. TAK-426 has also demonstrated safety and immunogenicity in clinical trials. It is expected to progress to Phase 2 trials to evaluate the long-term efficacy and durability of the immune response to determine its utility in broader populations [99,100].
DNA/RNA vaccines: VRC5283, GLS-5700, and mRNA-1893 have been demonstrated to induce strong neutralizing antibody responses in preclinical and clinical trials. VRC5283 achieved a 100% antibody response, as strong neutralizing antibody and T-cell responses were produced. Additionally, GLS-5700 exhibited a strong protective response, as participants developed antibodies to neutralize the infection. Both vaccines were well tolerated, with only minor local reactions reported. Furthermore, mRNA-1893 induced robust and long-lasting immune responses without serious adverse events [111,118].
Viral vector-based vaccines: ChAdOx1 and Ad26.ZIKV.001 have immense potential based on the results of preclinical and clinical trials. They have demonstrated the induction of specific immune responses against ZIKV, confirming their safety and tolerance in Phase 1 clinical trials. However, further research is needed to fully evaluate their long-term efficacy and ability to prevent ZIKV infection in diverse populations [123,124].
Although these vaccines present promising results, further research is required to assess the persistence of the provided protection and determine their effectiveness against natural exposure to ZIKV. This process involves conducting additional clinical trials in later stages and implementing long-term follow-up of participants to gain a more comprehensive understanding of the long-term effectiveness of these vaccines. In addition to safety and efficacy, it is essential to consider logistical and economic aspects in evaluating vaccines, such as ease of large-scale production, product stability, storage and distribution requirements, and the cost associated with each vaccine. These factors can significantly impact a vaccine’s implementation and long-term success in clinical practice. Table 3 provides a comprehensive comparison of all mentioned vaccine platforms, highlighting their primary advantages and disadvantages.

Table 3.
Benefits and drawbacks of vaccine platforms.
Selecting the most suitable vaccine against ZIKV will require a comprehensive evaluation considering each population’s specific needs, circumstances, and epidemiological situation. It may be necessary to implement a comprehensive vaccination strategy that combines different vaccines and approaches to effectively address the prevention and control of the disease in various contexts and populations. It is crucial to continue research and surveillance to obtain more data on the safety, efficacy, and durability of vaccines against ZIKV.
5. Challenges in ZIKV Vaccine Development
Efforts to develop a vaccine began quickly after the close association of ZIKV with severe neurological disorders and the increase in children born with congenital malformations among populations affected by the virus. Since then, a wide variety of strategies have been developed by researchers around the world to produce safe and effective vaccines against the virus. However, despite the efforts, several challenges must be solved and encompass several key areas. Firstly, pre-existing immunity to flaviviruses poses significant challenges due to the potential ADE effect, which could impact vaccine safety, immunogenicity, and clinical efficacy. Secondly, the possible association between ZIKV and CZS highlights the need to understand the immune responses necessary to prevent fetal infection and the devastating consequences of the virus. Thirdly, there is the theoretical concern that vaccine-induced immune responses might cause GBS. Finally, there are considerable challenges associated with vaccinating pregnant women and vulnerable populations. Vaccine safety is a priority, so thorough evaluations of possible adverse effects are required to evaluate long-term safety and effectiveness [154]. However, major challenges arise from the crucial need for well-characterized animal models that accurately reflect human diseases and from the significant decline in human ZIKV infection cases. The unpredictable nature of future outbreaks compounds this issue. In the absence of sustained viral transmission, evaluating the efficacy of candidate vaccines against the virus through traditional clinical trials becomes exceedingly complex [154].
To address this challenge, in 2016, the National Institute of Allergy and Infectious Diseases (NIAID) and the Walter Reed Army Institute of Research (WRAIR) engaged in discussions regarding the potential utilization of a ZIKV Controlled Human Infection Model (CHIM) to facilitate vaccine development. They deliberated on the conditions under which such a model could be ethically justified. It was emphasized that using a CHIM posed considerable risks due to the limited understanding of the virus at that time [155]. The declining number of natural Zika cases and challenges in conducting traditional clinical trials led to reconsideration of a ZIKV CHIM. Identifying critical endpoints for vaccine efficacy evaluation necessitated a comprehensive plan to address potential risks, including fetal infection, mosquito-borne transmission, sexual transmission, and GBS. Any study must exclusively recruit non-pregnant women to ensure fetal safety. Housing participants in a controlled environment can eliminate mosquito-borne transmission during the viremia period. This isolation also offers significant protection against sexual transmission. Furthermore, to reduce the risk of sexual transmission, all participants can be required to use barrier methods following discharge from the controlled environment. As an additional safety measure, the study must only enroll women because sexual transmission is more common in males. Finally, to mitigate the risk of GBS, participants should be limited to those aged 50 or younger with no history of GBS or autoimmune diseases [156].
Recently, as an alternative to this problem, the possibility of using new innovative biological modeling approaches has been raised, such as the use of organoids that simulate the infection to study the pathogenesis; detect new drugs; and, in the future, serve to test the vaccines that are under development against ZIKV [157]. Organoids are an exciting alternative, as they are three-dimensional scale models that can physiologically mimic the actual organ. In addition, data generated in vivo and in vitro to study the mechanisms of infection by pathogens using them exist. However, there are still limitations in developing organoids to fully model host immune responses [157]. It is unclear whether pre-existing antibodies to ZIKV could cause ADE after DENV reinfection in humans. However, the first patient with a fatal case of DENV associated with prior exposure to ZIKV was recently reported in the United States [96]. This suggests that pre-existing immunity to ZIKV may be highly determinant in causing ADE and increasing the risk of severe disease due to DENV. Therefore, future vaccines must consider the use of antigens that prevent ADE. Also, it is crucial to determine whether pre-existing cross-reactive antibodies may be involved in the fetal transmission of the virus or if they may modulate the immune response triggered by the virus and cause fetus malformations. The association of the virus with CZS remains a critical concern for vaccine development, as there is a need to develop a vaccine against the virus that can be administered independently to different target populations. An alternative would be nasal vaccines based on recombinant proteins because they are not infectious, can induce protective immune responses against ZIKV, and can be safe and effective options for high-risk populations, such as pregnant women, immunosuppressed individuals, and the elderly.
Despite declining reported cases, the true prevalence of ZIKV infections has been underestimated due to decreased vigilance in surveillance and monitoring efforts worldwide. A recent study conducted in Merida City, Yucatán, Mexico, during 2021–2022 revealed strong evidence of ongoing ZIKV transmission, estimating an incidence of 2.8–5.2 per 1000 person-years, with most cases being symptomatic. Moreover, this may still underestimate the actual prevalence due to potential waning of antibodies over time or undetectable antibodies in asymptomatic individuals because of test sensitivity. These findings highlight the importance of maintaining vigilance and awareness regarding the ongoing risk of ZIKV outbreaks. Furthermore, they emphasize the critical need for public health authorities to implement appropriate measures to prevent future outbreaks and improve diagnosis in transmission areas. Therefore, the development of vaccines against ZIKV remains a public health necessity [158].
6. Conclusions
In conclusion, this review underscores the imperative for the urgent development and licensing of an effective ZIKV vaccine despite a decline in reported cases since 2018 [154]. Persistent evidence indicates ongoing ZIKV transmission in high-risk areas, necessitating heightened concern within the public health system regarding potential future outbreaks. It is imperative to implement preventive measures capable of mitigating potential risks and improve diagnosis [158]. Various vaccine platforms have been explored, yet the outcomes of Phase I and Phase II clinical studies remain inconclusive, with some trials yet to publish their results. While endpoints for assessing immunogenicity and the durability of protective antibodies exist, additional considerations in ZIKV vaccine development include addressing safety concerns inherent in human clinical trials and eliciting robust innate and adaptive immune responses [77,155].
To achieve these goals, vaccine candidates must undergo rigorous animal testing before progressing to human trials. However, the limited global incidence of Zika infections poses challenges for large-scale clinical trials. Recognizing the urgency of addressing this infection disease as an imperative public health concern and the need to prevent future outbreaks, one promising approach involves establishing a controlled human infection model for ZIKV adhering rigorous risk assessment protocols, facilitating continued testing and data collection on ZIKV infections and immune responses. This model offers a significant advantage in expediting vaccine licensure [154,156]. Alternatively, exploring organoids as a surrogate for human participants in trials presents a potential avenue, although the technology’s limitations warrant further characterization [157].
Ongoing trials promise to provide additional insights to inform the selection of the most promising vaccine candidate and advance it toward Phase III clinical evaluation. Continued collaboration and innovation in vaccine development are essential to addressing the constant threat of ZIKV infection and safeguarding public health, preventing further outbreaks.
This exhaustive and meticulous review provides an in-depth understanding of all aspects of ZIKV and offers a detailed analysis of the current state of vaccines against the virus. From describing various vaccine platforms to identifying challenges in their development, this research stands out for its thoroughness and meticulousness in gathering and presenting relevant data.
Similarly, the information presented in this study is paramount to the scientific community, healthcare professionals, and policymakers, as it underscores the importance of international collaboration and continuous action in the fight against ZIKV to protect public health and prevent future health crises related to this emerging virus.
Author Contributions
Conceptualization, R.M.R.-A., J.A.C.-V., A.L.B.-P., S.R.-S.; Funding acquisition, G.A.-O., J.A.C.-V., A.J.-A. and R.M.R.-A.; Investigation, A.L.B.-P., S.R.-S., R.M.R.-A., J.A.C.-V., G.A.-O. and A.J.-A.; Methodology, A.L.B.-P., S.R.-S., J.A.C.-V., G.A.-O. and R.M.R.-A.; Resources, R.M.R.-A., J.A.C.-V., A.L.B.-P., S.R.-S.; Supervision, G.A.-O., J.A.C.-V., A.J.-A. and R.M.R.-A., Writing—original draft, A.L.B.-P., S.R.-S. and R.M.R.-A.; Writing—review and editing, G.A.-O., J.A.C.-V., A.J.-A., A.L.B.-P., S.R.-S. and R.M.R.-A. All authors have read and agreed to the published version of the manuscript.
Funding
The Instituto Politécnico Nacional supported this work through Secretaría de Investigación y Posgrado, grants 20242388 (J.A.C.-V.); 20242504 (A.J.-A.); 20242037 (G.A.-O.), and 20242379 (R.M.R.-A.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
A.L.B.-P. and S.R.-S. thank CONACyT and CONAHCyT, Mexico, and Instituto Politécnico Nacional (IPN) for graduate study fellowships. J.A.C.-V., A.J.-A., G.A.-O., and R.M.R.-A. are recipients of Comisión de Operación y Fomento de Actividades Académicas (COFAA-IPN) grants, and J.A.C.-V. is a recipient of the Programa de Estímulos al Desempeño de los Investigadores (EDI-IPN) grant. R.M.R.-A. and G.A.-O. are recipients of Programa de Estímulo al Desempeño Docente (EDD-IPN) fellowships, both granted by Instituto Politécnico Nacional, Mexico. J.A.C.-V. and R.M.R.-A. are members of the Sistema Nacional de Investigadores, CONAHCyT, Mexico.
Conflicts of Interest
We declare no conflicts of interest.
References
- Dick, G.W.A. Zika virus (II). Pathogenicity and physical properties. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Smithburn, K.C. Neutralizing Antibodies Against Certain Recently Isolated Viruses in the Sera of Human Beings Residing in East Africa. J. Immunol. 1952, 69, 223–234. [Google Scholar] [CrossRef]
- Moore, D.L.; Causey, O.R.; Carey, D.E.; Reddy, S.; Cooke, A.R.; Akinkugbe, F.M.; David-West, T.S.; Kemp, G.E. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann. Trop. Med. Parasitol. 1975, 69, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Fagbami, A.H. Zika virus infections in Nigeria: Virological and seroepidemiological investigations in Oyo State. J. Hyg. (Lond.) 1979, 83, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; DuBray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.-L.; Mallet, H.-P.; Sall, A.A.; Musso, D. Zika Virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- PAHO. Zika Cumulative Cases. 2016. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=12390%3Azika-cumulative-cases&catid=8424%3Acontents&Itemid=42090&lang=en (accessed on 22 February 2024).
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.-M. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361, Erratum in Emerg. Infect. Dis. 2015, 21, 552. [Google Scholar] [CrossRef]
- Gourinat, A.-C.; O’connor, O.; Calvez, E.; Goarant, C.; Dupont-Rouzeyrol, M. Detection of Zika Virus in Urine. Emerg. Infect. Dis. 2015, 21, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Stramer, S.L.; AABB Transfusion-Transmitted Diseases Committee; Busch, M.P.; International Society of Blood Transfusion Working Party on Transfusion-Transmitted Infectious Diseases. Zika virus: A new challenge for blood transfusion. Lancet 2016, 387, 1993–1994. [Google Scholar] [CrossRef] [PubMed]
- Besnard, M.; Lastère, S.; Teissier, A.; Cao-Lormeau, V.M.; Musso, D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Eurosurveillance 2014, 19, 20751. [Google Scholar] [CrossRef] [PubMed]
- Pomar, L.; Vouga, M.; Lambert, V.; Pomar, C.; Hcini, N.; Jolivet, A.; Benoist, G.; Rousset, D.; Matheus, S.; Malinger, G.; et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: Prospective cohort study in French Guiana. BMJ 2018, 363, k4431. [Google Scholar] [CrossRef]
- Hayes, E.B. Zika Virus Outside Africa. Emerg. Infect. Dis. 2009, 15, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Weger-Lucarelli, J.; Rückert, C.; Chotiwan, N.; Nguyen, C.; Luna, S.M.G.; Fauver, J.R.; Foy, B.D.; Perera, R.; Black, W.C.; Kading, R.C.; et al. Vector Competence of American Mosquitoes for Three Strains of Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0005101. [Google Scholar] [CrossRef] [PubMed]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, J.A.; Tran, L.T.; Li, J.; Luan, Y.; Siqueira-Neto, J.L.; Li, R. Drugs for the Treatment of Zika Virus Infection. J. Med. Chem. 2020, 63, 470–489. [Google Scholar] [CrossRef] [PubMed]
- WHO. Fifth Meeting of the Emergency Committee under the International Health Regulations (2005) Regarding Microcephaly, Other Neurological Disorders and Zika Virus. 2005. Available online: http://www.who.int/news-room/detail/18-11-2016-fifth-meeting-of-the-emergency-committee-under-the-international-health-regulations-(2005)-regarding-microcephaly-other-neurological-disorders-and-zika-virus (accessed on 22 February 2024).
- Lee, L.J.; Komarasamy, T.V.; Adnan, N.A.A.; James, W.; Balasubramaniam, V.R. Hide and Seek: The Interplay Between Zika Virus and the Host Immune Response. Front. Immunol. 2021, 12, 750365. [Google Scholar] [CrossRef]
- Wen, Z.; Song, H.; Ming, G.-L. How does Zika virus cause microcephaly? Genes Dev. 2017, 31, 849–861. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.-F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–126. [Google Scholar] [CrossRef]
- Magnani, D.M.; Rogers, T.F.; Maness, N.J.; Grubaugh, N.D.; Beutler, N.; Bailey, V.K.; Gonzalez-Nieto, L.; Gutman, M.J.; Pedreño-Lopez, N.; Kwal, J.M.; et al. Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nat. Commun. 2018, 9, 1624. [Google Scholar] [CrossRef]
- Rothan, H.A.; Fang, S.; Mahesh, M.; Byrareddy, S.N. Zika virus and the metabolism of neuronal cells. Mol. Neurobiol. 2018, 56, 2551–2557, Erratum in Mol. Neurobiol. 2018, 56, 2558. [Google Scholar] [CrossRef] [PubMed]
- Jabrane-Ferrat, N.; Veas, F. Zika Virus targets multiple tissues and cell types during the first trimester of pregnancy. Methods Mol. Biol. 2020, 2142, 235–249. [Google Scholar] [CrossRef]
- Marchi, S.; Viviani, S.; Montomoli, E.; Tang, Y.; Boccuto, A.; Vicenti, I.; Zazzi, M.; Sow, S.; Diallo, A.; Idoko, O.T.; et al. Zika Virus in West Africa: A Seroepidemiological Study between 2007 and 2012. Viruses 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Mier, Y.T.-R.L.; Delorey, M.J.; Sejvar, J.J.; Johansson, M.A. Guillain–Barré syndrome risk among individuals infected with Zika virus: A multi-country assessment. BMC Med. 2018, 16, 67. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population Incidence of Guillain-Barré Syndrome: A Systematic Review and Meta-Analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef]
- Schonberger, L.B.; Bregman, D.J.; Sullivan-Bolyai, J.Z.; Keenlyside, R.A.; Ziegler, D.W.; Retailliau, H.F.; Eddins, D.L.; Bryan, J.A. Guillain-Barre Syndrome Following Vaccination in The National Influenza Immunization Program, United States, 1976–19771. Am. J. Epidemiol. 1979, 110, 105–123. [Google Scholar] [CrossRef]
- Lewis, R.A.; Arcila-Londono, X. Guillain-Barré Syndrome. Semin. Neurol. 2012, 32, 179–186. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Rapid Risk Assessment. Zika Virus Disease Epidemic. Tenth Update, 4 April 2017. 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-zika-virus-disease-epidemic-10th-update-4-april-2017 (accessed on 22 February 2024).
- Styczynski, A.R.; Malta, J.M.A.S.; Krow-Lucal, E.R.; Percio, J.; Nóbrega, M.E.; Vargas, A.; Lanzieri, T.M.; Leite, P.L.; Staples, J.E.; Fischer, M.X.; et al. Increased rates of Guillain-Barré syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0005869. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Lizarazo, J.; Jiménez-Arango, J.A.; Zea-Vera, A.F.; González-Manrique, G.; Vargas, J.; Angarita, J.A.; Zuñiga, G.; Lopez-Gonzalez, R.; Beltran, C.L.; et al. Guillain–Barré Syndrome Associated with Zika Virus Infection in Colombia. N. Engl. J. Med. 2016, 375, 1513–1523. [Google Scholar] [CrossRef]
- Del Carpio-Orantes, L.; Moguel, K.P.; Díaz, J.S.; Pola-Ramirez, M.d.R.; Miranda, M.d.P.M.; García-Méndez, S.; Perfecto-Arroyo, M.; Solís-Sánchez, I.; Trujillo-Ortega, B.; González-Flores, E. Síndrome de Guillain-Barré asociado a zika; análisis de la cohorte delegacional en la región Veracruz norte durante 2016–2017. Neurologia 2018, 35, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Gongora-Rivera, F.; Grijalva, I.; Infante-Valenzuela, A.; Cámara-Lemarroy, C.; Garza-González, E.; Paredes-Cruz, M.; Grajales-Muñiz, C.; Guerrero-Cantera, J.; Vargas-Ramos, I.; Soares, J.; et al. Zika Virus infection and Guillain-Barré syndrome in Northeastern Mexico: A case-control study. PLoS ONE 2020, 15, e0230132. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, G.F. Structural Biology of the Zika Virus. Trends Biochem. Sci. 2017, 42, 443–456. [Google Scholar] [CrossRef]
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, B.; Fu, Z.F.; Chen, H.; Cao, S. Immune evasion strategies of flaviviruses. Vaccine 2013, 31, 461–471. [Google Scholar] [CrossRef]
- Culshaw, A.; Mongkolsapaya, J.; Screaton, G. The immunology of Zika Virus. F1000Research 2018, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, D.; Chen, Z.; Sun, L.; Klose, T.; Pierson, T.C.; Rossmann, M.G.; Kuhn, R.J. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 2016, 352, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, D.; Kuhn, R.J. Zika Virus Structure, Maturation, and Receptors. J. Infect. Dis. 2017, 216 (Suppl. S10), S935–S944. [Google Scholar] [CrossRef] [PubMed]
- Kostyuchenko, V.A.; Lim, E.X.Y.; Zhang, S.; Fibriansah, G.; Ng, T.-S.; Ooi, J.S.G.; Shi, J.; Lok, S.-M. Structure of the thermally stable Zika virus. Nature 2016, 533, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Sevvana, M.; Long, F.; Miller, A.S.; Klose, T.; Buda, G.; Sun, L.; Kuhn, R.J.; Rossmann, M.G. Refinement and Analysis of the Mature Zika Virus Cryo-EM Structure at 3.1 Å Resolution. Structure 2018, 26, 1169–1177.e3. [Google Scholar] [CrossRef] [PubMed]
- Carbaugh, D.L.; LaZear, H.M. Flavivirus Envelope Protein Glycosylation: Impacts on Viral Infection and Pathogenesis. J. Virol. 2020, 94, 94. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Huang, X.Y.; Liu, Z.-Y.; Zhang, F.; Zhu, X.-L.; Yu, J.-Y.; Ji, X.; Xu, Y.-P.; Li, G.; Li, C.; et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef]
- Ávila-Pérez, G.; Nogales, A.; Park, J.-G.; Márquez-Jurado, S.; Iborra, F.J.; Almazan, F.; Martínez-Sobrido, L. A natural polymorphism in Zika virus NS2A protein responsible of virulence in mice. Sci. Rep. 2019, 9, 19968. [Google Scholar] [CrossRef]
- Beaver, J.T.; Lelutiu, N.; Habib, R.; Skountzou, I. Evolution of Two Major Zika Virus Lineages: Implications for Pathology, Immune Response, and Vaccine Development. Front. Immunol. 2018, 9, 1640. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Balasubramaniam, V.R.; Brown, J.A.; Mena, I.; Grant, A.; Bardina, S.V.; Maringer, K.; Schwarz, M.C.; Maestre, A.M.; Sourisseau, M.; et al. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017, 13, e1006258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hou, S.; Airo, A.M.; Limonta, D.; Mancinelli, V.; Branton, W.; Power, C.; Hobman, T.C. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016, 17, 1766–1775. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Serman, T.M.; Gack, M.U. Evasion of Innate and Intrinsic Antiviral Pathways by the Zika Virus. Viruses 2019, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Kennedy, R.B.; Ovsyannikova, I.G.; Palacios, R.; Ho, P.L.; Kalil, J. Development of vaccines against Zika virus. Lancet Infect. Dis. 2018, 18, e211–e219. [Google Scholar] [CrossRef]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.-Q.; Musyoki, A.M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef]
- Dowd, K.A.; DeMaso, C.R.; Pelc, R.S.; Speer, S.D.; Smith, A.R.; Goo, L.; Platt, D.J.; Mascola, J.R.; Graham, B.S.; Mulligan, M.J.; et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep. 2016, 16, 1485–1491. [Google Scholar] [CrossRef]
- Wang, J.; Bardelli, M.; Espinosa, D.A.; Pedotti, M.; Ng, T.-S.; Bianchi, S.; Simonelli, L.; Lim, E.X.; Foglierini, M.; Zatta, F.; et al. A Human Bi-specific Antibody against Zika Virus with High Therapeutic Potential. Cell 2017, 171, 229–241.e15. [Google Scholar] [CrossRef]
- Abbink, P.; LaRocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’Ang’A, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Sapparapu, G.; Fernandez, E.; Kose, N.; Cao, B.; Fox, J.M.; Bombardi, R.G.; Zhao, H.; Nelson, C.A.; Bryan, A.L.; Barnes, T.; et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 2016, 540, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, J.; Gao, G.F. Monoclonal Antibodies against Zika Virus: Therapeutics and Their Implications for Vaccine Design. J. Virol. 2017, 91, e01049-17. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Miller, A.; Sapparapu, G.; Fernandez, E.; Klose, T.; Long, F.; Fokine, A.; Porta, J.C.; Jiang, W.; Diamond, M.S.; et al. A human antibody against Zika virus crosslinks the E protein to prevent infection. Nat. Commun. 2017, 8, 14722. [Google Scholar] [CrossRef]
- Zhao, H.; Fernandez, E.; Dowd, K.A.; Speer, S.D.; Platt, D.J.; Gorman, M.J.; Govero, J.; Nelson, C.A.; Pierson, T.C.; Diamond, M.S.; et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell 2016, 166, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajew, D.; Avila-Rios, S.; Nogueira, L.; Patel, R.; et al. Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell 2017, 169, 597–609.e11. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dent, M.; Lai, H.; Sun, H.; Chen, Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine 2017, 35, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91, e01469-17. [Google Scholar] [CrossRef]
- Grubor-Bauk, B.; Wijesundara, D.K.; Masavuli, M.; Abbink, P.; Peterson, R.L.; Prow, N.A.; Larocca, R.A.; Mekonnen, Z.A.; Shrestha, A.; Eyre, N.S.; et al. NS1 DNA vaccination protects against Zika infection through T cell–mediated immunity in immunocompetent mice. Sci. Adv. 2019, 5, eaax2388. [Google Scholar] [CrossRef]
- Pardy, R.D.; Rajah, M.M.; Condotta, S.A.; Taylor, N.G.; Sagan, S.M.; Richer, M.J. Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice. PLoS Pathog. 2017, 13, e1006184. [Google Scholar] [CrossRef]
- Huang, H.; Li, S.; Zhang, Y.; Han, X.; Jia, B.; Liu, H.; Liu, D.; Tan, S.; Wang, Q.; Bi, Y.; et al. CD8 + T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J. Virol. 2017, 91, e00900-17. [Google Scholar] [CrossRef] [PubMed]
- Ngono, A.E.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and Role of the CD8 + T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.W.; Myers, L.M.; Woods, T.A.; Messer, R.J.; Carmody, A.B.; McNally, K.L.; Scott, D.P.; Hasenkrug, K.J.; Best, S.M.; Peterson, K.E. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J. Immunol. 2017, 198, 3526–3535. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.G.O.; Kitoko, J.Z.; Ferreira, F.M.; Suzart, V.G.; Papa, M.P.; Coelho, S.V.A.; Cavazzoni, C.B.; Paula-Neto, H.A.; Olsen, P.C.; Iwasaki, A.; et al. Critical role of CD4+ T cells and IFNγ signaling in antibody-mediated resistance to Zika virus infection. Nat. Commun. 2018, 9, 3136. [Google Scholar] [CrossRef] [PubMed]
- Hassert, M.; Wolf, K.J.; Schwetye, K.E.; DiPaolo, R.J.; Brien, J.D.; Pinto, A.K. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018, 14, e1007237. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.; Anderson, R.; Gupta, S. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. Proc. Natl. Acad. Sci. USA 1999, 96, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.A.; Pierson, T.C. Antibody-mediated neutralization of flaviviruses: A reductionist view. Virology 2011, 411, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Crill, W.D.; Chang, G.-J.J. Localization and Characterization of Flavivirus Envelope Glycoprotein Cross-Reactive Epitopes. J. Virol. 2004, 78, 13975–13986. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Nascimento, E.J.M.; Cynthia, B.; Cordeiro, M.T.; De Carvalho, O.V.; De Mendonça, L.R.; Azevedo, E.A.N.; França, R.F.O.; Rafael, D.; Marques, E.T.A. Dengue virus-specific antibodies enhance Brazilian Zika virus infection. J. Infect. Dis. 2017, 215, 781–785. [Google Scholar] [CrossRef]
- Paul, L.M.; Carlin, E.R.; Jenkins, M.M.; Tan, A.L.; Barcellona, C.M.; Nicholson, C.O.; Michael, S.F.; Isern, S. Dengue virus antibodies enhance Zika virus infection. Clin. Transl. Immunol. 2016, 5, e117. [Google Scholar] [CrossRef] [PubMed]
- Langerak, T.; Mumtaz, N.; Tolk, V.I.; van Gorp, E.C.M.; Martina, B.E.; Rockx, B.; Koopmans, M.P.G. The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis. PLoS Pathog. 2019, 15, e1007640. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef]
- Garg, H.; Yeh, R.; Watts, D.M.; Mehmetoglu-Gurbuz, T.; Resendes, R.; Parsons, B.; Gonzales, F.; Joshi, A. Enhancement of Zika virus infection by antibodies from West Nile virus seropositive individuals with no history of clinical infection. BMC Immunol. 2021, 22, 5. [Google Scholar] [CrossRef]
- Wessel, A.W.; Kose, N.; Bombardi, R.G.; Roy, V.; Chantima, W.; Mongkolsapaya, J.; Edeling, M.A.; Nelson, C.A.; Bosch, I.; Alter, G.; et al. Antibodies targeting epitopes on the cell-surface form of NS1 protect against Zika virus infection during pregnancy. Nat. Commun. 2020, 11, 5278. [Google Scholar] [CrossRef]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.-J.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef]
- Valiant, W.G.; Huang, Y.-J.S.; Vanlandingham, D.L.; Higgs, S.; Lewis, M.G.; Mattapallil, J.J. Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection. Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177, Erratum in Nat. Immunol. 2015, 16, 544; Erratum in Nat. Immunol. 2015, 16, 785. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.-C.; Medits, I.; Sharma, A.; Simon-Lorière, E.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Haouz, A.; et al. Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature 2016, 536, 48–53, Erratum in Nature 2016, 539, 314. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Xu, K.; Li, J.; Huang, Q.; Song, J.; Han, Y.; Zheng, T.; Gao, P.; Lu, X.; Yang, H.; et al. Protective Zika vaccines engineered to eliminate enhancement of dengue infection via immunodominance switch. Nat. Immunol. 2021, 22, 958–968. [Google Scholar] [CrossRef]
- Bonheur, A.N.; Thomas, S.; Soshnick, S.H.; McGibbon, E.; Dupuis, A.P., 2nd; Hull, R.; Slavinski, S.; Del Rosso, P.E.; Weiss, D.; Hunt, D.T.; et al. A fatal case report of antibody-dependent enhancement of dengue virus type 1 following remote Zika virus infection. BMC Infect. Dis. 2021, 21, 749. [Google Scholar] [CrossRef]
- LaRocca, R.A.; Abbink, P.; Peron, J.P.S.; de A. Zanotto, P.M.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’Ang’A, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.E.; Tan, C.S.; Walsh, S.R.; Hale, A.; Ansel, J.L.; Kanjilal, D.G.; Jaegle, K.; Peter, L.; Borducchi, E.N.; Nkolola, J.P.; et al. Safety and immunogenicity of a Zika purified inactivated virus vaccine given via standard, accelerated, or shortened schedules: A single-centre, double-blind, sequential-group, randomised, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 1061–1070. [Google Scholar] [CrossRef]
- Modjarrad, K.; Lin, L.; George, S.L.; Stephenson, K.E.; Eckels, K.H.; De La Barrera, R.A.; Jarman, R.G.; Sondergaard, E.; Tennant, J.; Ansel, J.L.; et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: Phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 2017, 391, 563–571, Erratum in Lancet 2020, 395, 1906. [Google Scholar] [CrossRef]
- Han, H.-H.; Diaz, C.; Acosta, C.J.; Liu, M.; Borkowski, A. Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in healthy adults: An observer-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Bharat Biotech International Limited. Safety and Immunogenicity of BBV121. 2020. Available online: https://clinicaltrials.gov/study/NCT04478656 (accessed on 2 May 2023).
- Kim, I.-J.; Blackman, M.A.; Lin, J.-S. Pre-Clinical Pregnancy Models for Evaluating Zika Vaccines. Trop. Med. Infect. Dis. 2019, 4, 58. [Google Scholar] [CrossRef]
- Castanha, P.M.S.; Marques, E.T.A. A Glimmer of Hope: Recent Updates and Future Challenges in Zika Vaccine Development. Viruses 2020, 12, 1371. [Google Scholar] [CrossRef]
- NIH. Evaluation of the Safety and Immunogenicity of the Live Attenuated Zika Vaccine rZIKV/D4Δ30-713 in Fla-vivirus-naïve Adults. ClinicalTrials. 2023. Available online: https://clinicaltrials.gov/study/NCT03611946?id=NCT03611946&rank=1 (accessed on 29 August 2023).
- Zhong, D.; Kirkpatrick, B.; Pierce, K.; Patel, U.; Blunt, M.R.; Durbin, A. 2138. Evaluating the Safety and Immunogenicity of the rZIKV/D4Δ30-713 Live Attenuated Chimeric Zika Candidate Vaccine in Healthy Flavivirus-Naïve Adults. Open Forum Infect. Dis. 2022, 9 (Suppl. S2), ofac492-1758. [Google Scholar] [CrossRef]
- Dowd, K.A.; Ko, S.-Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.S.; Chang, G.-J.J.; Cropp, B.; Roehrig, J.T.; Martin, D.A.; Mitchell, C.J.; Bowen, R.; Bunning, M.L. West Nile Virus Recombinant DNA Vaccine Protects Mouse and Horse from Virus Challenge and Expresses In Vitro a Noninfectious Recombinant Antigen That Can Be Used in Enzyme-Linked Immunosorbent Assays. J. Virol. 2001, 75, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, J.E.; Pierson, T.C.; Hubka, S.A.; Desai, N.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Nelson, S.; Nason, M.; Gu, W.; et al. A West Nile Virus DNA Vaccine Utilizing a Modified Promoter Induces Neutralizing Antibody in Younger and Older Healthy Adults in a Phase I Clinical Trial. J. Infect. Dis. 2011, 203, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Gaudinski, M.R.; Houser, K.V.; Morabito, K.M.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2018, 391, 552–562. [Google Scholar] [CrossRef] [PubMed]
- NIH. VRC 705: A Zika Virus DNA Vaccine in Healthy Adults and Adolescents (DNA). Zika Virus Infection Clinical Trials. ClinicalTrials. 2023. Available online: https://clinicaltrials.gov/study/NCT03110770?cond=Zika%20Virus%20Infection&aggFilters=phase:2&rank=2 (accessed on 29 August 2023).
- Muthumani, K.; Griffin, B.D.; Agarwal, S.; Kudchodkar, S.B.; Reuschel, E.L.; Choi, H.; Kraynyak, K.A.; Duperret, E.K.; Keaton, A.A.; Chung, C.; et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. npj Vaccines 2016, 1, 16021. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine—Preliminary Report. N. Engl. J. Med. 2017, 385, e35. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10, Erratum in Cell 2017, 169, 176. [Google Scholar] [CrossRef]
- Schrauf, S.; Tschismarov, R.; Tauber, E.; Ramsauer, K. Current Efforts in the Development of Vaccines for the Prevention of Zika and Chikungunya Virus Infections. Front. Immunol. 2020, 11, 592. [Google Scholar] [CrossRef]
- Essink, B.; Chu, L.; Seger, W.; Barranco, E.; Le Cam, N.; Bennett, H.; Faughnan, V.; Pajon, R.; Paila, Y.D.; Bollman, B.; et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 2023, 23, 621–633. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Guo, Q.; Chan, J.F.-W.; Poon, V.K.-M.; Wu, S.; Chan, C.C.-S.; Hou, L.; Yip, C.C.-Y.; Ren, C.; Cai, J.-P.; Zhao, M.; et al. Immunization with a Novel Human Type 5 Adenovirus-Vectored Vaccine Expressing the Premembrane and Envelope Proteins of Zika Virus Provides Consistent and Sterilizing Protection in Multiple Immunocompetent and Immunocompromised Animal Models. J. Infect. Dis. 2018, 218, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, D.; de Queiroz, N.M.G.P.; Xia, T.; Ahn, J.; Barber, G.N. Cutting Edge: Innate Immune Augmenting Vesicular Stomatitis Virus Expressing Zika Virus Proteins Confers Protective Immunity. J. Immunol. 2017, 198, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, C.; Bodmer, B.S.; Fiedler, A.H.; Gabriel, G.; Mühlebach, M.D. A Measles Virus-Based Vaccine Candidate Mediates Protection against Zika Virus in an Allogeneic Mouse Pregnancy Model. J. Virol. 2019, 93, e01485-18. [Google Scholar] [CrossRef] [PubMed]
- Themis Bioscience GmbH. Zika-Vaccine Dose Finding Study Regarding Safety, Immunogenicity and Tolerability (V186-001). 2022. Available online: https://clinicaltrials.gov/study/NCT02996890 (accessed on 2 May 2023).
- López-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Harrison, K.; Preciado-Llanes, L.; Lopez, F.R.; Bittaye, M.; Kim, Y.C.; Flaxman, A.; Bellamy, D.; Makinson, R.; Sheridan, J.; et al. A single dose of ChAdOx1 Chik vaccine induces neutralizing antibodies against four chikungunya virus lineages in a phase 1 clinical trial. Nat. Commun. 2021, 12, 4636. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.; Van Der Fits, L.; Abbink, P.; Larocca, R.A.; Van Huizen, E.; Saeland, E.; Verhagen, J.; Peterson, R.; Tolboom, J.; Kaufmann, B.; et al. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PLoS ONE 2018, 13, e0202820. [Google Scholar] [CrossRef] [PubMed]
- Salisch, N.C.; Stephenson, K.E.; Williams, K.; Cox, F.; van der Fits, L.; Heerwegh, D.; Truyers, C.; Habets, M.N.; Kanjilal, D.G.; Larocca, R.A.; et al. A Double-Blind, Randomized, Placebo-Controlled Phase 1 Study of Ad26.ZIKV.001, an Ad26-Vectored Anti–Zika Virus Vaccine. Ann. Intern. Med. 2021, 174, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Porier, D.L.; Adam, A.; Kang, L.; Michalak, P.; Tupik, J.; Santos, M.A.; Lee, C.; Allen, I.C.; Wang, T.; Auguste, A.J. Humoral and T-cell-mediated responses to a pre-clinical zika vaccine candidate that utilizes a unique insect-specific flavivirus platform. bioRxiv 2023. [Google Scholar] [CrossRef]
- Liang, H.; Yang, R.; Liu, Z.; Li, M.; Liu, H.; Jin, X. Recombinant Zika virus envelope protein elicited protective immunity against Zika virus in immunocompetent mice. PLoS ONE 2018, 13, e0194860. [Google Scholar] [CrossRef]
- Han, J.-F.; Qiu, Y.; Yu, J.-Y.; Wang, H.-J.; Deng, Y.-Q.; Li, X.-F.; Zhao, H.; Sun, H.-X.; Qin, C.-F. Immunization with truncated envelope protein of Zika virus induces protective immune response in mice. Sci. Rep. 2017, 7, 10047. [Google Scholar] [CrossRef]
- Slon-Campos, J.L.; Dejnirattisai, W.; Jagger, B.W.; López-Camacho, C.; Wongwiwat, W.; Durnell, L.A.; Winkler, E.S.; Chen, R.E.; Reyes-Sandoval, A.; Rey, F.A.; et al. A protective Zika virus E-dimer-based subunit vaccine engineered to abrogate antibody-dependent enhancement of dengue infection. Nat. Immunol. 2019, 20, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-S.; Hsu, C.-W.; Tu, L.-L.; Chai, K.M.; Yu, L.-L.; Wu, C.-C.; Chen, M.-Y.; Chiang, C.-Y.; Liu, S.-J.; Liao, C.-L.; et al. Intranasal Vaccination with Recombinant Antigen-FLIPr Fusion Protein Alone Induces Long-Lasting Systemic Antibody Responses and Broad T Cell Responses. Front. Immunol. 2021, 12, 751883. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sahoo, B.R.; Struble, L.R.; Borgstahl, G.E.O.; Zhou, Y.; Franco, R.; Barletta, R.G.; Osorio, F.A.; Petro, T.M.; Pattnaik, A.K. A Ferritin Nanoparticle-Based Zika Virus Vaccine Candidate Induces Robust Humoral and Cellular Immune Responses and Protects Mice from Lethal Virus Challenge. Vaccines 2023, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, J.; Gòdia, F.; Cervera, L. Production of virus-like particles for vaccines. New Biotechnol. 2017, 39 Pt B, 174–180. [Google Scholar] [CrossRef]
- Boigard, H.; Alimova, A.; Martin, G.R.; Katz, A.; Gottlieb, P.; Galarza, J.M. Zika virus-like particle (VLP) based vaccine. PLoS Negl. Trop. Dis. 2017, 11, e0005608. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Miranda, G.; Lim, S.M.; Mohsen, M.O.; Pobelov, I.V.; Roesti, E.S.; Heath, M.D.; Skinner, M.A.; Kramer, M.F.; Martina, B.E.E.; Bachmann, M.F. Zika Virus-Derived E-DIII Protein Displayed on Immunologically Optimized VLPs Induces Neutralizing Antibodies without Causing Enhancement of Dengue Virus Infection. Vaccines 2019, 7, 72, Erratum in Vaccines 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Diamos, A.G.; Pardhe, M.D.; Sun, H.; Hunter, J.G.; Mor, T.; Meador, L.; Kilbourne, J.; Chen, Q.; Mason, H.S. Codelivery of improved immune complex and virus-like particle vaccines containing Zika virus envelope domain III synergistically enhances immunogenicity. Vaccine 2020, 38, 3455–3463. [Google Scholar] [CrossRef]
- Shanmugam, R.K.; Ramasamy, V.; Shukla, R.; Arora, U.; Swaminathan, S.; Khanna, N. Pichia pastoris-expressed Zika virus envelope domain III on a virus-like particle platform: Design, production and immunological evaluation. Pathog. Dis. 2019, 77, ftz026. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, T.; Zhang, Y.; Wang, H.; Deng, F. Zika Virus Baculovirus-Expressed Virus-Like Particles Induce Neutralizing Antibodies in Mice. Virol. Sin. 2018, 33, 213–226. [Google Scholar] [CrossRef]
- Cuevas-Juárez, E.; Liñan-Torres, A.; Hernández, C.; Kopylov, M.; Potter, C.S.; Carragher, B.; Ramírez, O.T.; Palomares, L.A. Mimotope discovery as a tool to design a vaccine against Zika and dengue viruses. Biotechnol. Bioeng. 2023, 120, 2658–2671. [Google Scholar] [CrossRef] [PubMed]
- Parvizpour, S.; Pourseif, M.M.; Razmara, J.; Rafi, M.A.; Omidi, Y. Epitope-based vaccine design: A comprehensive overview of bioinformatics approaches. Drug Discov. Today 2020, 25, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Soria-Guerra, R.E.; Nieto-Gomez, R.; Govea-Alonso, D.O.; Rosales-Mendoza, S. An overview of bioinformatics tools for epitope prediction: Implications on vaccine development. J. Biomed. Inform. 2015, 53, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Backert, L.; Kohlbacher, O. Immunoinformatics and epitope prediction in the age of genomic medicine. Genome Med. 2015, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Dorigatti, E.; Schubert, B. Graph-theoretical formulation of the generalized epitope-based vaccine design problem. PLoS Comput. Biol. 2020, 16, e1008237. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Ali, S.; Ahamad, S.; Malik, Z.; Ishrat, R. From ZikV genome to vaccine: In silico approach for the epitope-based peptide vaccine against Zika virus envelope glycoprotein. Immunology 2016, 149, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Neto, E.; Rosa, D.S.; Harris, P.E.; Olson, T.; Morrow, A.; Ciotlos, S.; Herst, C.V.; Rubsamen, R.M. An Approach for a Synthetic CTL Vaccine Design against Zika Flavivirus Using Class I and Class II Epitopes Identified by Computer Modeling. Front. Immunol. 2017, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.U.; Rafique, S.; Ali, A.; Munir, M.; Ikram, N.; Manan, A.; Salo-Ahen, O.M.H.; Idrees, M. Towards peptide vaccines against Zika virus: Immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of Zika viral proteins. Sci. Rep. 2016, 6, 37313, Erratum in Sci. Rep. 2017, 7, 44633. [Google Scholar] [CrossRef]
- Prow, N.A.; Liu, L.; Nakayama, E.; Cooper, T.H.; Yan, K.; Eldi, P.; Hazlewood, J.E.; Tang, B.; Le, T.T.; Setoh, Y.X.; et al. A vaccinia-based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nat. Commun. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Shahid, F.; Ashfaq, U.A.; Javaid, A.; Khalid, H. Immunoinformatics guided rational design of a next generation multi epitope based peptide (MEBP) vaccine by exploring Zika virus proteome. Infect. Genet. Evol. 2020, 80, 104199. [Google Scholar] [CrossRef]
- Pandey, R.K.; Ojha, R.; Mishra, A.; Prajapati, V.K. Designing B- and T-cell multi-epitope based subunit vaccine using immunoinformatics approach to control Zika virus infection. J. Cell. Biochem. 2018, 119, 7631–7642. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Zhai, L.; Contreras, A.; Tumban, E. Immunization with phage virus-like particles displaying Zika virus potential B-cell epitopes neutralizes Zika virus infection of monkey kidney cells. Vaccine 2018, 36, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Hu, J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Giesker, K.; Hensel, M. Bacterial Vaccines. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 213–224. ISBN 9780128012383. [Google Scholar]
- Khoshnood, S.; Arshadi, M.; Akrami, S.; Koupaei, M.; Ghahramanpour, H.; Shariati, A.; Sadeghifard, N.; Heidary, M. An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. J. Clin. Lab. Anal. 2022, 36, e24418. [Google Scholar] [CrossRef] [PubMed]
- VaccinesWork. What Are Protein Subunit Vaccines and How Could They Be Used against COVID-19? 2022. Available online: https://www.gavi.org/vaccineswork/what-are-protein-subunit-vaccines-and-how-could-they-be-used-against-covid-19 (accessed on 14 February 2024).
- Vannice, K.S.; Cassetti, M.C.; Eisinger, R.W.; Hombach, J.; Knezevic, I.; Marston, H.D.; Wilder-Smith, A.; Cavaleri, M.; Krause, P.R. Demonstrating vaccine effectiveness during a waning epidemic: A WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates. Vaccine 2019, 37, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Seema, K.; Shah, J.D.; Kimmelman, J.; Lyerly, A.D.; Lynch, H.F.; McCutchan, F.; Miller, F.G.; Palacios, R.; Pardo-Villamizar, C.; Zorrilla, C. Ethical Considerations for Zika Virus Human Challenge Trials: Report and Recommendations. 2017. Available online: https://www.niaid.nih.gov/sites/default/files/EthicsZikaHumanChallengeStudiesReport2017.pdf (accessed on 14 February 2024).
- Durbin, A.P.; Whitehead, S.S. Zika Vaccines: Role for Controlled Human Infection. J. Infect. Dis. 2017, 216, S971–S975. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; Cricca, M.; Nastasi, C. Are the Organoid Models an Invaluable Contribution to ZIKA Virus Research? Pathogens 2021, 10, 1233. [Google Scholar] [CrossRef]
- Earnest, J.T.; Ciau-Carillo, K.J.; Kirstein, O.D.; Che-Mendoza, A.; Espinoza, D.O.; Puerta-Guardo, H.; Yam-Trujillo, K.; Parra-Cardeña, M.; Barrera-Fuentes, G.A.; Pavia-Ruz, N.; et al. Evidence of Ongoing Transmission of Zika Virus in Mérida, Mexico. Am. J. Trop. Med. Hyg. 2024, 110, 724–730. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).