1. Introduction
Dunaliella salina is a unicellular green alga that is known for its ability to tolerate extreme environmental conditions, including high salinity, nutrient deprivation (nitrogen or phosphorous limitation), extreme temperature, intense light and exposure to ultraviolet B (UV-B) radiation or toxic substances such as cadmium, arsenic, lead and microplastics [
1,
2,
3,
4,
5,
6,
7,
8]. Due to its ability to endure extreme environmental conditions,
D. salina is a promising candidate for large-scale and open-air cultivation to produce large amounts of sustainable raw materials such as proteins, carbohydrates and lipids and make the transition from a fossil-fuel-based refinery to a biorefinery.
Dunaliella salina is also valued for its high nutritional content and the presence of various bioactive compounds such as β-carotene, the secondary carotenoids, α-tocopherol, ascorbic acid and glutathione [
1]. Abd El-Baky et al. [
1] and Al-Rashed et al. [
9] reported that nitrogen deficiency, high salinity and UV-B radiation increase the content of lipophilic (carotenoids and tocopherol) and hydrophilic (glutathione and ascorbic acid) antioxidants.
Glutathione (L-γ-glutamyl-L-cysteinyl-glycine; GSH), a low-molecular-weight tripeptide (307 g/mol), is the most abundant peptide and the dominant thiol in plants and animals [
2]. Glutathione is synthesized from several amino acids, L-glutamate, L-cysteine and glycine, through two consecutive enzymatic reactions, and catalyzed by two enzymes, γ-glutamyl-cysteine synthase and glutathione synthase [
10]. Glutathione attracted attention in the pharmaceutical, food production and cosmetic industries due to its crucial role against the oxidative damage induced by reactive oxygen species (ROS), which is attributed to its capacity to easily undergo oxidation and reduction reactions [
11,
12,
13,
14]. It also has the power to chelate heavy metals such as cadmium, copper and lead [
2].
Reduced glutathione (GSH) and oxidized glutathione disulfide (GSSG) are two forms of the tripeptide molecule glutathione, which play important roles in the antioxidant defense system of
D. salina. GSH acts as an electron donor, which can neutralize free radicals and protect cells from oxidative damage. In contrast, GSSG forms when GSH is oxidized by ROS and loses two electrons. However, GSSG can be recycled back to GSH by the enzyme glutathione reductase (GR), which helps to maintain the redox balance in the cell [
7,
15,
16]. Maintaining a high GSH/GSSG ratio is crucial to facilitate the dual role of glutathione as an antioxidant and reductant and to enable thiol/sulfide exchange reactions with cellular compounds while preventing enzyme oxidation [
17].
The high glutathione concentration in
D. salina is believed to contribute to its ability to survive in harsh environments [
11]. Previous research reported that the glutathione in
Dunaliella spp., including
D. salina, is 25–361 µmol/10
12 cell [
2,
18]. Muhaemin [
18] studied glutathione concentration changes during daytime and night and found that they fluctuate between 25 and 50 µmol/10
12 cells, with the highest values under light conditions. The author attributed this result to the need to eliminate or reduce the ROS produced during the saturation of photosynthesis at intense light levels.
The majority of existing studies on the glutathione content in D. salina lack specific details about the growth phases at which their glutathione measurements were taken. Moreover, the concentration of glutathione within D. salina could potentially fluctuate in accordance with its growth stages. Consequently, the identification of optimal culture conditions and harvesting timings becomes necessary to maximize glutathione levels. To the best of our knowledge, there has been no previous research that measured the change in glutathione in D. salina across its various growth phases. Therefore, in this study, as a preliminary step for subsequent investigations, we aimed to determine whether the glutathione concentration varies according to the growth stage of D. salina.
2. Materials and Methods
The microalga
D. salina (strain CS-744/01) was used in the experiments. It was cultivated in f/2 medium [
19,
20] with a salinity of 12% (2 M NaCl). The f/2 medium was prepared by mixing all chemicals in a sea salt solution (Red Sea Salt as used in aquariums, Red Sea Fish Pharm Ltd., Eilat, Israel). For this study, we selected conditions so that the microalga was cultured at 25 °C under continuous illumination of 200 µmol/m²/s (white LED) without agitation. Since
Dunaliella salina lacks a rigid polysaccharide cell wall, we avoided mixing or stirring to prevent any potential damage. The initial cell concentration of
D. salina was set at 5 × 10
4 cell/mL. The cell concentration was periodically monitored by taking a 25 µL sample and measuring it in an Invitrogen Tali Image-Based Cytometer (Thermo Fisher Scientific, Waltham, MA, USA). The glutathione concentration was monitored for 15 days. Experiments were performed in 1.5 mL clear tubes using the luminescence-based GSH/GSSG-Glo Assay Kit (Promega Corporation, Madison, WI, USA), which measures the total glutathione (GSH + GSSG) and oxidized forms of glutathione (GSSG), as well as GSH/GSSG ratios [
21]. We chose this test because it is simple and rapid, allows for real-time monitoring and was employed in several scientific publications. Luminescence was measured in relative light units (RLU) using a luminometer (Gene Light GL-200, Microtech Co. Ltd., Chiba, Japan). Microalgal samples were concentrated before performing the GSH/GSSG-Glo Assay by centrifugation at 3000 rpm for 15 s and discarding the supernatant; this step produced a cell concentration of around 5 × 10
6 cell/mL. The pH was measured using a portable pH meter (Hanna Instruments HI98130, Woonsocket, RI, USA), and the salinity was measured with a portable salt refractometer (Tekcoplus Refractometer ATC, Kwun Tong, Kowloon, Hong Kong). The experiments were repeated three times to ensure that the results were consistent.
3. Results
We indirectly measured the glutathione concentration based on the relative luciferin luminescence. We used the GSH/GSSG-Glo Assay, which is a simple and rapid method in which luminescent signals are correlated with either the total glutathione or oxidized glutathione concentrations of the sample. Most of the intracellular glutathione exists in the reduced form (GSH), while a small percentage corresponds to the oxidized form (GSSG). The total glutathione, expressed in GSH equivalents, indicates the GSH level plus two times the GSSG level; this is because GSSG is a disulfide that originates from the combination of two glutathione molecules. To measure the total glutathione, a reducing agent first converts all of the glutathione in a cell lysate to GSH. Next, a reaction that utilizes a GSH probe, called Luciferin-NT, is converted to luciferin by glutathione-S-transferase in the presence of GSH. The reaction is then coupled to a firefly luciferase reaction, resulting in a luminescent signal that is proportional to the amount of GSH that is present. To measure GSSG, a reagent that blocks all GSH while leaving GSSG intact is added, followed by a reducing step that converts GSSG to GSH for quantification.
The initial cell concentration of the
D. salina culture was 5.0 × 10
4 cell/mL, and the initial pH was 7.42. After 15 days, the cell concentration was around 5 × 10
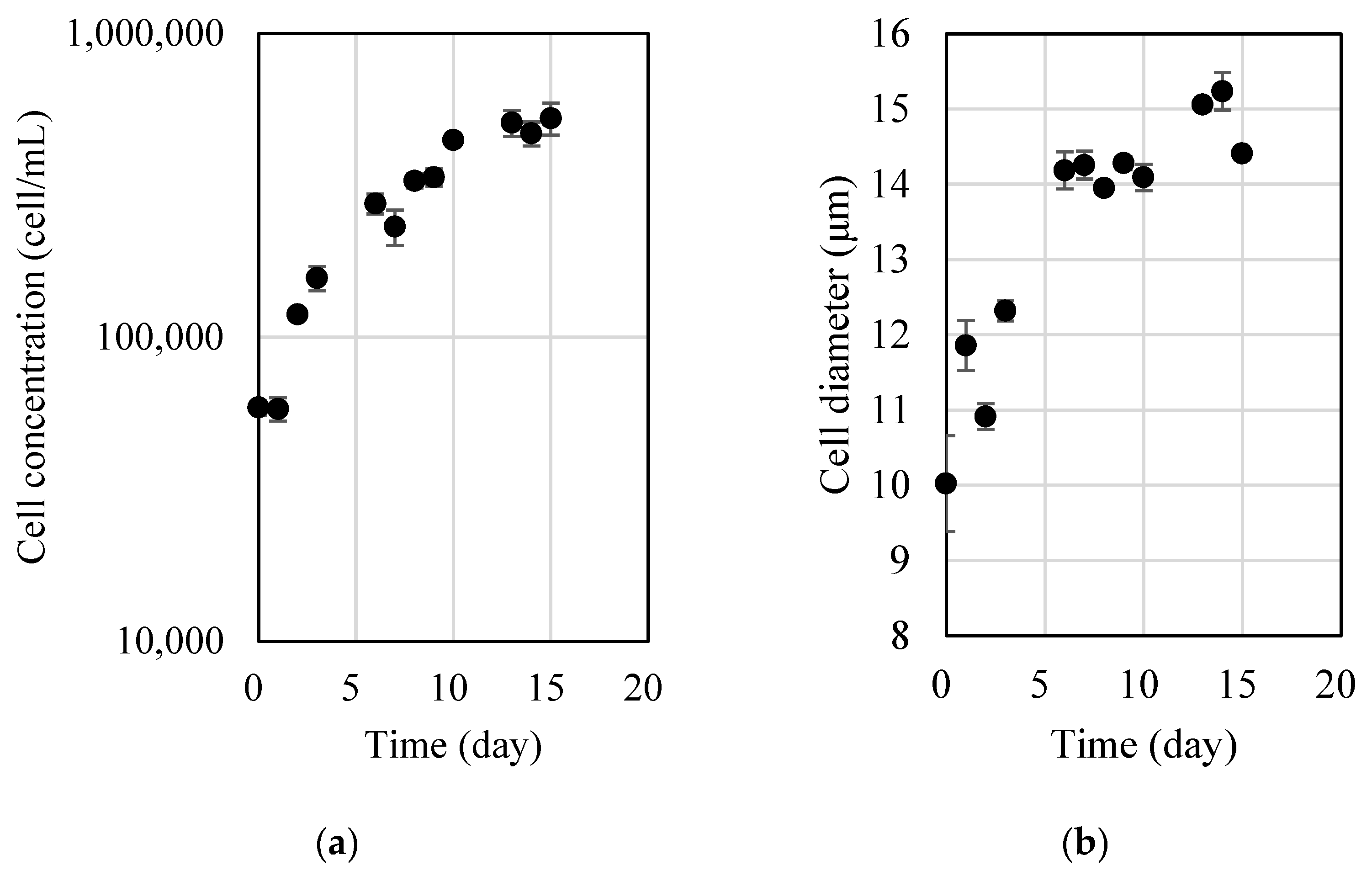
5 cell/mL, and the pH was 8.81. The nitrogen and phosphorus concentrations were continuously monitored, and their concentrations gradually decreased and were depleted after 10 days from the start of the experiments (results not shown). Three phases can be recognized from the growth curve of
D. salina (
Figure 1a): the initial lag phase, the exponential log phase and the stationary phase. For
D. salina cultivated in f/2 medium at 25 °C and under 200 µmol/m
2/s of continuous illumination, the cell concentration was between 5 × 10
4 and 1 × 10
5 cell/mL during the initial lag phase, between 2 × 10
5 and 5 × 10
5 cell/mL during the exponential log phase and around 5 × 10
5 cell/mL during the stationary phase. The cell size was between 10 and 15 µm throughout the three phases (
Figure 1b), specifically between 10 and 12 µm during the initial lag phase, around 14 µm during the exponential log phase and around 15 µm during the stationary phase.
We evaluated the glutathione concentration during the three growth phases; it is expressed as µmol of glutathione per 10
12 microalgal cells (
Figure 2a). The glutathione concentration increased during the exponential phase but slightly decreased during the stationary phase. During the initial lag phase, it was between 190 and 280 µmol/10
12 cells, whereas it was between 280 and 500 µmol/10
12 cells and between 320 and 370 µmol/10
12 cells during the exponential log and stationary phases, respectively. There was a significant difference between the groups (Tukey’s test,
p < 0.001): the glutathione levels were the highest during the exponential growth phase. It should be noted that the data presented in
Figure 2a represent the mean ± standard error of three experiments. The results from each experiment were within 10% of the mean.
In addition, the GSH/GSSG ratio also changed during the growth cycle: it was higher during the lag and exponential phases (
Figure 2b). This indicates that GSH decreased during the stationary phase, whereas GSSG increased over time. This change is probably due to the decrease in glutathione synthesis caused by nutrient limitation, and/or the culture had reached senescence [
2]. The results are consistent with the previous research, which reported a reduction in the GSH/GSSG ratio caused by a decrease in glutathione synthesis and its utilization to safeguard cells against oxidative-stress-induced damage [
4,
9,
12].
Taken together, the results suggest that the glutathione content of D. salina is regulated by the growth cycle and may play a crucial role in the alga’s ability to adapt to and survive in changing environmental conditions.
4. Discussion
The glutathione content in D. salina was shown to change depending on the growth phases. During the early logarithmic growth phase, D. salina had relatively low levels of glutathione, which gradually increased as the cells entered the stationary phase. In addition, the GSH/GSSG ratio changed during the growth cycle, with GSH being the predominant form during the exponential growth phase and GSSG becoming more abundant during the stationary phase. These findings suggest that the glutathione content of D. salina is tightly regulated during the growth cycle and may play a crucial role in the alga’s ability to adapt to and survive in changing environmental conditions.
The fact that the exponential phase had the highest glutathione concentration (280–500 µmol/10
12 cell) can probably be attributed to the increased metabolic activity of the cells during this phase, which leads to the higher production of peroxides as by-products [
15]. Due to its ability to act as a reducing agent, glutathione is known to be involved in a range of biological processes such as cell growth and division, protein and nucleic acid synthesis, the regulation of cellular metabolism and the detoxification of harmful substances [
12,
16,
17]. Thus, glutathione plays a crucial role in maintaining cellular homeostasis and protecting cells from damage caused by oxidative stress and toxic substances [
13].
Dunaliella salina has high levels of glutathione, with the GSH/GSSG ratio varying depending on the growth stage (as observed in this study), culture settings and environmental conditions [
7]. In this study, the GSH/GSSH ratio decreased over time, probably as a result of senescence or a lack of nutrients. During the early growth phase, the ratio was relatively high, indicating a predominantly reducing environment. As the cells entered the stationary phase or were exposed to stressors such as high salinity or intense light, the GSH/GSSG ratio decreased, which is an indication of an increase in oxidative stress. This shift in the redox balance may trigger the activation of other antioxidant enzymes and signaling pathways to protect the cells from damage. Overall, the interplay between GSH and GSSG in
D. salina is a crucial aspect of the alga’s response to oxidative stress and its ability to survive in harsh environments.
Our results provide insights into the relationship between glutathione concentrations and the various growth phases of D. salina, emphasizing the importance of monitoring the glutathione levels throughout its growth stages. These data can serve as a reference for future studies exploring the role of glutathione in other microalgae. While this study represents the initial step in investigating this biological feature of D. salina, further research is necessary, such as elucidating the optimal cultivation conditions required to maximize the glutathione concentrations in D. salina. Additionally, for practical applications, further studies should investigate extraction methods and assess the economic feasibility of potential industrial applications, including agriculture, nutraceuticals, cosmetics and more.
5. Conclusions
We observed a significant variation in the glutathione concentration based on the growth stage. In the initial lag growth phase, D. salina exhibited comparatively lower levels of glutathione (190–280 µmol/1012 cells). As it transitioned into the log phase, the glutathione levels gradually increased (280–500 µmol/1012 cells) but, subsequently, decreased when entering the stationary phase (320–370 µmol/1012 cells). Our results suggest that the glutathione content of D. salina is regulated by the growth cycle and that the best time to harvest the microalga and to extract glutathione is during the exponential phase, when the alga is cultivated using the conditions established in this study (25 °C, 200 µmol/m²/s and without agitation).
Author Contributions
Conceptualization, M.K.; methodology, M.K.; validation, M.K.; formal analysis, K.E. and Y.M.; investigation, M.K. and A.N.; resources, M.K.; data curation, K.E., Y.M. and A.N.; writing—original draft preparation, M.K. and A.N.; writing—review and editing, M.K. and A.N.; visualization, M.K. and A.N.; supervision, M.K.; project administration, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Acknowledgments
We would like to express our gratitude to the reviewers for their valuable comments and feedback on our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abd El-Baky, H.H.; El Baz, F.K.; El-Baroty, G.S. Production of antioxidant by the green alga Dunaliella salina. Int. J. Agric. Biol. 2004, 6, 49–57. [Google Scholar]
- Ahner, B.A.; Wei, L.; Oleson, J.R.; Ogura, N. Glutathione and other low molecular weight thiols in marine phytoplankton under metal stress. Mar. Ecol. Prog. Ser. 2002, 232, 93–103. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zheng, Y.; Ge, Y. Phytochelatin synthesis in Dunaliella salina induced by arsenite and arsenate under various phosphate regimes. Ecotoxicol. Environ. Saf. 2017, 136, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, M.; Bao, J.; Liu, J. Physiological, metabolomic, and transcriptomic analyses reveal the dynamic redox homeostasis upon extended exposure of Dunaliella salina GY-H13 cells to Cd. Ecotoxicol. Environ. Saf. 2021, 223, 112593. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Liu, G.Z.; Liu, F.F. Physiological and metabolic toxicity of polystyrene microplastics to Dunaliella salina. Environ. Pollut. 2022, 316, 120544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, X.; Wang, M.; Zhang, W.; Zhou, B.; Wang, Y. ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J. Photochem. Photobiol. B Biol. 2017, 173, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Tammam, A.A.; Fakhry, E.M.; El-Sheekh, M. Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliella salina and Dunaliella tertiolecta. Afr. J. Biotechnol. 2011, 10, 3795–3808. [Google Scholar]
- Haghjou, M.M.; Shariati, M.; Pozveh, M.H. The effect of low light intensities on oxidative stress induced by short-term chilling in Dunaliella salina teod. Pak. J. Biol. Sci. 2006, 9, 2048–2054. [Google Scholar]
- Al-Rashed, S.A.; Ibrahim, M.M.; El-Gaaly, G.A.; Al-Shehri, S.; Mostafa, A. Evaluation of radical scavenging system in two microalgae in response to interactive stresses of UV-B radiation and nitrogen starvation. Saudi J. Biol. Sci. 2016, 23, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Tian, J.; Yu, J. Changes in ultrastructure and responses of antioxidant systems of algae (Dunaliella salina) during acclimation to enhanced ultraviolet-B radiation. J. Photochem. Photobiol. B Biol. 2009, 97, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, G.; Chen, J. Glutathione: A review on biotechnological production. Appl. Microbiol. Biotechnol. 2004, 66, 233–242. [Google Scholar] [CrossRef] [PubMed]
- May, M.J.; Vernoux, T.; Leaver, C.; Montagu, M.V.; Inzé, D. Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J. Exp. Bot. 1998, 49, 649–667. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Muhaemin, M. The glutathione diurnal cycling in Dunaliella salina. J. Coast. Dev. 2008, 12, 41–46. [Google Scholar]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Promega Corporation, GSH/GSSG-Glo™ Assay Technical Manual. Available online: https://www.promega.jp/-/media/files/resources/protocols/technical-manuals/101/gsh-gssg-glo-assay-protocol.pdf?rev=cfd04feb992845f298e63d80cc9c4ed8&la=en (accessed on 1 August 2023).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).