Abstract
The achlorophyllous unicellular microalga of the genus Prototheca, a causative agent of bovine mammary gland infection, is receiving increasing attention in the field of veterinary medicine. Mastitis caused by these algae leads to significant economic losses for farmers worldwide and represents a source of threat to raw milk quality and dairy food-chain safety. This case report describes an outbreak of bovine mastitis in dairy cattle caused by Prototheca bovis and the on-farm practices that resulted in the recovery of the affected herd and elimination of the presence of Prototheca spp. in the farm environment. Effective management strategies that played a crucial role in protothecosis eradication included regular screening, timely identification, strict separation of Prototheca-positive cows, a change of housing regime associated with the utilisation of straw mattresses and removal of deep bedding and, finally, the introduction of intermediate disinfection of teat cups with peracetic acid to prevent the spread of infection to other healthy individuals. The eradication process lasted approximately three years and required the removal of 139 cows from the farm. The corrective and preventive measures described in this case report provide guidance to farmers on how to successfully deal with protothecal mastitis on farms.
1. Introduction
Bovine mammary gland infection caused by members of the genus Prototheca has been reported with increasing incidence worldwide in recent decades [1], and this is accompanied by a growing awareness and interest in this organism in veterinary medicine and dairy practice. Prototheca spp., saprophytic achlorophyllous unicellular microalgae [2], are distributed ubiquitously in the environment inhabiting plants, soil, sludge, the faeces of domestic and wild animals, drinking and marine water, barn floors, animal bedding, and meat products [3]. Some species may be opportunistic pathogens of both humans and animals including dairy cattle, dogs, cats, and goats, causing various inflammatory diseases from cutaneous to systemic forms [4]. In a veterinary context, bovine mastitis is the predominant animal infection caused by this microalga [1], with the species P. bovis (formerly P. zopfii genotype 2) most commonly associated with the disease [5].
Predisposing factors for the development of bovine mastitis in dairy cattle include, first and foremost, deficient animal care and poor milking hygiene [3]. These algae do not respond to antibiotic treatment, and there is currently no effective method of therapy, for which reason the only control measure is the elimination of infected animals from breeding. Ultimately, mastitis caused by Prototheca spp. leads to significant economic losses for farmers, which is associated with reduced milk production, premature culling of infected animals, and veterinary expenses [1], while its zoonotic potential is no less serious in terms of public health [6]. The optimal and effective management of affected dairy cattle breeding aimed at the eradication of protothecal mastitis on farms remains unclear, and to the best of our knowledge, there is currently no published paper dealing with the recovery of dairy farming from protothecosis. This case report therefore describes an outbreak of protothecal mastitis on a dairy farm and subsequent procedures applied to recover the herd and eliminate the presence of Prototheca spp. from the farm. It also offers a set of recommendations for preventive measures for breeders, which aims to support the stabilisation of the mastitis situation associated with Prototheca algae on a global scale.
2. Case Description
This case report describes the development of the protothecal mastitis situation on a dairy cattle farm in the period from October 2019 to August 2022. The farm, numbering 400 dairy cows of the Bohemian Spotted breed and located in the Czech Republic, had a long history of mastitis. Persistently high somatic cell counts (SCC) were observed in milk (Figure 1). The farm faced problems with the treatment of mastitis cows that did not respond to the antibiotics applied—the secretion of thin, watery milk with white flakes from the mastitis quarter persisted, and in some cases, there was even a worsening of the condition. As it later turned out, the cause of the ineffectiveness of the antibiotic therapy was incorrect assessment of the result of the on-farm culture tests, with the presence of bacterial agents being mistakenly assumed. Consequently, inadequate and ineffective organisational measures were applied. Mastitis was manifested clinically only sporadically, by the aforementioned alterations in milk appearance and consistency. In the case of an acute clinical status (loss of appetite and milk production) the cows were taken immediately to the slaughterhouse, where all the cows that had a liver pathology were found to have algal or fungal pathogens detected by standard cultivation methods either in the mammary gland or in other internal organs.
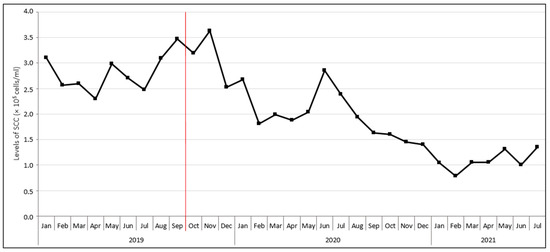
Figure 1.
Development of somatic cell counts (SCC) in bulk tank milk during the protothecosis eradication process on the farm. The beginning of the eradication process is indicated in red. To illustrate the situation before the beginning of eradication, the chart is supplemented with SCC data from January to September 2019.
The farm practised a loose-housing system with bedding operation. The basis of the bedding was fresh manure, and wet sawdust with a disinfectant was used to sprinkle the bedding. Lactating cows were managed in sections, heifers at a high pregnancy level and calving cows on deep bedding, while other pregnant heifers and dry cows were kept outdoors in the grazing area from April to November. The antibiotic Drycloxa-kel (KELA Laboratoria n.v., Hoogstraten, Belgium) was applied intramammary to the entire herd in order to drying of lactating cows.
An insufficient level of cow health management was practiced on the farm without thorough compliance with the principles of milk collection hygiene (e.g., no application of any variant of milking unit disinfection between individual milkings, inconsistent use of teat plugs). An excessive amount of water was applied to prepare the mammary gland for milking. Calved and lactating mastitis cows were milked into bucket milking units, which had a devastating effect on their udders. There was a roughly two-year experience with poor-quality feed that was wetted with utility water not originating from a verified water source; calves were fed waste milk (including mastitic) acidified with formic acid. In addition, only methods that were not entirely accurate and reliable were used routinely for the diagnosis of mastitis in cows, such as the Natural Killer Test (the Czech equivalent of the California Mastitis Test) and on-farm culturing—the Pure Milk Test (PM).
The microbiological quality of milk on the farm was evaluated at an accredited diagnostic veterinary laboratory in October 2019, with a bulk tank milk (BTM) sample being culturally examined for the presence of major mastitis pathogens, including Prototheca spp. (Table 1). The culture examination revealed the presence of P. bovis and yeasts at quantities of 1.0 × 103 and 1.3 × 103 CFU/mL, respectively. The SCC value decreased slightly from the previous measurement to 3.2 × 105 cells/mL (Figure 1). In response to these findings, a comprehensive screening of algal and fungal agents in the herd was performed, following the diagnostic protocol schematically shown in Figure 2. In addition, a set of measures was recommended to the breeder, including thorough disinfection and bleaching of stalls, bedding removal in production stalls and its replacement with straw mattresses regularly sprinkled with a Dekamix alkaline biocide powder (DüKa Czech s.r.o., Domazlice, Czech Republic) having the effect of drying and increasing the pH of the bedding and reducing the infectious pressure of pathogenic microorganisms. The cows with confirmed protothecal mastitis were separated from healthy individuals and calved cows and heifers were incorporated into the herd only after a negative result of microbiological tests carried out by an accredited laboratory. Cows showing clinical signs of mastitis were left in the herd until infection with Prototheca spp. was confirmed via microbiological tests. The feeding of mastitic milk to calves was prohibited, as was the excessive use of water in the milking parlour during the milking process and the administration of antibiotics to Prototheca-positive pregnant cows. Furthermore, preventive measures, such as regular microbiological control of feed sources, separate milking of calved and mastitis cows, and an intermediate disinfection regime of teat cups with manual spray application after the milking of each cow were implemented.

Table 1.
Prototheca bovis cell counts determined in bulk tank milk by culture examination during the protothecosis eradication process on the farm.
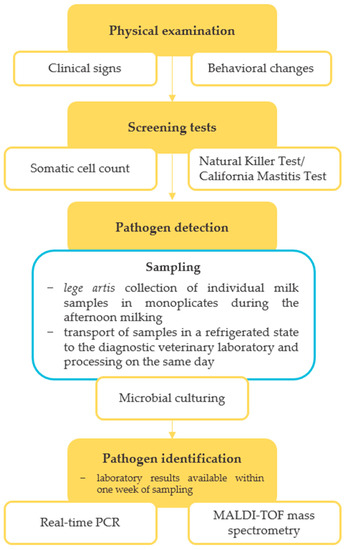
Figure 2.
Diagnostic protocol for non-bacterial bovine mastitis.
A culture examination of 432 individual milk samples from the whole mammary gland of all lactating cows on the farm revealed the presence of 126 animals infected with P. bovis, representing a prevalence of 29.2% in the herd. The presence of moulds and yeast (Candida parapsilosis) was also detected in a small proportion of samples (4.3 and 3%, respectively). The milk samples were collected from calved cows in four stages (monthly from October 2019 to January 2020) to map the entire herd. In relation to individual milk collections, the presence of P. bovis in milk was detected in 29.5, 31.7, 32.3, and 24.1% of tested cows, respectively (Table 2). The non-decreasing trend in the number of Prototheca-positive cows in the herd was surprising given that all animals included in production were checked via microbiological tests for algal presence. Possible causes appeared to be the common housing of primiparous cows with positive cows, the inappropriate sequence of milking cows in the milking parlour (mastitis cows with bacterial and non-bacterial agents followed by calved cows), and uncompleted replacement of all potentially contaminated rubber components at the milking parlour. Pregnant high-production Prototheca-positive cows were consequently separated from the rest of the herd in an extra stall with a strict veterinary and hygiene regime; positive barren cows were removed from the farm. All positive cows were marked on insemination cards. The recommendation for the breeder was to feed Butycell (Bodit Tachov s.r.o., Stribro, Czech Republic), a supplementary mineral feed containing substances supporting liver function and increasing the immune defence capacity of cows (herbal extracts, methionine, vitamin E, zinc chelate, selenium). Other newly introduced regime measures included the separation of problem cows into two groups depending on the pathogen detected (bacterial and non-bacterial agents), the strict hygienic storage of teat plugs, and the usage of one disinfectant for teat dipping exclusively for each of the groups (with iodine as an active substance).

Table 2.
Development of the number of Prototheca-positive calved cows determined by culture examination of individual milk samples during the protothecosis eradication process on the farm.
As part of the routine farm performance inspection carried out in November 2019, P. bovis was detected in a herd-level sample from BTM with an average quantity of 6.8 × 102 CFU/mL. The presence of yeasts was also determined at a quantity of 2.5 × 102 CFU/mL. Both values represented a slight improvement over the previous month’s analysis. In addition, the level of environmental contamination on the farm was assessed, and sources of algal infection were sought. Swab samples from the environment were collected during the working process and submitted for culture examination. The evaluated samples included swabs from milking equipment, including its internal surfaces and pipelines, teat plugs, milking parlour fencing, water used on the farm, and all feed components such as hay, corn silage, and grain scrap. None of the examined samples was found to be positive for the presence of Prototheca spp.; however, moulds and yeasts were detected in great abundance. Therefore, regular weekly disinfection of the back part of straw mattresses with peracetic acid was introduced, as this compound was previously observed to have high disinfection efficiency against non-bacterial agents of mastitis [7].
In addition, water quality was also investigated with regard to selected chemical indicators that could be related to the issue of potential algae reservoirs in the milking parlour pipes or cooling tanks (silicon dioxide, hydrogen carbonates, and hydrogen phosphates). The chemical analysis revealed that the concentrations of the last two mentioned compounds meet the drinking water requirements set by the Czech legislation (Decree No. 252/2004 Col.). Nevertheless, silicon dioxide, although not bound by this decree, was found at high concentrations (9.4–9.8 mg/L). Considering the higher probability of algal occurrence in places where the silicon dioxide concentration exceeds 2.5 mg/L, the presence of a biofilm formed by Prototheca spp. was suspected [8]. It was therefore recommended that the internal surfaces of the milking parlour and cooling equipment, in addition to standard sanitation, were regularly washed with sodium hydroxide with the aim of removing biofilms and siliceous salts surfaces, which may represent a significant reservoir of non-bacterial agents of mastitis. The compound sodium hydroxide is part of standard cleaning procedures in many food and dairy manufacturing plants and is capable of eliminating biofilms [9].
At the beginning of 2020, the disinfection efficiency of pre- and post-milking teat dips against P. bovis and Candida krusei yeast was laboratory analysed. Dips with chlorhexidine and chlorine dioxide as active substances were found to be ineffective against these pathogens. On the other hand, a dip containing iodine and peracetic acid showed the greatest possible efficiency and was therefore recommended to the farmer as an effective means of combating the pathogens mentioned above. Furthermore, the effectiveness of the manual spray intermediate disinfection of teat cups with a disinfectant containing peracetic acid during the milking process and the quality of mammary gland preparation for milking were verified and assessed. A total of 7 out of 10 swab samples taken from the milking unit at the time of mastitis cow section milking were found to be positive for P. bovis. However, all 10 swab samples from the inner surface of teat cups taken after intermediate disinfection had been performed gave a negative result for the presence of Prototheca spp., demonstrating sufficient efficiency of the selected disinfectant against this agent. The results were in agreement with further testing a year later, when none of 28 swab samples taken from the inner surface of teat cups and from the skin surface of teats after intermediate disinfection with peracetic acid was found to be positive for Prototheca spp. Only bacterial mastitis pathogens and rarely yeasts were detected through cultivation.
In February 2020, 48 individual milk samples were taken from the production section of the cows and examined, seven of which tested positive for algae (14.5%). A total of 80 protothecal mastitis cows were slaughtered from the initiation of the resolution of the on-farm problem with protothecal mastitis in October 2019 until March 2020. Milk samples and organ swabs were examined from five of these cows. Although P. bovis was detected in all milk samples, nasal, rectal, and tongue surface swab samples were culturally negative. However, granulomatous tissue was found in the ovaries, kidneys, and liver in all cases.
The comprehensive screening of the Prototheca spp. presence in lactating cows was performed in May 2020. Overall, 14 out of 254 individual milk samples culturally examined (5.5%) gave a positive result, all representing new cases. No representatives of moulds or yeasts were detected. In a herd-level BTM sample, the P. bovis cell load was, on average, 5.3 × 103 CFU/mL. Control measures introduced in this period included replacing all rubber components and changing the order of animal milking—bacterial mastitis cows essentially milked before protothecal mastitis cows. In preparation for milking, the first stream of milk from each teat was fundamentally squirted only into strip cups. The barn was disinfected once every two weeks until July 2020, when the last Prototheca-positive cow calved. Manure was removed from the stalls twice a day, and straw bedding was changed. After the last Prototheca-positive cow parturition, the deep bedding was disposed of and the calving barn was thoroughly disinfected. Calves were fed exclusively with milk replacer. The farm continued feeding Butycell as a significant increase in performance has been recorded since the beginning of this supplementary feed administration to cows. Control of the health status of 44 and 36 cows newly calved in July 2020 and August 2020, respectively, did not show the presence of Prototheca spp. using an on-farm PM test, the results of which were validated by an accredited diagnostic veterinary laboratory. At that time, there were 24 cows with protothecal mastitis on the farm, which were planned to be removed by the end of October 2020. These cows were milked separately from cows with bacterial mastitis. After eliminating all Prototheca-positive cows from the farm, the bedding in the extra stall for cows with protothecosis was removed, and deep disinfection of the stall was performed.
A fundamental measure for the next period was the introduction of regular herd-level sample examination from BTM. BTM samples were screened at approximately weekly intervals between October 2020 and March 2021 and were mostly free of P. bovis; in other cases, the quantity of P. bovis cells ranged from 5 to 150 CFU/mL. In relation to the milk of individual animals, the examination of 69 and 84 individual milk samples from calved cows in December 2020 and March 2021, respectively, revealed no P. bovis occurrence. Only in six cases was the presence of yeasts in low quantities recorded. However, 1 cow in the first lactation out of the 86 examined was diagnosed with protothecosis immediately after calving in July 2021. The investigation showed that the cow had been fed with milk from a Prototheca-positive dam as a heifer.
From May to July 2021, the load of P. bovis cells in BTM samples dropped to <10 CFU/mL and from August 2021 to August 2022, P. bovis was no longer detected in this type of sample. Only low levels of yeasts (quantity range 1–5 × 102 CFU/mL) were recorded. It could be stated that since November 2021, after the removal of the last positive cow from the farm, the herd was free of positive animals, with no Prototheca-positive results detected in individual milk samples, for which reason the process of protothecosis eradication on the farm was considered successful. Monitoring was terminated in August 2022 and required the removal of 139 cows from the farm.
3. Laboratory Examination
Milk (individual and BTM), swabs and environmental samples were examined for the presence of non-bacterial mastitis agents at an accredited diagnostic veterinary laboratory (Vedia s.r.o., Strakonice, Czech Republic) using standard cultivation methods. The identification of yeast and fungal isolates was performed via the MALDI-TOF mass spectrometry method at Jihlava State Veterinary Institute (Jihlava, Czech Republic). Isolates suspected for the presence of Prototheca spp. based on colony morphology and microscopic appearance (Figure 3) were identified using multiplex real-time PCR [10].
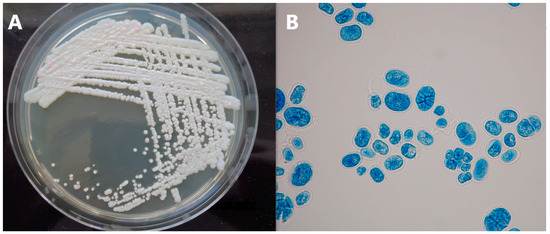
Figure 3.
Colonies of Prototheca sp. on Sabouraud dextrose agar (A), lactophenol cotton blue stained Prototheca sp. cells in 1000× magnification (B).
The disinfection efficiency of biocidal preparations was tested either via the drop-based method or by sprinkling, with tested preparations being applied to an algal or fungal pathogen previously inoculated on an agar plate. The clear areas (growth inhibition) in the lawn of confluent growth of the tested pathogen were observed following incubation at 25 and 37 °C for 24 h to 5 days.
Water sample analysis was performed in accordance with the standards valid in the Czech Republic for the examination of the chemical and physical parameters of water (ČSN 75 7481, ČSN EN ISO 9963-1) at the Department of Water Technology and Environmental Engineering at the University of Chemistry and Technology (Prague, Czech Republic).
4. Discussion and Conclusions
Mastitis represents one of the most widespread and economically intensive diseases in dairy cattle worldwide. In addition to the most frequent bacterial mastitis pathogens, non-bacterial agents such as yeasts and Prototheca algae can also contribute to the development of this disease on dairy cattle farms [1,11]. Although generally saprophytic, the second agent mentioned has been reported as causing bovine mastitis with growing incidence throughout the world [1]. The Prototheca genus comprises several species, but only P. bovis, P. blaschkeae, and rarely P. wickerhamii are currently associated with bovine mastitis of non-bacterial origin [3]. Protothecal mastitis manifests predominantly as a subclinical infection with an increased number of leukocytes in milk or udder and reduced milk production due to udder damage. In rare cases, it passes into a clinical form accompanied by fever, mammary gland swelling, or anorexia [1]. The biggest challenge complicating the recovery of the cattle herd from protothecosis is the impossibility of treatment of protothecal mastitis infections with antibiotics. Although ineffective, antibiotic therapy is often applied due to incorrect diagnosis of infected animals; however, this leads to the elimination of the protective commensal microbiota and ultimately worsens the course of the disease [12]. At present, the only possible corrective measure is the gradual removal of infected animals to slaughterhouses, which underlines the importance of a high level of hygiene management and animal care on the farm. For this reason, this case study reveals management weaknesses of one dairy farming predisposing the herd to the development of protothecal infection and offers farmers a set of recommendations to eliminate this agent from the farm.
A key point in the eradication of protothecal mastitis on the farm is the early and accurate diagnosis of subclinically infected individuals, which allows corrective measures to be taken on time to prevent the spread of infection to the rest of the herd. Currently, the diagnosis of Prototheca spp. infections is based on histological and microscopic identification, pathogen cultivation, or qPCR [10]. In our study, strict separation of Prototheca-positive cows, diagnosed on the basis of continuous individual milk sample examination, into extra stalls and their gradual elimination in slaughterhouses proved effective in this regard. However, due to the fact that it is not always possible to detect all infected individuals in the herd due to intermittent shedding, it is recommended that suspected cows are re-examined [13]. It is generally believed that Prototheca spp. cells enter the udder through the teat orifice, originating, for example, from contaminated milking equipment or various environmental sources [1]. In this regard, it is necessary to ensure thorough and routine intermediate disinfection of teat cups after the milking of each cow with a peracetic acid, which has been shown to be one of the most effective sanitising agents against Prototheca spp. [7]. Its effectiveness was also demonstrated in our study. We also stress the absolute necessity of regular sanitation interventions in stalls, the removal of deep bedding as a potential source of infection, and its replacement with straw mattresses regularly sprinkled with a Dekamix alkaline powder and disinfected with peracetic acid. Although BTM sample examination for the presence of Prototheca spp. gave negative results throughout the year (August 2021–August 2022), and also, no Prototheca spp. was detected in individual milk samples from November 2021, and we thus considered the herd as Prototheca free, the risk that the infection reappears here exists. Hypothetically, there is a risk of infection in pastures where previously positive dairy cows grazed. This implies the necessity of continuous checks and testing at certain intervals for the presence of Prototheca spp.
Attention should be paid to the impact of Prototheca spp. infection on the technological processing of milk into dairy products and especially milk safety. Although milk is heat treated in the vast majority of cases, Prototheca spp. presence was revealed in milk even after pasteurisation, posing a potential risk of intestinal infections and enteritis in humans [10]. The consequences of bovine mastitis on public health still need to be determined.
To our knowledge, this is the first report of successful eradication of protothecal mastitis on a dairy farm. The corrective measures that have been shown to be effective in the protothecosis eradication process are summarized in Table 3, providing guidance to farmers on how to successfully deal with a protothecal mastitis situation on farms. The described control strategy can ultimately contribute to reducing the incidence of this disease globally.

Table 3.
Guidance for farmers on the prevention of outbreaks and successful eradication of protothecal mastitis on a dairy farm.
Author Contributions
Conceptualization, R.S. and M.M.; methodology, R.S.; formal analysis, M.B. and R.S.; investigation, R.S.; writing—original draft preparation, M.B.; writing—review and editing, M.C. and R.S.; project administration, M.M. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture of the Czech Republic, grant numbers QK1910092 and RO0523.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Libisch, B.; Picot, C.; Ceballos-Garzon, A.; Moravkova, M.; Klimesova, M.; Telkes, G.; Chuang, S.T.; Le Pape, P. Prototheca infections and ecology from a One Health perspective. Microorganisms 2022, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Bakuła, Z.; Gawor, J.; Maciszewski, K.; Kusber, W.-H.; Dyląg, M.; Nowakowska, J.; Gromadka, R.; Karnkowska, A. The genus Prototheca (Trebouxiophyceae, Chlorophyta) revisited: Implications from molecular taxonomic studies. Algal Res. 2019, 43, 101639. [Google Scholar] [CrossRef]
- Thompson, G.; Silva, E.; Marques, S.; Müller, A.; Carvalheira, J. Algaemia in a dairy cow by Prototheca blaschkeae. Med. Mycol. 2009, 47, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Dyląg, M.; Roesler, U.; Murugaiyan, J. Isolation of infectious microalga Prototheca wickerhamii from a carp (Cyprinus carpio)—A first confirmed case report of protothecosis in a fish. J. Fish Dis. 2017, 40, 1417–1421. [Google Scholar] [CrossRef]
- Ricchi, M.; De Cicco, C.; Buzzini, P.; Cammi, G.; Arrigoni, N.; Cammi, M.; Garbarino, C. First outbreak of bovine mastitis caused by Prototheca blaschkeae. Vet. Microbiol. 2013, 162, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Silva, E.; Carvalheira, J.; Thompson, G. Short communication: In vitro antimicrobial susceptibility of Prototheca wickerhamii and Prototheca zopfii isolated from bovine mastitis. J. Dairy Sci. 2006, 89, 4202–4204. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Lee, S.H.; de Paula Arruda, E.; Pedroso Galles, D.; Camargo Caetano, V.; Fernandes de Oliveira, C.A.; Fernandes, A.M.; Veiga dos Santos, M. Biofilm-producing ability and efficiency of sanitizing agents against Prototheca zopfii isolates from bovine subclinical mastitis. J. Dairy Sci. 2015, 98, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J. Biofilm formation by pathogenic Prototheca algae. Lett. Appl. Microbiol. 2015, 61, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Flint, S.H.; Brooks, J.D. Evaluation of the effect of cleaning regimes on biofilms of thermophilic bacilli on stainless steel. J. Appl. Microbiol. 2004, 96, 110–116. [Google Scholar] [CrossRef]
- Bacova, R.; Kralik, P.; Kucharovicova, I.; Seydlova, R.; Moravkova, M. A novel TaqMan qPCR assay for rapid detection and quantification of pro-inflammatory microalgae Prototheca spp. in milk samples. Med. Mycol. 2021, 59, 784–792. [Google Scholar] [CrossRef]
- Moravkova, M.; Huvarova, V.; Vlkova, H.; Kostovova, I.; Bacova, R. Raw bovine milk as a reservoir of yeast with virulence factors and decreased susceptibility to antifungal agents. Med. Mycol. 2021, 59, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [PubMed]
- Park, H.S.; Moon, D.C.; Hyun, B.H.; Lim, S.K. Short Communication: Occurrence and persistence of Prototheca zopfii in dairy herds of Korea. J. Dairy Sci. 2019, 102, 2539–2543. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).