Geobacter grbiciae—A New Electron Donor in the Formation of Co-Cultures via Direct Interspecies Electron Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Media, and Culturing Conditions
2.2. Analytical Techniques
3. Results
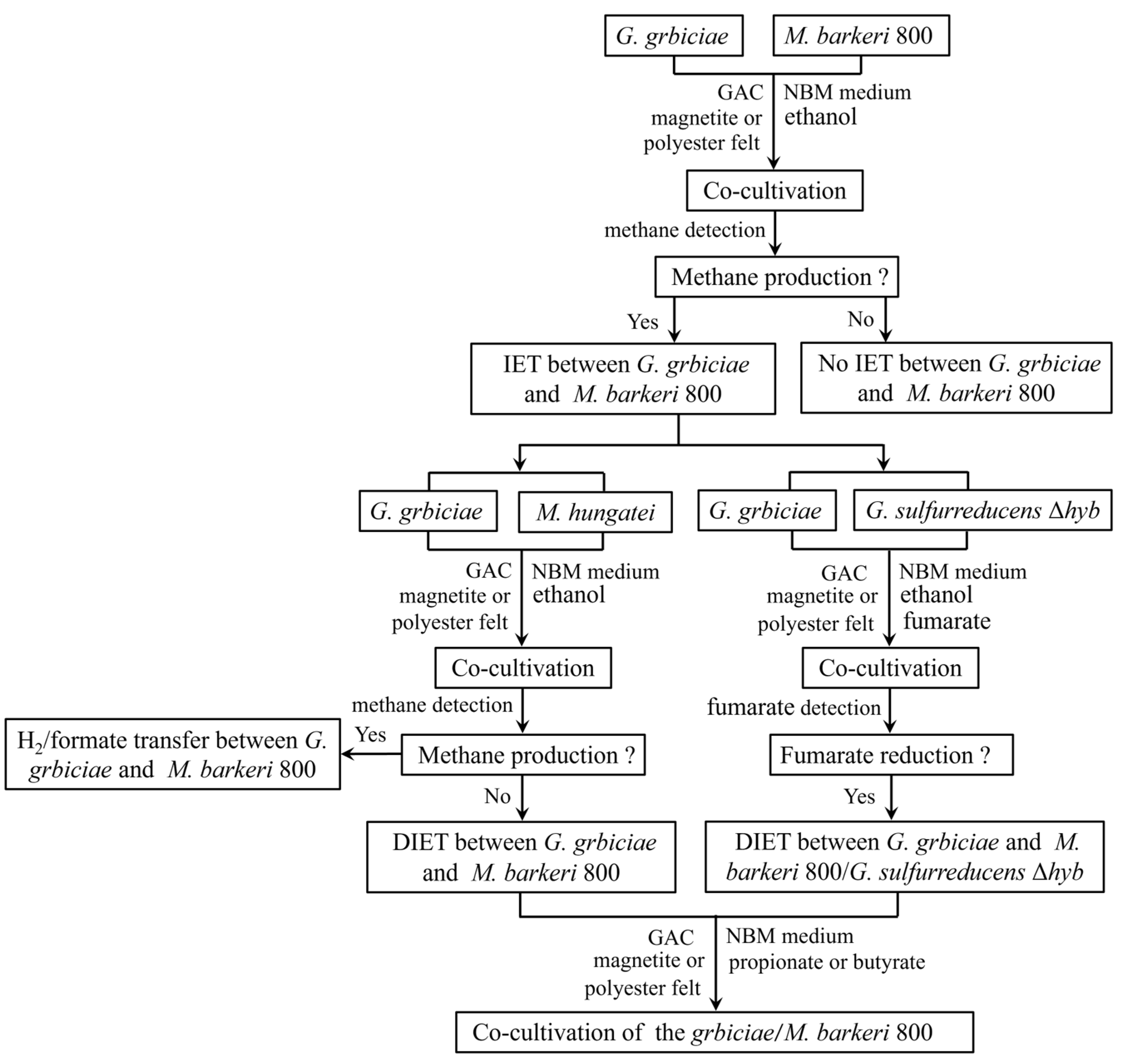
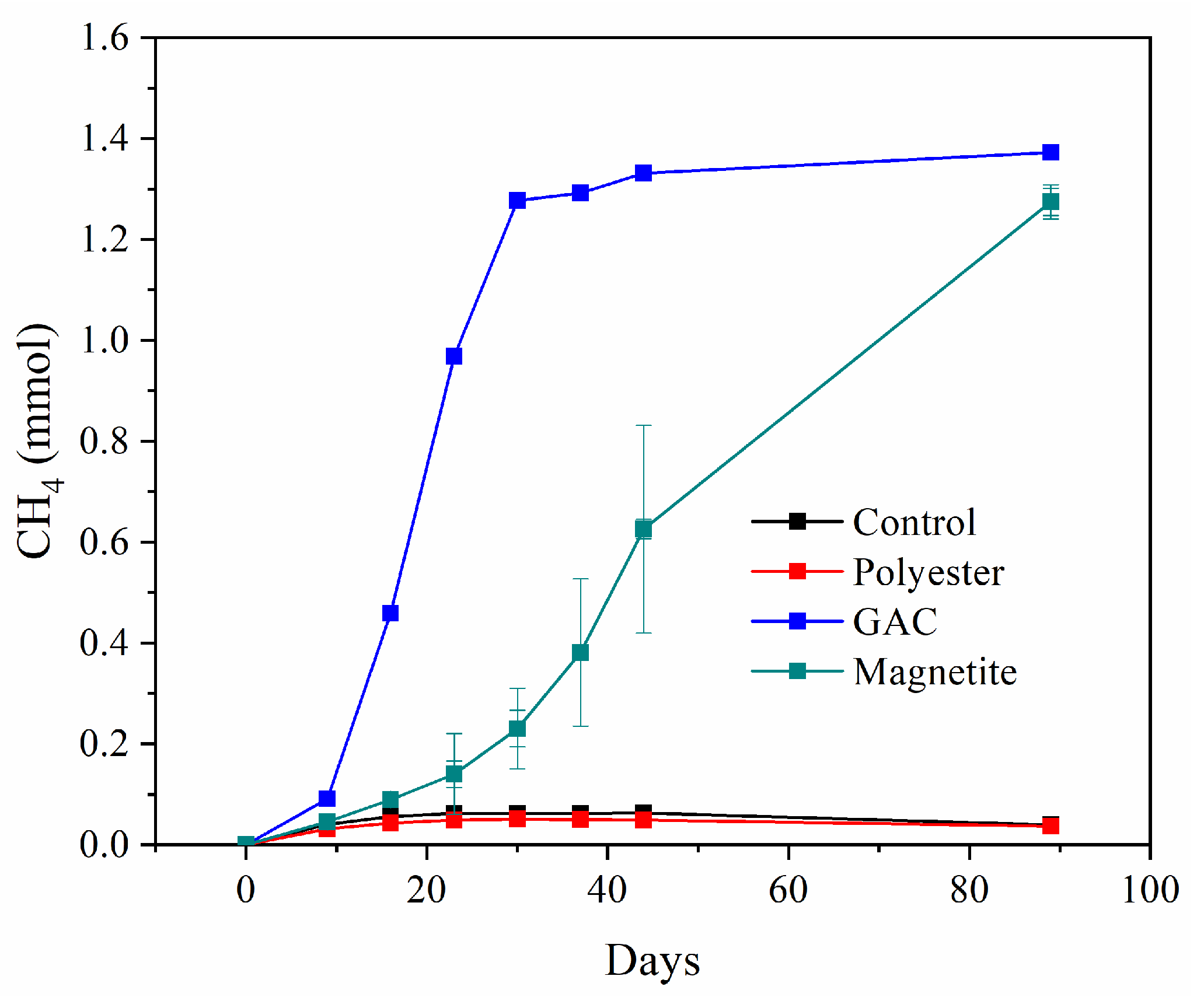
3.1. Experiments with G. grbiciae and M. barkeri 800
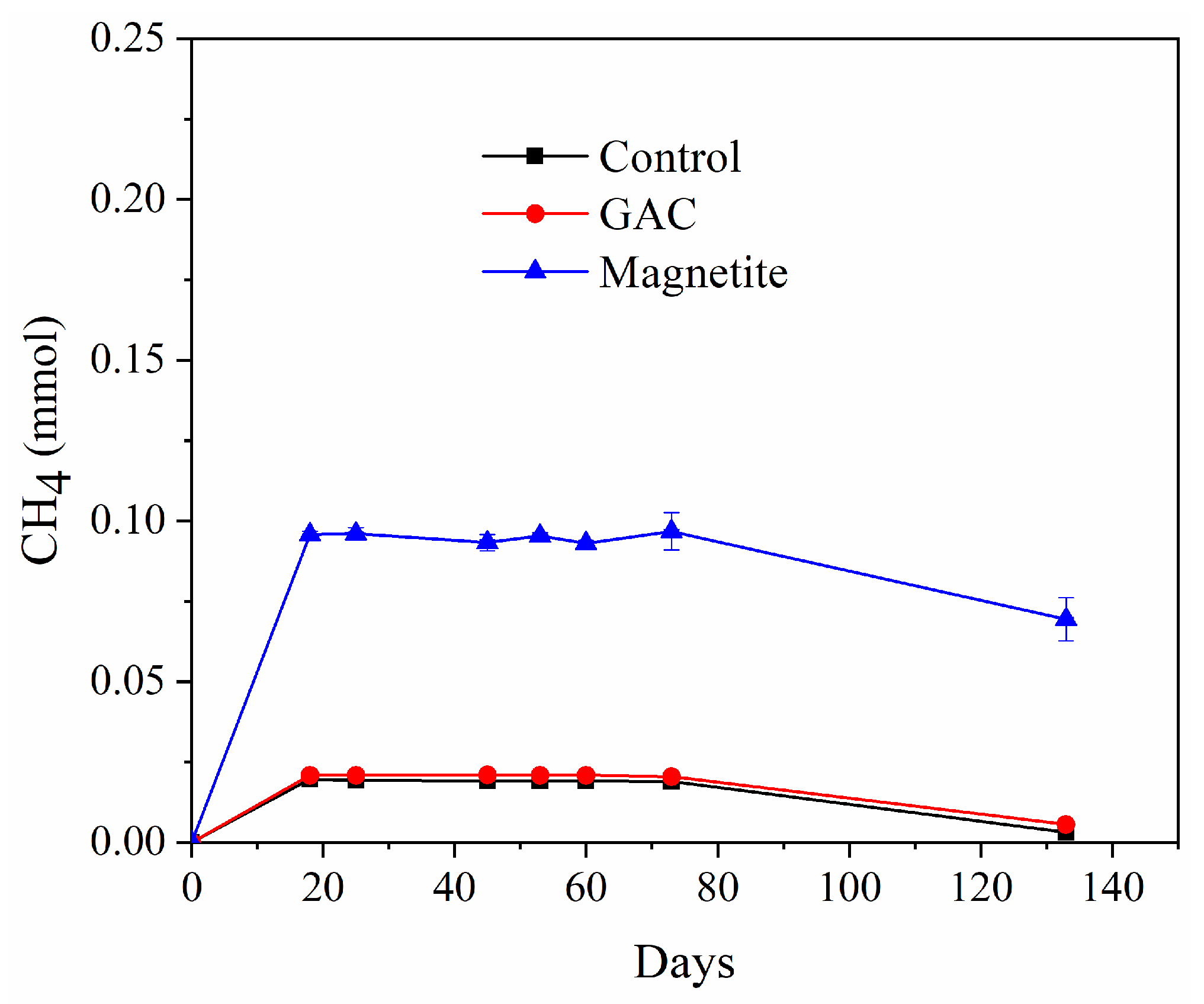
3.2. Experiments with G. grbiciae and M. hungatei
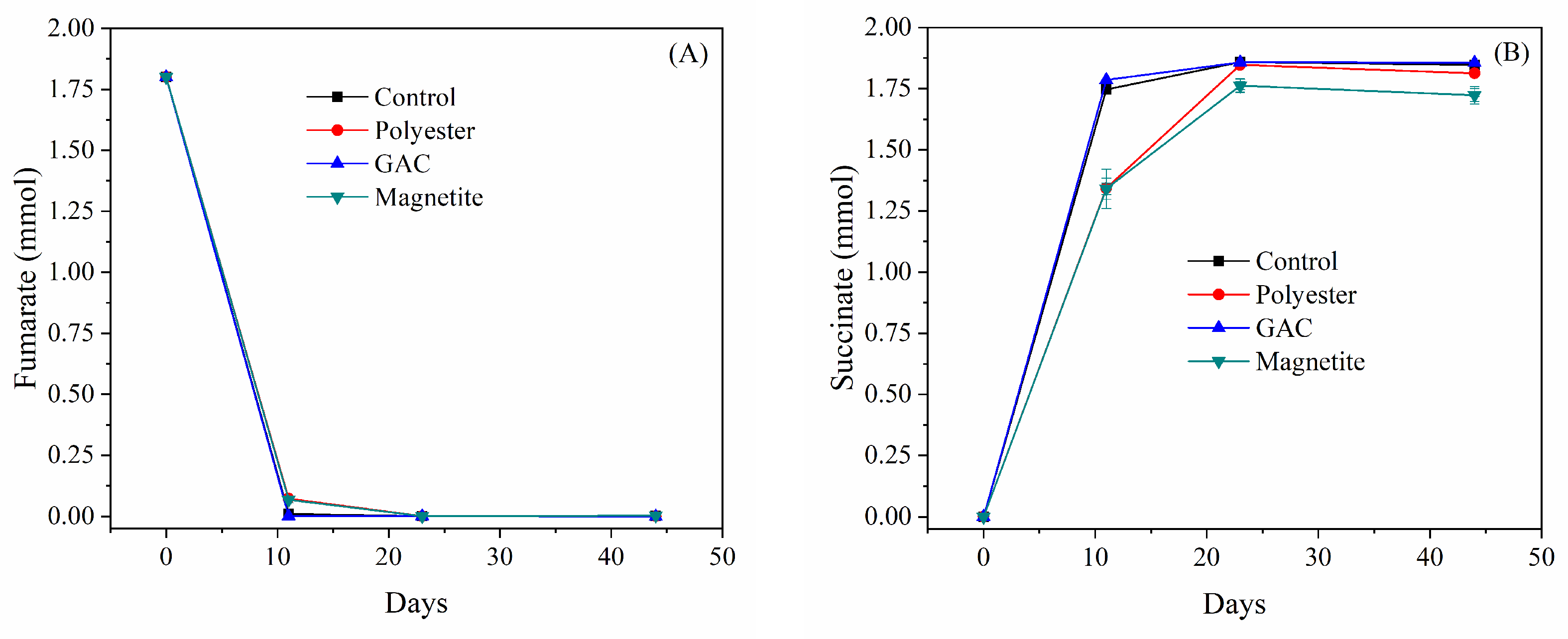
3.3. Co-Culturing G. grbiciae with the G. sulfurreducens Δhyb Mutant
3.4. Syntrophic Metabolism of Propionate and Butyrate in Co-Cultures of G. grbiciae and M. barkeri 800
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De-Bok, F.A.; Luijten, M.L.; Stams, A.J. Biochemical Evidence for Formate Transfer in Syntrophic Propionate-Oxidizing Cocultures of Syntrophobacter Fumaroxidans and Methanospirillum Hungatei. Appl. Environ. Microbiol. 2002, 68, 4247–4252. [Google Scholar] [CrossRef] [PubMed]
- Sieber, J.R.; McInerney, M.J.; Gunsalus, R.P. Genomic Insights into Syntrophy: The Paradigm for Anaerobic Metabolic Cooperation. Annu. Rev. Microbiol. 2012, 66, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Stams, A.J.; Plugge, C.M. Electron Transfer in Syntrophic Communities of Anaerobic Bacteria and Archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Yu, Q.; Li, Y.; Zhao, Z.; Fan, S.; Zhang, Y. Effects of Magnetite on Nitrate-Dependent Anaerobic Oxidation of Methane in an Anaerobic Membrane Biofilm Reactor: Metatranscriptomic Analysis and Mechanism Prediction. Environ. Technol. Innov. 2023, 32, 103288. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Lovley, D.R. A New Model for Electron Flow During Anaerobic Digestion: Direct Interspecies Electron Transfer to Methanosaeta for The Reduction of Carbon Dioxide to Methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Wang, G.; Fu, P.; Zhang, B.; Zhang, J.; Huang, Q.Y.; Yao, G.F.; Dzakpasu, M.; Zhang, J.F.; Li, Y.Y.; Chen, R. Biochar Facilitates Methanogens Evolution by Enhancing Extracellular Electron Transfer to Boost Anaerobic Digestion of Swine Manure Under Ammonia Stress. Bioresour. Technol. 2023, 388, 129773. [Google Scholar] [CrossRef]
- Jung, H.J.; Yu, H.Y.J.; Lee, C.S. Direct Interspecies Electron Transfer Enables Anaerobic Oxidation of Sulfide to Elemental Sulfur Coupled with CO2-Reducing Methanogenesis. iScience 2023, 26, 107504. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Wang, S.Y.; Ping, Q.; Li, Y. A Novel Approach to Enhance Methane Production During Anaerobic Digestion of Waste Activated Sludge by Combined Addition of Trypsin, Nano-Zero-Valent Iron and Activated Carbon. Chemosphere 2023, 341, 140007. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Z.H.; Jiang, T.B.; Zhang, W.W.; Wang, Z.W.; Hou, Q.X. Enhancing Sludge Methanogenesis with Changed Micro-Environment of Anaerobic Microorganisms by Fenton Iron Mud. Chemosphere 2023, 341, 139884. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhang, H.W.; Tian, K.X.; Zhang, Y.; Zhang, J.S. Novel Lanthanum-Iron Oxide Nanoparticles Alleviate the Inhibition of Anaerobic Digestion by Carbamazepine through Adsorption and Bioaugmentation. J. Environ. Manag. 2023, 340, 117975. [Google Scholar] [CrossRef]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct Exchange of Electrons within Aggregates of an Evolved Syntrophic Coculture of Anaerobic Bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial Interspecies Electron Transfer via Electric Currents through Conductive Minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct Interspecies Electron Transfer between Geobacter metallireducens and Methanosarcina Barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Nevin, K.P.; Woodard, T.L.; Mu, B.Z.; Lovley, D.R. Expanding The Diet for DIET: Electron Donors Supporting Direct Interspecies Electron Transfer (DIET) in Defined Co-Cultures. Front. Microbiol. 2016, 7, 236. [Google Scholar] [CrossRef]
- Ha, P.T.; Lindemann, S.R.; Shi, L.; Dohnalkova, A.C.; Fredrickson, J.K.; Madigan, M.T.; Beyenal, H. Syntrophic Anaerobic Photosynthesis via Direct Interspecies Electron Transfer. Nat. Commun. 2017, 8, 13924. [Google Scholar] [CrossRef]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intercellular Wiring Enables Electron Transfer between Methanotrophic Archaea and Bacteria. Nature 2015, 526, 587–590. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Link Between Capacity for Current Production and Syntrophic Growth in Geobacter Species. Front. Microbiol. 2015, 6, 744. [Google Scholar] [CrossRef]
- Jin, H.Y.; Yang, L.; Ren, Y.X.; Tang, C.C.; Zhou, A.J.; Liu, W.Z.; Wang, A.J.; He, Z.W. Insights into the Roles and Mechanisms of a Green-Prepared Magnetic Biochar in Anaerobic Digestion of Waste Activated Sludge. Sci. Total Environ. 2023, 896, 165170. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q.; He, X.; Yu, R.; He, C.; Shen, D.; Jiao, Y. Investigation on The Effect of Different Additives on Anaerobic Co-Digestion of Corn Straw and Sewage Sludge: Comparison of Biochar, Fe3O4, and Magnetic Biochar. Bioresour. Technol. 2022, 345, 126532. [Google Scholar] [CrossRef]
- Zhuravleva, E.A.; Shekhurdina, S.V.; Kotova, I.B.; Loiko, N.G.; Popova, N.M.; Kryukov, E.; Litti, Y.V. Effects of Various Materials Used to Promote the Direct Interspecies Electron Transfer on Anaerobic Digestion of Low-Concentration Swine Manure. Sci. Total Environ. 2022, 839, 156073. [Google Scholar] [CrossRef]
- Yuan, T.G.; Sun, R.; Miao, Q.M.; Wang, X.; Xu, Q. Analysing the Mechanism of Food Waste Anaerobic Digestion Enhanced by Iron Oxide in A Continuous Two-Stage Process. Waste Manag. 2023, 171, 610–620. [Google Scholar] [CrossRef]
- Mou, A.Q.; Yu, N.J.W.; Yang, X.Y.; Liu, Y. Enhancing Methane Production and Organic Loading Capacity from High Solid-Content Wastewater in Modified Granular Activated Carbon (GAC)-Amended Up-Flow Anaerobic Sludge Blanket (UASB). Sci. Total Environ. 2023, 906, 167609. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.M.; Ma, H.; Shen, Y.X.; Shen, Y.; Shu, J.; Zeng, X.Y.; Liu, M.; Wang, H.Y. Magnetic Biochar Derived from Peanut Shells Facilitates Antibiotic Degradation and Fouling Control in Anaerobic Membrane Bioreactor for Antibiotic Pharmaceutical Wastewater Treatment. J. Water Process Eng. 2023, 55, 104249. [Google Scholar] [CrossRef]
- Alghashm, S.; Song, L.; Liu, L.L.; Ouyang, C.; Zhou, J.L.; Li, X.W. Improvement of Biogas Production using Biochar from Digestate at Different Pyrolysis Temperatures during OFMSW Anaerobic Digestion. Sustainability 2023, 15, 11917. [Google Scholar] [CrossRef]
- Wang, M.W.; Ren, T.F.; Yin, M.X.; Lu, K.C.; Xu, H.; Huang, X.; Zhang, X.Y. Enhanced Anaerobic Wastewater Treatment by a Binary Electroactive Material: Pseudocapacitance/Conductance-Mediated Microbial Interspecies Electron Transfer. Environ. Sci. Technol. 2023, 57, 12072–12082. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Ding, X.; Zhu, X.; Wang, L.; Zhang, Y.; Zhao, C. Effects of Different Types of Granular Activated Carbon on Methanogenesis of Carbohydrate-Rich Food Waste: Performance, Microbial Communities and Optimization. Sci. Total Environ. 2023, 895, 165173. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wei, C.H.; Yu, H.R.; Qu, F.S.; Rong, H.W.; He, J.G.; Ngo, H.H. Preparation and Mechanism of Carbon Felt Supported Iron Trioxide and Zero-Valent Iron for Enhancing Anaerobic Digestion Performance. Chem. Eng. J. 2023, 468, 143565. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Lovley, D.R. Promoting Interspecies Electron Transfer with Biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef]
- Cruz-Viggi, C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite Particles Triggering a Faster and More Robust Syntrophic Pathway of Methanogenic Propionate Degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef]
- Morita, M.; Malvankar, N.S.; Franks, A.E.; Summers, Z.M.; Giloteaux, L.; Rotaru, A.E.; Lovley, D.R. Potential for Direct Interspecies Electron Transfer in Methanogenic Wastewater Digester Aggregates. MBio 2011, 2, e00159-11. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, H.; Li, Y.; Zhang, H.; Wang, O.; Zhang, J.; Liu, F. Co-Occurrence of Methanosarcina Mazei and Geobacteraceae in an Iron (III)-Reducing Enrichment Culture. Front. Microbiol. 2015, 6, 941. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Shi, Z.J.; Cai, Y.F.; Zhang, S.C.; Luo, G. Microbial Insights towards Understanding the Role of Hydrochar in Enhancing Phenol Degradation in Anaerobic Digestion. Environ. Pollut. 2023, 330, 121779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.K.; Liu, Q.H.; Yang, Z.M. Nano Magnetite-Loaded Biochar Boosted Methanogenesis through Shifting Microbial Community Composition and Modulating Electron Transfer. Sci. Total Environ. 2023, 861, 160597. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Tao, Q.; Sun, D.; Dang, Y. Carbon Cloth Self-Forming Dynamic Membrane Enhances Anaerobic Removal of Organic Matter from Incineration Leachate via Direct Interspecies Electron Transfer. Chem. Eng. J. 2022, 445, 136732. [Google Scholar] [CrossRef]
- Li, R.; Lu, H.; Fu, Z.; Wang, X.; Li, Q.; Zhou, J. Effect of Riboflavin and Carbon Black Co-Modified Fillers Coupled with Alkaline Pretreatment on Anaerobic Digestion of Waste Activated Sludge. Environ. Res. 2023, 224, 115531. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.J.; He, J.G.; Zhang, P.F.; Zou, X.; Pan, X.L.; Zhang, J. Novel Nitrogen-Doped Biochar Supported Magnetite Promotes Anaerobic Digestion: Material Characterization and Metagenomic Analysis. Bioresour. Technol. 2023, 369, 128492. [Google Scholar] [CrossRef]
- Zhuravleva, E.; Kovalev, A.; Kovalev, D.; Kotova, I.; Shekhurdina, S.; Laikova, A.; Krasnovsky, A.; Pygamov, T.; Vivekanand, V.; Li, L.H.; et al. Does Carbon Cloth Really Improve Thermophilic Anaerobic Digestion Performance on a Larger Scale? Focusing on Statistical Analysis and Microbial Community Dynamics. J. Environ. Manag. 2023, 341, 118124. [Google Scholar] [CrossRef]
- Zheng, S.C.; Yang, F.; Huang, W.L.; Lei, Z.F.; Zhang, Z.Y.; Huang, W.W. Combined Effect of Zero Valent Iron and Magnetite on Semi-Dry Anaerobic Digestion of Swine Manure. Bioresour. Technol. 2022, 346, 126438. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, Y.; Wang, M.; Zhu, Y.; Sun, C.; Zhang, Y.; Zhao, Z. Enhancing Anaerobic Digestion of Kitchen Wastes via Combining Ethanol-Type Fermentation with Magnetite: Potential for Stimulating Secretion of Extracellular Polymeric Substances. Waste Manag. 2021, 127, 10–17. [Google Scholar] [CrossRef]
- Li, Y.C.; He, C.H.; Dong, F.; Yuan, S.J.; Hu, Z.H.; Wang, W. Performance of Anaerobic Digestion of Phenol using Exogenous Hydrogen and Granular Activated Carbon and Analysis of Microbial Community. Environ. Sci. Pollut. Res. 2023, 30, 45077–45087. [Google Scholar] [CrossRef]
- Zhou, N.; Zhou, J.; Huang, W.; Hu, Q.; Qiu, B. Improved Methane Production from Anaerobic Wastewater Treatment by Conductive Zero-Valent Iron@ Carbon@ Polyaniline. Int. Biodeterior. Biodegrad. 2023, 176, 105524. [Google Scholar] [CrossRef]
- Xie, S.H.; Li, X.; Wang, C.D.; Kulandaivelu, J.; Jiang, G.M. Enhanced Anaerobic Digestion of Primary Sludge with Additives: Performance and Mechanisms. Bioresour. Technol. 2020, 316, 123970. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Xia, X.; Yang, G.Q.; Zhuang, L. The Role of Exopolysaccharides in Direct Interspecies Electron Transfer. Front. Microbiol. 2022, 13, 927246. [Google Scholar] [CrossRef]
- Zhou, J.J.; Smith, J.A.; Li, M.; Holmes, D.E. Methane Production by Methanothrix Thermoacetophila via Direct Interspecies Electron Transfer with Geobacter metallireducens. MBio 2023, 14, e00360-23. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, S.E.; Chadwick, G.L.; Kempes, C.P.; Orphan, V.J. Single Cell Activity Reveals Direct Electron Transfer in Methanotrophic Consortia. Nature 2015, 526, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.M.; Rotaru, A.E.; Aklujkar, M.; Liu, F.; Shrestha, M.; Summers, Z.M.; Lovley, D.R. Syntrophic Growth with Direct Interspecies Electron Transfer as the Primary Mechanism for Energy Exchange. Environ. Microbiol. Rep. 2013, 5, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Holmes, D.E.; Woodard, T.L.; Li, Y.; Liu, X.Y.; Wang, L.Y.; Meier, D.; Schwarz, I.A.; Lovley, D.R. Detrimental Impact of the Geobacter metallireducens Type VI Secretion System on Direct Interspecies Electron Transfer. Microbiol. Spectr. 2023, 11, e0094123. [Google Scholar] [CrossRef]
- Rao, R.; Hu, J.; Lee, P.H. Theoretical Characterisation of Electron Tunnelling from Granular Activated Carbon to Electron Accepting Organisms in Direct Interspecies Electron Transfer. Sci. Rep. 2022, 12, 12426. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Rotaru, A.E.; Ueki, T.; Shrestha, P.M.; Ferry, J.G.; Lovley, D.R. Electron and Proton Flux for Carbon Dioxide Reduction in Methanosarcina Barkeri During Direct Interspecies Electron Transfer. Front. Microbiol. 2018, 9, 3109. [Google Scholar] [CrossRef]
- Yee, M.O.; Rotaru, A.E. Extracellular Electron Uptake in Methanosarcinales is Independent of Multiheme C-Type Cytochromes. Sci. Rep. 2020, 10, 372. [Google Scholar] [CrossRef]
- Yee, M.O.; Snoeyenbos-West, O.L.; Thamdrup, B.; Ottosen, L.D.M.; Rotaru, A.E. Extracellular Electron Uptake by Two Methanosrcina Species. Front. Energy Res. 2019, 7, 29. [Google Scholar] [CrossRef]
- Holmes, D.E.; Zhou, J.J.; Ueki, T.; Woodard, T.; Lovley, D.R. Mechanisms for Electron Uptake by Methanosarcina Acetivorans during Direct Interspecies Electron Transfer. MBio 2021, 12, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Liu, F.; Wang, B.C.; Zhang, Y.C.; Lovley, D.R. Methanobacterium Capable of Direct Interspecies Electron Transfer. Environ. Sci. Technol. 2020, 54, 15347–15354. [Google Scholar] [CrossRef] [PubMed]
- Wegener, G.; Krukenberg, V.; Ruff, S.E.; Kellermann, M.Y.; Knittel, K. Metabolic Capabilities of Microorganisms Involved in and Associated with the Anaerobic Oxidation of Methane. Front. Microbiol. 2016, 7, 46. [Google Scholar] [CrossRef]
- Coates, J.D.; Bhupathiraju, V.K.; Achenbach, L.A.; Mclnerney, M.J.; Lovley, D.R. Geobacter hydrogenophilus, Geobacter chapellei and Geobacter grbiciae, Three New, Strictly Anaerobic, Dissimilatory Fe (III)-Reducers. Int. J. Syst. Evol. Microbiol. 2001, 51, 581–588. [Google Scholar] [CrossRef]
- Coppi, M.V.; Leang, C.; Sandler, S.J.; Lovley, D.R. Development of a Genetic System for Geobacter sulfurreducens. Appl. Environ. Microbiol. 2001, 67, 3180–3187. [Google Scholar] [CrossRef]
- Tremblay, P.L.; Aklujkar, M.; Leang, C.; Nevin, K.P.; Lovley, D. A Genetic System for Geobacter metallireducens: Role of the Flagellin and Pilin in the Reduction of Fe (III) Oxide. Environ. Microbiol. Rep. 2012, 4, 82–88. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Magnetite Compensates for the Lack of a Pilin-Associated C-Type Cytochrome in Extracellular Electron Exchange. Environ. Microbiol. 2015, 17, 648–655. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting Direct Interspecies Electron Transfer with Activated Carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Butler, J.E.; Glaven, R.H.; Esteve-Núnez, A.; Núnez, C.; Shelobolina, E.S.; Bond, D.R.; Lovley, D.R. Genetic Characterization of a Single Bifunctional Enzyme for Fumarate Reduction and Succinate Oxidation in Geobacter sulfurreducens and Engineering of Fumarate Reduction in Geobacter metallireducens. J. Bacteriol. 2006, 188, 450–455. [Google Scholar] [CrossRef]
- Coppi, M.V.; O’Neil, R.A.; Lovley, D.R. Identification of an Uptake Hydrogenase Required for Hydrogen-Dependent Reduction of Fe (III) and other Electron Acceptors by Geobacter sulfurreducens. J. Bacteriol. 2004, 186, 3022–3028. [Google Scholar] [CrossRef]
- Caccavo, F.J.; Lonergan, D.J.; Lovley, D.R.; Davis, M.; Stolz, J.F.; McInerney, M.J. Geobacter sulfurreducens sp. nov., a Hydrogen- and Acetate-Oxidizing Dissimilatory Metal-Reducing Microorganism. Appl. Environ. Microbiol. 1994, 60, 3752–3759. [Google Scholar]
- Yan, Y.; Zhang, J.; Tian, L.; Yan, X.; Du, L.; Leininger, A.; Zhang, M.; Li, N.; Ren, Z.J.; Wang, X. DIET-Like Mutualism of Geobacter and Methanogens at Specific Electrode Potential Boosts Production of both Methane and Hydrogen from Propionate. Water Res. 2023, 235, 119911. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.B.; Zhang, X.X.; Qiu, S.W.; Zhou, X.Y.; Tian, Z.X.; Liang, Y.J.; Zhang, Y.F.; Ma, L.P. Pyrite-Enhanced Sludge Digestion via Stimulation of Direct Interspecies Electron Transfer between Syntrophic Propionate-and Sulfur-Oxidizing Bacteria and Methanogens: Genome-Centric Metagenomics Evidence. Chem. Eng. J. 2023, 456, 141089. [Google Scholar] [CrossRef]
- Wu, N.; Liu, T.; Li, Q.; Quan, X. Enhancing Anaerobic Methane Production in Integrated Floating-Film Activated Sludge System Filled with Novel MWCNTs-Modified Carriers. Chemosphere 2022, 299, 134483. [Google Scholar] [CrossRef]
- Li, L.L.; Gao, Q.W.; Liu, X.P.; Zhao, Q.; Wang, W.Y.; Wang, K.; Zhou, H.M.; Jiang, J.Q. Insights into High-Solids Anaerobic Digestion of Food Waste Enhanced by Activated Carbon via Promoting Direct Interspecies Electron Transfer. Bioresour. Technol. 2022, 351, 127008. [Google Scholar] [CrossRef]
- Ma, W.D.; Li, H.; Zhang, W.D.; Shen, C.C.; Wang, L.Y.; Li, Y.X.; Li, Q.B.; Wang, Y.P. TiO2 Nanoparticles Accelerate Methanogenesis in Mangrove Wetlands Sediment. Sci. Total Environ. 2022, 713, 136602. [Google Scholar] [CrossRef]
- Deng, Y.F.; Xia, J.; Zhao, R.; Xu, J.X.; Liu, X.Y. Iron-Coated Biochar Alleviates Acid Accumulation and Improves Methane Production under Ammonium Enrichment Conditions. Sci. Total Environ. 2022, 809, 151154. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Ma, Y.Q.; Ji, D.D.D.; Li, X.Y.; Zhang, J.S.; Zang, L.H. Synergetic Promotion of Direct Interspecies Electron Transfer for Syntrophic Metabolism of Propionate and Butyrate with Graphite Felt in Anaerobic Digestion. Bioresour. Technol. 2019, 287, 121373. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Metal Reduction. Annu. Rev. Microbiol. 1993, 47, 263–290. [Google Scholar] [CrossRef]


| Microbes | References |
|---|---|
| Geobacter metallireducens GS-15 | [14,43,44] |
| Geobacter hydrogenophilus | [17] |
| Geobacter sulfurreducens PCA | [15] |
| Anaerobic methanotrophic archaea (ANME) | [16,45] |
| Microbes | References |
|---|---|
| Geobacter sulfurreducens PCA | [14,43,46,47,48] |
| Methanosarcina barkeri 800 | [48,49] |
| Methanosaeta harundinacea 8Ac | [5,14] |
| Methanosarcina mazei Gö1 | [50] |
| Methanosarcina mazei 633 k.o. | [50] |
| Methanosarcina barkeri Fusaro | [50] |
| Methanosarcina barkeri 227 | [50] |
| Methanosarcina horonobensis HB-1 | [50,51] |
| Methanothrix thermoacetophila | [44] |
| Methanosarcina acetivorans | [52] |
| Methanobacterium | [53] |
| Thiobacillus denitrificans | [12] |
| SRB HotSeep-1 | [16,54] |
| Prosthecochloris aestaurii | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, P.; Wang, L.; Li, X.; Zhang, J.; Jiang, H. Geobacter grbiciae—A New Electron Donor in the Formation of Co-Cultures via Direct Interspecies Electron Transfer. Microbiol. Res. 2023, 14, 1774-1787. https://doi.org/10.3390/microbiolres14040122
Deng P, Wang L, Li X, Zhang J, Jiang H. Geobacter grbiciae—A New Electron Donor in the Formation of Co-Cultures via Direct Interspecies Electron Transfer. Microbiology Research. 2023; 14(4):1774-1787. https://doi.org/10.3390/microbiolres14040122
Chicago/Turabian StyleDeng, Panbo, Lulu Wang, Xia Li, Jinshan Zhang, and Haiming Jiang. 2023. "Geobacter grbiciae—A New Electron Donor in the Formation of Co-Cultures via Direct Interspecies Electron Transfer" Microbiology Research 14, no. 4: 1774-1787. https://doi.org/10.3390/microbiolres14040122
APA StyleDeng, P., Wang, L., Li, X., Zhang, J., & Jiang, H. (2023). Geobacter grbiciae—A New Electron Donor in the Formation of Co-Cultures via Direct Interspecies Electron Transfer. Microbiology Research, 14(4), 1774-1787. https://doi.org/10.3390/microbiolres14040122





