Novel Vesicular Formulation Based on a Herbal Extract Loaded with Niosomes and Evaluation of Its Antimicrobial and Anticancer Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Materials
2.3. Extract Preparation
2.4. Gas Chromatography/Mass Spectrometry (GC-MS) Analysis of the Extracts
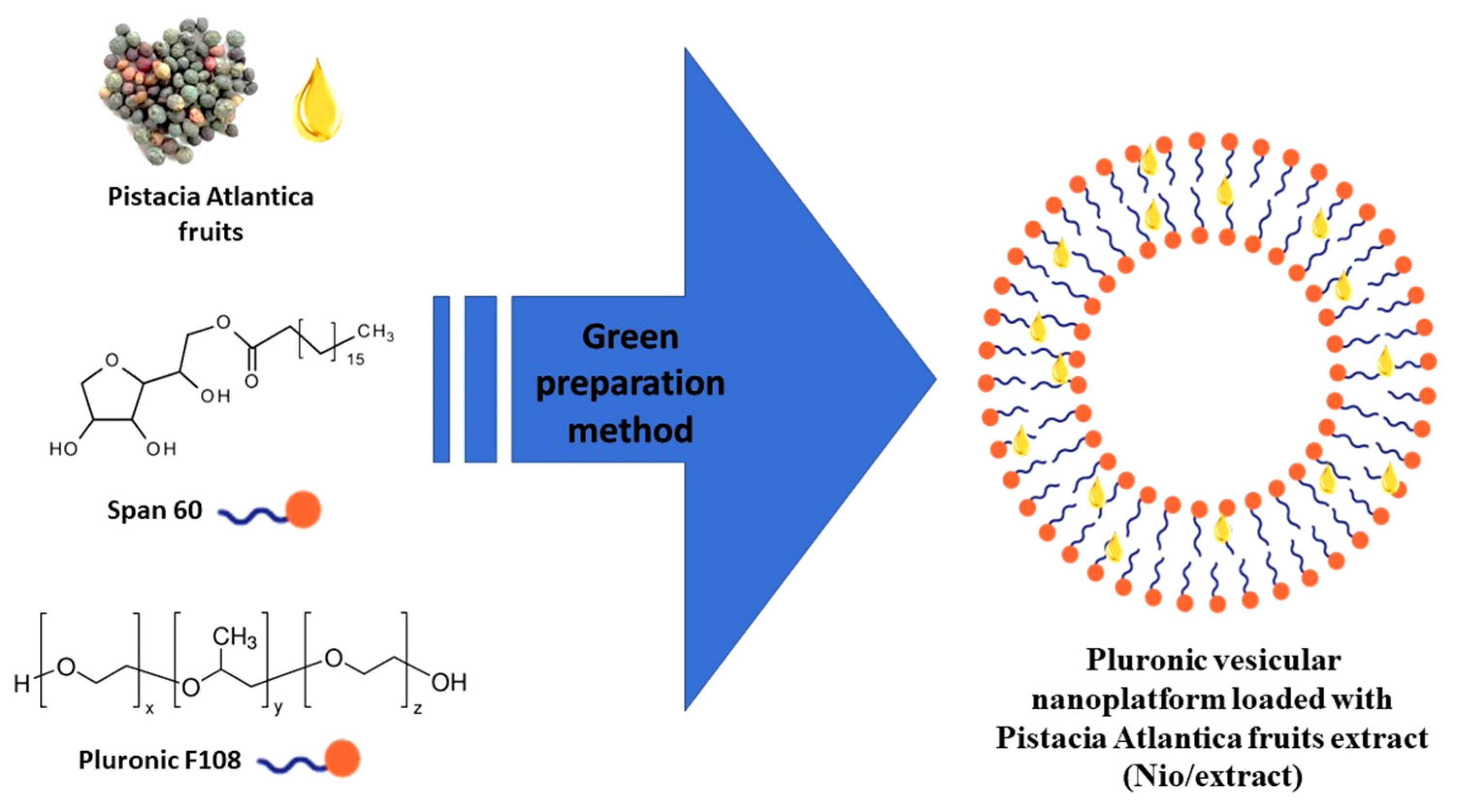
2.5. Green Preparation of the Nio/Extract by Ultrasonic Processing Technique
2.6. Characterization of the Nio/Extract
2.6.1. Size and Polydispersity Index (PDI)
2.6.2. Morphology
2.6.3. Encapsulation Efficiency
2.6.4. Stability Studies
2.6.5. Study of Drug Release
2.7. Cytotoxic Activity
2.7.1. Cell Culture
2.7.2. MTT Assay
2.8. Antibacterial Efficacy
2.9. Statistical Analysis
3. Results
3.1. Content and Identification of the Chemical Composition of P. atlantica Extract
3.2. Physicochemical Properties of Nanocarrier
3.2.1. Size Distribution
3.2.2. TEM Analysis
3.2.3. Encapsulation Efficiency of the Nio/Extract Formulation
3.2.4. Stability Study
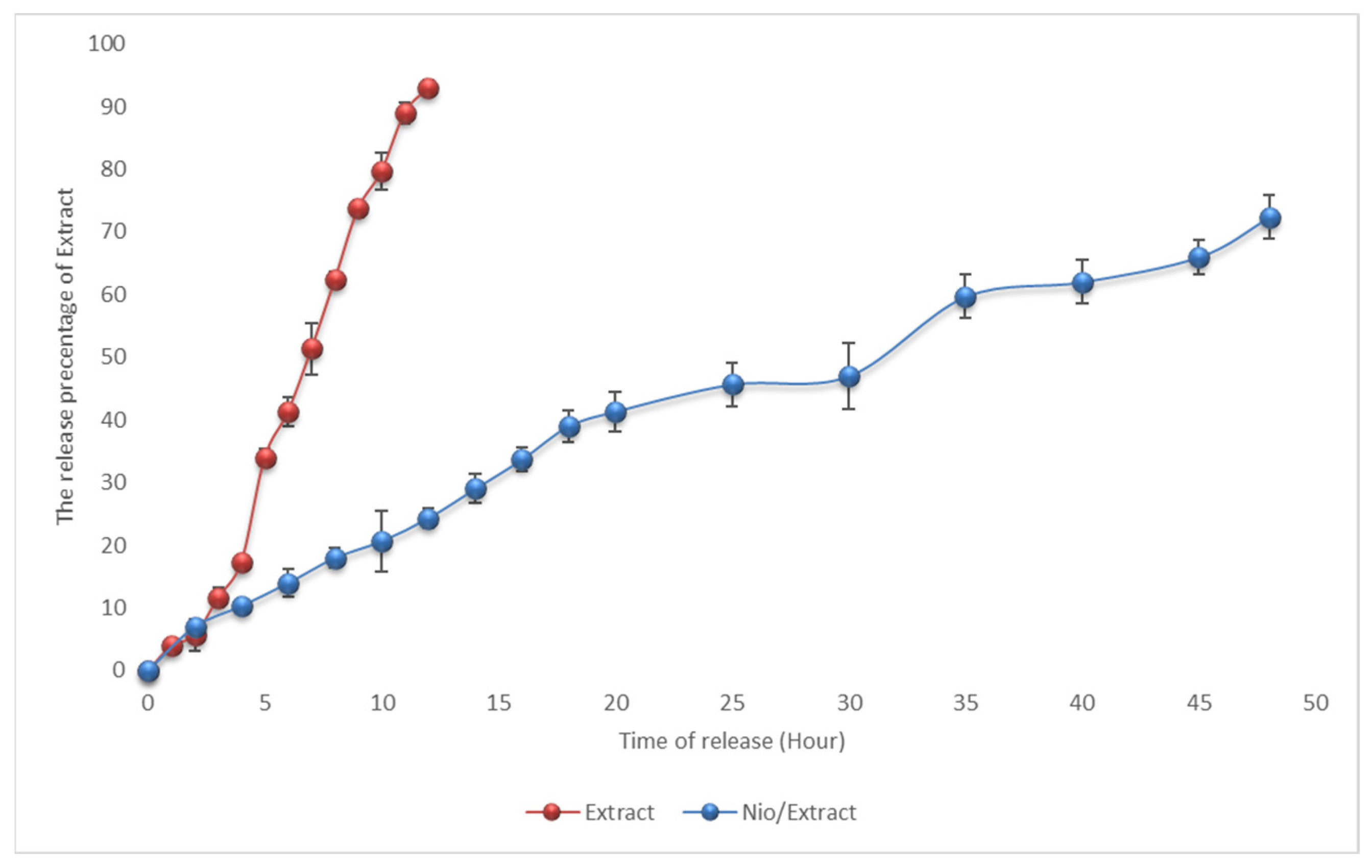
3.2.5. In Vitro Release Behavior
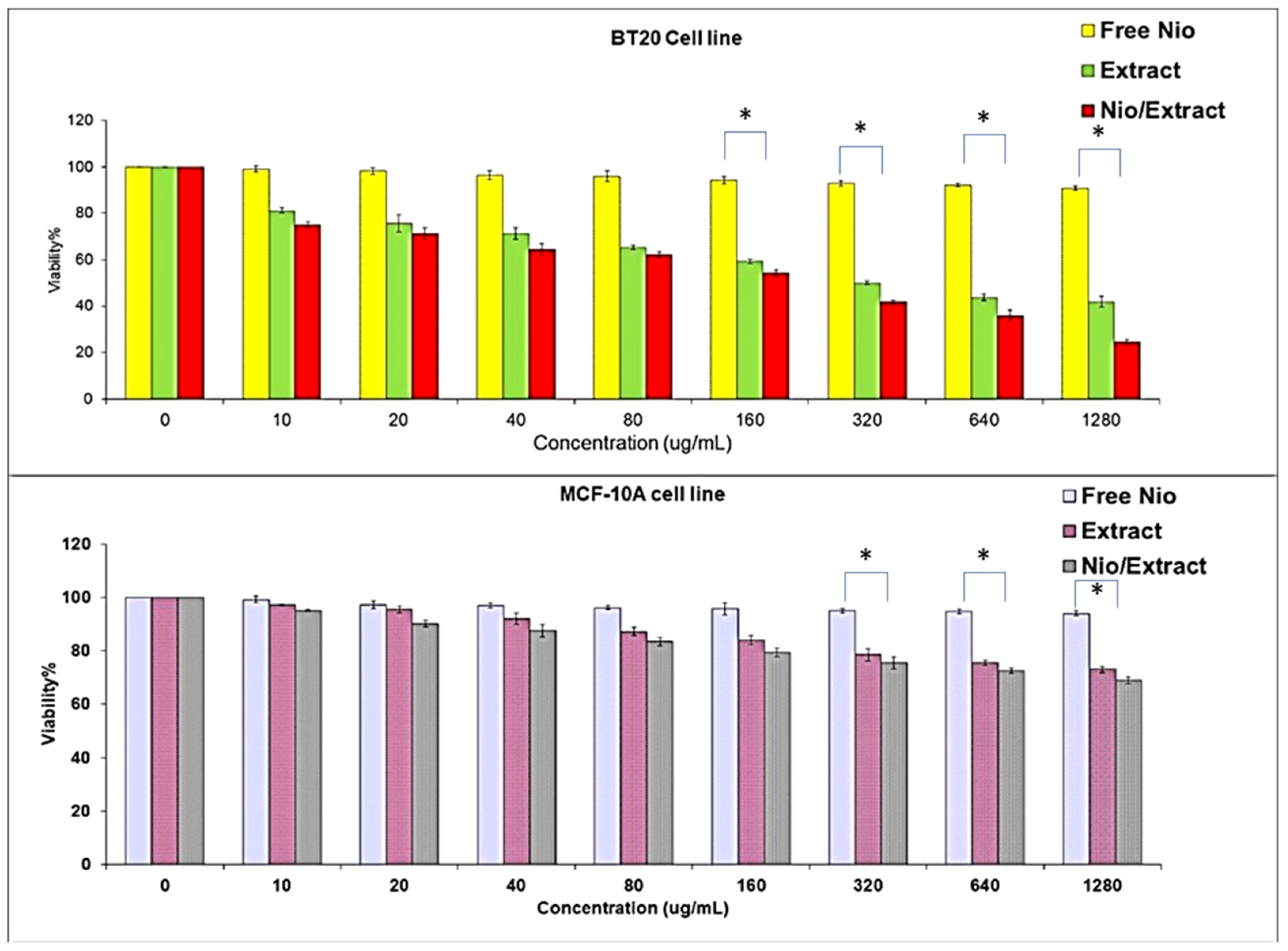
3.3. Cytotoxicity Assay
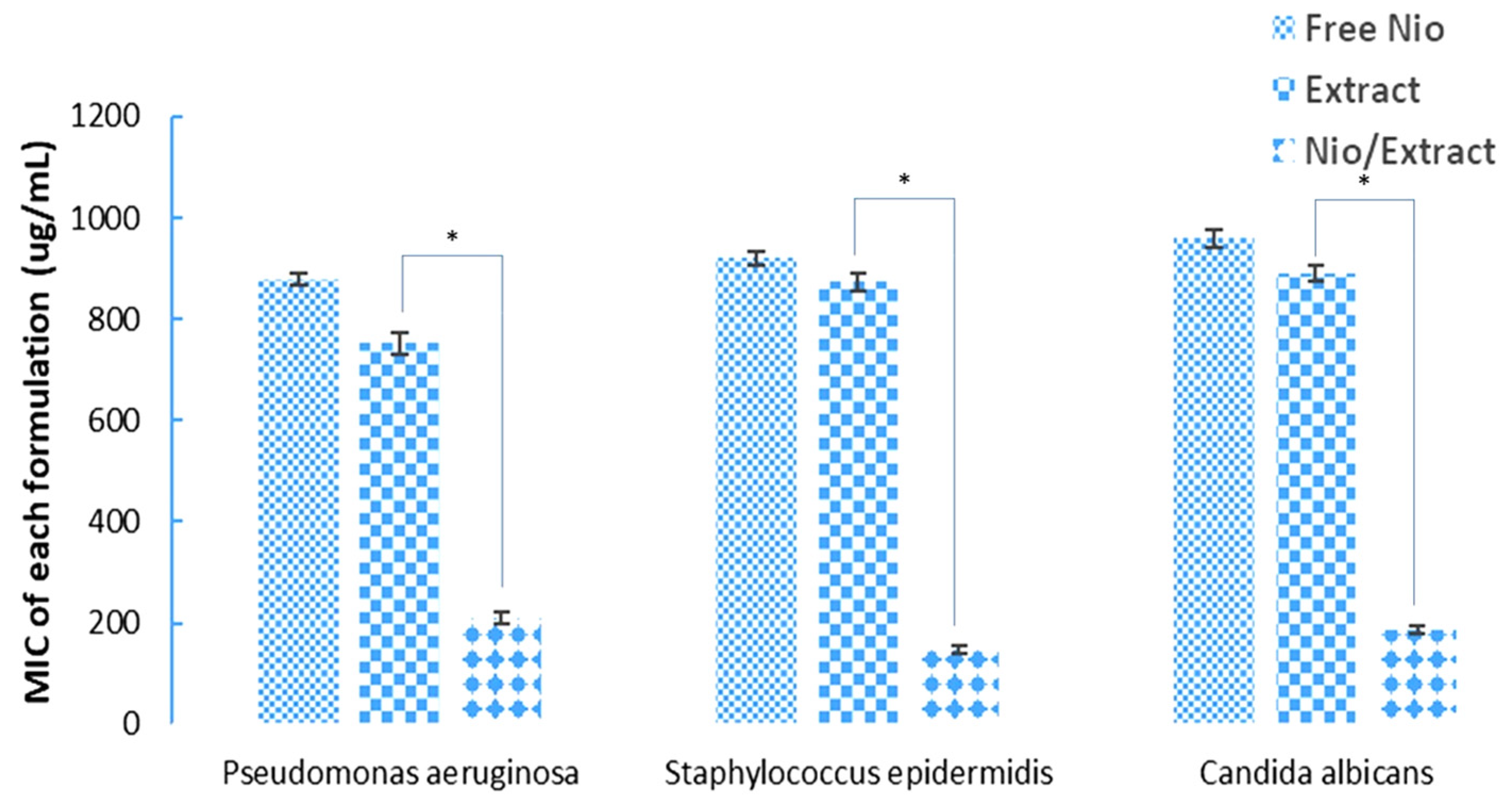
3.4. Antimicrobial Activity of the Nio/Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AlNadhari, S.; Al-Enazi, N.M.; Alshehrei, F.; Ameen, F. A review on biogenic synthesis of metal nanoparticles using marine algae and its applications. Environ. Res. 2021, 194, 110672. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Saravanan, M.; Gopinath, V.; Chaurasia, M.K.; Syed, A.; Ameen, F.; Purushothaman, N. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb. Pathog. 2018, 115, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Preethi, J.; Vijayan, R.; Yusoff, A.R.M.; Ameen, F.; Suresh, S.; Balagurunathan, R.; Palvannan, T. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. J. Photochem. Photobiol. B Biol. 2016, 163, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.B.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Vander Heyden, Y. Four Pistacia atlantica subspecies (atlantica, cabulica, kurdica and mutica): A review of their botany, ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 265, 113329. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; ALNadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef] [PubMed]
- Benmahieddine, A.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; El Zerey-Belaskri, A.; Gismondi, A.; Di Marco, G.; Canini, A.; Bechlaghem, N.; Bekkara, F.A.; Djebli, N. Influence of plant and environment parameters on phytochemical composition and biological properties of Pistacia atlantica Desf. Biochem. Syst. Ecol. 2021, 95, 104231. [Google Scholar] [CrossRef]
- Mahjoub, F.; Rezayat, K.A.; Yousefi, M.; Mohebbi, M.; Salari, R. Pistacia atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology. J. Med. Life 2018, 11, 180. [Google Scholar] [CrossRef]
- Ameen, F.; AlYahya, S.; Govarthanan, M.; ALjahdali, N.; Al-Enazi, N.; Alsamhary, K.; Alshehri, W.; Alwakeel, S.; Alharbi, S. Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J. Mol. Struct. 2020, 1202, 127233. [Google Scholar] [CrossRef]
- Ameen, F.; Dawoud, T.; AlNadhari, S. Ecofriendly and low-cost synthesis of ZnO nanoparticles from Acremonium potronii for the photocatalytic degradation of azo dyes. Environ. Res. 2021, 202, 111700. [Google Scholar] [CrossRef]
- Ameen, F.; Srinivasan, P.; Selvankumar, T.; Kamala-Kannan, S.; Al Nadhari, S.; Almansob, A.; Dawoud, T.; Govarthanan, M. Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg. Chem. 2019, 88, 102970. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, K.; You, K.; Gao, Z.; Kakuchi, T.; Feng, B.; Duan, Q. Synthesis and characterization of phenylboronic acid-containing polymer for glucose-triggered drug delivery. Sci. Technol. Adv. Mater. 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, T.; Chen, Y.; Sun, N.; Liu, Q.; Huang, Z.; Yang, Y.; Cheng, H.; Yue, K. Biodegradable and Cytocompatible Hydrogel Coating with Antibacterial Activity for the Prevention of Implant-Associated Infection. ACS Appl. Mater. Interfaces 2023, 15, 11507–11519. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Li, Y.; He, C.; Yong, C.; Wang, L.; Fu, H.; He, X.L.; Wang, Z.Y.; Liu, D.F.; Zhang, Y.Y. Synthesis and Biological Activities of Oxazolidinone Pleuromutilin Derivatives as a Potent Anti-MRSA Agent. ACS Infect. Dis. 2023, 9, 1711–1729. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Abbasi, M.; Alijani, H.Q.; Akbarizadeh, M.R.; Iravani, S.; Barani, M.; Najafi, K.; Khatami, S.; Mehrdad, K. Ceramic magnetic ferrite nanoribbons: Eco-friendly synthesis and their antifungal and parasiticidal activity. Ceram. Int. 2022, 48, 3448–3454. [Google Scholar] [CrossRef]
- Khatami, M.; Iravani, P.; Jamalipour Soufi, G.; Iravani, S. MXenes for antimicrobial and antiviral applications: Recent advances. Mater. Technol. 2022, 37, 1890–1905. [Google Scholar] [CrossRef]
- Cui, G.; Bai, Y.; Li, W.; Gao, Z.; Chen, S.; Qiu, N.; Satoh, T.; Kakuchi, T.; Duan, Q. Synthesis and characterization of Eu (III) complexes of modified D-glucosamine and poly (N-isopropylacrylamide). Mater. Sci. Eng. C 2017, 78, 603–608. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG macromolecule crosslinked chitosan hydrogels as antibacterial wound dressing. Carbohydr. Polym. 2022, 277, 118871. [Google Scholar] [CrossRef]
- Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Sudhakar, C.; Ameen, F.; Al-Sabri, A.; Selvam, K.; Govarthanan, M.; Kim, H. Utilization of market vegetable waste for silver nanoparticle synthesis and its antibacterial activity. Mater. Lett. 2018, 225, 101–104. [Google Scholar] [CrossRef]
- Yang, R.; Hou, E.; Cheng, W.; Yan, X.; Zhang, T.; Li, S.; Yao, H.; Liu, J.; Guo, Y. Membrane-targeting neolignan-antimicrobial peptide mimic conjugates to combat methicillin-resistant Staphylococcus aureus (MRSA) infections. J. Med. Chem. 2022, 65, 16879–16892. [Google Scholar] [CrossRef]
- Sonbol, H.; Ameen, F.; AlYahya, S.; Almansob, A.; Alwakeel, S. Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef] [PubMed]
- Kashkooli, F.M.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef] [PubMed]
- Mangalgiri, A.; Shaikh, A.; Matole, V.; Ingale, S. A brief review on niosome drug delivery system. Res. J. Pharm. Dos. Forms Technol. 2021, 13, 23–24. [Google Scholar]
- Yeo, L.K.; Olusanya, T.O.; Chaw, C.S.; Elkordy, A.A. Brief effect of a small hydrophobic drug (cinnarizine) on the physicochemical characterisation of niosomes produced by thin-film hydration and microfluidic methods. Pharmaceutics 2018, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.H.; Bashir, S.; Figueiredo, P.; Santos, H.A.; Khan, M.I.; Peltonen, L. Process optimization of ecological probe sonication technique for production of rifampicin loaded niosomes. J. Drug Deliv. Sci. Technol. 2019, 50, 27–33. [Google Scholar] [CrossRef]
- Khan, M.I.; Madni, A.; Hirvonen, J.; Peltonen, L. Ultrasonic processing technique as a green preparation approach for diacerein-loaded niosomes. AAPS PharmSciTech 2017, 18, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Sternberg, B.; Florence, A.T. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85). Int. J. Pharm. 1994, 105, 1–6. [Google Scholar] [CrossRef]
- Alexandridis, P.; Alan Hatton, T. Poly(ethylene oxide)poly(propylene oxide)poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Tavano, L.; de Cindio, B.; Picci, N.; Ioele, G.; Muzzalupo, R. Drug compartmentalization as strategy to improve the physico-chemical properties of diclofenac sodium loaded niosomes for topical applications. Biomed. Microdevices 2014, 16, 851–858. [Google Scholar] [CrossRef]
- Falahati, M.; Sepahvand, A.; Mahmoudvand, H.; Baharvand, P.; Jabbarnia, S.; Ghojoghi, A.; Yarahmadi, M. Evaluation of the antifungal activities of various extracts from Pistacia atlantica Desf. Curr. Med. Mycol. 2015, 1, 25. [Google Scholar] [CrossRef]
- David Sparkman, O. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Jo, H.; Cha, B.; Kim, H.; Brito, S.; Kwak, B.M.; Kim, S.T.; Bin, B.H.; Lee, M.-G. α-pinene enhances the anticancer activity of natural killer cells via ERK/AKT pathway. Int. J. Mol. Sci. 2021, 22, 656. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh Moghaddam, F.; Akbarzadeh, I.; Marzbankia, E.; Farid, M.; Reihani, A.H.; Javidfar, M.; Mortazavi, P. Delivery of melittin-loaded niosomes for breast cancer treatment: An in vitro and in vivo evaluation of anti-cancer effect. Cancer Nanotechnol. 2021, 12, 14. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. Nanomater. Drug Deliv. Ther. 2019, 91–116. [Google Scholar]
- De Silva, L.; Fu, J.Y.; Htar, T.T.; Muniyandy, S.; Kasbollah, A.; Kamal, W.H.B.W.; Chuah, L.H. Characterization, optimization, and in vitro evaluation of Technetium-99m-labeled niosomes. Int. J. Nanomed. 2019, 14, 1101. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, M.U.; Khan, S.; Javed, F.; Khan, A.A. Nanoniosome-encapsulated levoflaxicin as an antibacterial agent against Brucella. J. Basic Microbiol. 2020, 60, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Khan, M.I.; Rehman, M.U.; Abbas, G.; Aslam, N.; Ahmad, S.; Abbas, K.; Shah, P.A.; Iqbal, M.; Al Subari, A.M.A. In vitro characterization and release studies of combined nonionic surfactant-based vesicles for the prolonged delivery of an immunosuppressant model drug. Int. J. Nanomed. 2020, 15, 7937. [Google Scholar] [CrossRef]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Nematollahi, M.H. Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: A Nano-herbal treatment for Cancer. DARU J. Pharm. Sci. 2018, 26, 11–17. [Google Scholar] [CrossRef]
- Diva, B.; Ishwarya, K.; Naganjaneyulu, R. In vitro drug release profile of aceclofenac niosomes formed with different ratios of cholestrol using sorbitan esters. Int. J. Chem. Sci. 2014, 12, 237–247. [Google Scholar]
- Ganjooei, N.A.; Ohadi, M.; Mostafavi, S.M.A.; Behnam, B.; Pardakhty, A. Preparing and assessing the physiochemical properties of curcumin niosomes and evaluating their cytotoxicity in 3T3 and MCF-7 cell lines. Avicenna J. Phytomedicine 2021, 11, 417. [Google Scholar]
- Mansouri, M.; Khayam, N.; Jamshidifar, E.; Pourseif, T.; Kianian, S.; Mirzaie, A.; Akbarzadeh, I.; Ren, Q. Streptomycin sulfate–loaded niosomes enables increased antimicrobial and anti-biofilm activities. Front. Bioeng. Biotechnol. 2021, 9, 745099. [Google Scholar] [CrossRef] [PubMed]
- Kashef, M.T.; Saleh, N.M.; Assar, N.H.; Ramadan, M.A. The antimicrobial activity of ciprofloxacin-loaded niosomes against ciprofloxacin-resistant and biofilm-forming Staphylococcus aureus. Infect. Drug Resist. 2020, 13, 1619. [Google Scholar] [CrossRef] [PubMed]

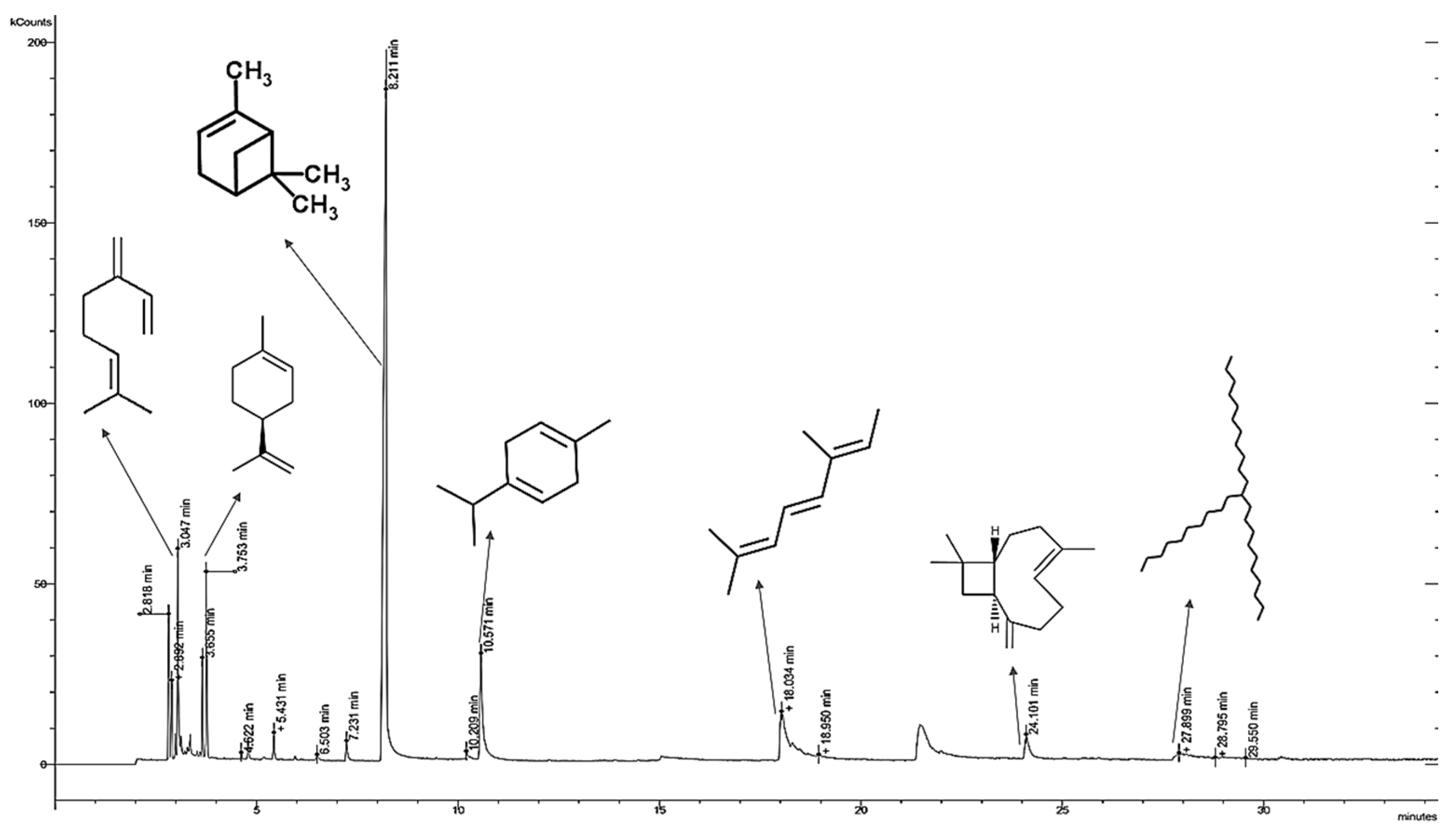


| No. | Compound | Retention Time | Structure | Percent % |
|---|---|---|---|---|
| 1 | β-myrcene | 3.05 | 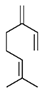 | 6.0 |
| 2 | Limonene | 3.65 | 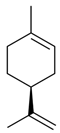 | 2.2 |
| 3 | α-pinene | 8.2 | 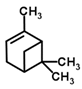 | 54.5 |
| 4 | γ-terpinene | 10.5 | 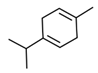 | 8.4 |
| 5 | Allo-ocimene | 18.0 |  | 6.0 |
| 6 | Trans-caryophyllene | 24.1 | 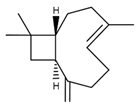 | 2.4 |
| 7 | 11-n-decyldocosane | 27.8 | 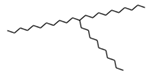 | 0.5 |
| Parameters | Temperature | 0 Day | 20 Days | 50 Days | 70 Days | 90 Days |
|---|---|---|---|---|---|---|
| Size (nm) | 25 °C | 103.2 | 107.2 | 110.4 | 124.8 | 157.3 |
| 4 °C | 103.2 | 104.8 | 104.6 | 106.1 | 109.1 | |
| Encapsulation efficiency (%) | 25 °C | 90.3 | 84.5 | 82.6 | 78.5 | 74.6 |
| 4 °C | 90.3 | 88.9 | 87.1 | 85.6 | 84.9 |
| Kinetic Model | Zero Order | First Order | Higuchi Model | Korsemeyer Peppas Models | |
|---|---|---|---|---|---|
| Kinetic parameters | R2 | R2 | R2 | R2 | n |
| Nio/Extract | 0.9582 | 0.6603 | 09515 | 0.9605 | 0.513 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Enazi, N.M.; Alsamhary, K.; Ameen, F.; Nobre, M.A.L. Novel Vesicular Formulation Based on a Herbal Extract Loaded with Niosomes and Evaluation of Its Antimicrobial and Anticancer Potential. Microbiol. Res. 2023, 14, 2133-2147. https://doi.org/10.3390/microbiolres14040144
Al-Enazi NM, Alsamhary K, Ameen F, Nobre MAL. Novel Vesicular Formulation Based on a Herbal Extract Loaded with Niosomes and Evaluation of Its Antimicrobial and Anticancer Potential. Microbiology Research. 2023; 14(4):2133-2147. https://doi.org/10.3390/microbiolres14040144
Chicago/Turabian StyleAl-Enazi, Nouf M., Khawla Alsamhary, Fuad Ameen, and Marcos Augusto Lima Nobre. 2023. "Novel Vesicular Formulation Based on a Herbal Extract Loaded with Niosomes and Evaluation of Its Antimicrobial and Anticancer Potential" Microbiology Research 14, no. 4: 2133-2147. https://doi.org/10.3390/microbiolres14040144
APA StyleAl-Enazi, N. M., Alsamhary, K., Ameen, F., & Nobre, M. A. L. (2023). Novel Vesicular Formulation Based on a Herbal Extract Loaded with Niosomes and Evaluation of Its Antimicrobial and Anticancer Potential. Microbiology Research, 14(4), 2133-2147. https://doi.org/10.3390/microbiolres14040144






