Antiviral Potential of Antillogorgia americana and elisabethae Natural Products against nsp16–nsp10 Complex, nsp13, and nsp14 Proteins of SARS-CoV-2: An In Silico Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Target Proteins
2.2. Obtaining Ligands
2.3. Visualization
2.4. Pre-Docking Preparations
2.5. Molecular Docking
2.6. Docking Validation
2.7. Molecular Dynamics Simulation
2.8. Toxicity Assessment
2.9. Pharmacokinetics Studies
3. Results and Discussion
3.1. Binding Affinity Analyses
3.1.1. nsp16–nsp10 Docking
| Categorization of Ligands nsp16–nsp10 | |||
|---|---|---|---|
| Low | Moderate | High | |
| −4.2 to −5.9 kcal/mol | −6.0 to 6.9 kcal/mol | −7.0 to 7.9 kcal/mol | −8.0 kcal/mol and above |
| 5-Hydroperoxyicosa-2,4,6,8-tetraenoic acid | 1-Monoacetate | 1β,3β-dihydroxy-5α,6α-epoxy-9-oxo-9,11-secogorgostan-11-ol | Pseudopterosin A |
| (+)-Aristolone | 3-β-Hydroxy-5α,6α-epoxy-9-oxo-9,11-secogorgostan-11-ol | 3-O-Methylquercetin | Pseudopterosin H |
| (+)-α-Terpineol | 3-epi-Elisabanolide | 15-α-(Angeloyloxy)kaura-16-ene-18-oicacid | Pseudopterosin I |
| (−)-β-Chamigrene | 4-Acetylamphilectolide | Ameristerol A | Pseudopterosin R |
| β-Gorgonene | 7-Hydroxyerogorgiaene | Ameristerenol A | Pseudopterosin T |
| β-Selinene | 7-Hydroxyerogorgiaenone | Amphilectosin A | Pseudopterosin V |
| Calarene | 7,14-Erogorgiaenediol | Amphilectosin B | Pseudopterosin X |
| Cumbiasin B | 8-epi-Americanolide C | Americanolide D | Pseudopterosin Y |
| Cumbiasin C | 8-epi-Methoxyamericanolide A | Americanolide E | Sandresolide B |
| Cyperene | 10-epi-Americanolide C | Americanolide F | |
| Elisabethadienol | 10-epi-Methoxyamericanolide A | Amphiphenalone | |
| Elisabethatrienol | 12-Acetoxypseudopterolide | Bis-7-Hydroxyerogorgiaene | |
| Elisabethin B | Aberrarone | Caribenol A | |
| Elisabethin C | Ameristerenol B | CMNPD12140 | |
| Elisabethin D | Americanolide A | CMNPD12142 | |
| Elisabethin G | Americanolide B | CMNPD16184 | |
| Elisabetholide | Americanolide C | CMNPD16186 | |
| Elisapterosin A | Amphilectolide | Elisabethol | |
| Furanotriene | β-Eudesmol | Elisabatin A | |
| γ-Maaliene | Bicyclogermacrene | Elisabatin B | |
| Ichthyothereol acetate | Biformene | Elisabatin C | |
| Preclavulone A | Caribenol B | Elisabethin A acetate | |
| Calamenene | Elisabethin D acetate | ||
| Caridiene | Elisapterosin F | ||
| Curcuhydroquinone | Grandifloric acid | ||
| (−)-Curcuquinone | Homopseudopteroxazole | ||
| Colombiasin A | Hyperoside | ||
| Cumbiasin A | Isoquercitrin | ||
| CMNPD5089 | Ileabethin | ||
| CMNPD11416 | Ileabethoxazole | ||
| CMNPD12137 | iso-Pseudopterosin E | ||
| CMNPD12138 | Kaempferol | ||
| CMNPD12139 | Methoxyamericanolide B | ||
| CMNPD16185 | O-Methylelisabethadione | ||
| CMNPD16187 | O-Methyl-nor-elisabethadione | ||
| CMNPD17188 | Pseudopterosin B | ||
| ent-Kaur-16-en-19-ol | Pseudopterosin C | ||
| Elisabanolide | Pseudopterosin D | ||
| Elisabethamine | Pseudopterosin E | ||
| Elisabethin A | Pseudopterosin F | ||
| Elisabethin E | Pseudopterosin G | ||
| Elisabethin F | Pseudopterosin J | ||
| Elisabethin H | Pseudopterosin K | ||
| Elisapterosin B | Pseudopterosin L | ||
| Elisapterosin C | Pseudopterosin M | ||
| Elisapterosin D | Pseudopterosin N | ||
| Elisapterosin E | Pseudopterosin O | ||
| Erogorgiaene | Pseudopterosin P | ||
| Furanogermacrene | Pseudopterosin Q | ||
| Furanoguaian-4-ene | Pseudopterosin U | ||
| Germacrene D | Pseudopterosin W | ||
| Isofuranotriene | Pseudopterosin Z | ||
| Kaurane-16,18-diol18-acetate | Pseudopterosin G-J aglycone | ||
| Kaurenoicacid | Pseudopteroxazole | ||
| Methoxyamericanolide A | Quercetin | ||
| Methoxyamericanolide E | Taxifolin | ||
| Methoxyamericanolide G | seco-Pseudopterosin A | ||
| Methoxyamericanolide H | seco-Pseudopterosin C | ||
| Methoxyamericanolide I | seco-Pseudopterosin D | ||
| Pseudopterolide | seco-Pseudopterosin E | ||
| Pseudopterosin S | seco-Pseudopterosin F | ||
| Pseudopterosin-MA | seco-Pseudopterosin G | ||
| Quercetin-3-O-β-d-arabinofuranoside | seco-Pseudopterosin H | ||
| seco-Pseudopterosin B | seco-Pseudopterosin I | ||
| seco-Pseudopterosin J | seco-Pseudopterosin K | ||
| seco-Gorgosterol | Sandresolide A | ||
| Sandresolide C | |||
| seco-pseudopteroxazole | |||
3.1.2. nsp13 Docking
| Binding Affinity Values—nsp13 | ||
|---|---|---|
| Low | Moderate | High |
| −5.0 to −5.9 kcal/mol | −6.0 to −6.9 kcal/mol | −7.0 to −8.2 kcal/mol |
| 5-Hydroperoxyicosa-2,4,6,8-tetraenoicacid | 1-Monoacetate | 3-epi-Elisabanolide |
| 7,14-Erogorgiaenediol | 1β,3β-dihydroxy-5α,6α-epoxy-9-oxo-9,11-secogorgostan-11-ol | 4-Acetylamphilectolide |
| 12-Acetoxypseudopterolide | 3β-Hydroxy-5α,6α-epoxy-9-oxo-9,11-secogorgostan-11-ol | 10-epi-Americanolide C |
| (+)-α-Terpineol | 3-O-Methylquercetin | Aberrarone |
| β-Eudesmol | 7-Hydroxyerogorgiaene | Ameristerenol B |
| β-Selinene | 7-Hydroxyerogorgiaenone | Americanolide A |
| Bicyclogermacrene | 8-epi-Americanolide C | Americanolide B |
| β-Gorgonene | 8-epi-Methoxyamericanolide A | Amphilectosin A |
| Cyperene | 10-epi-Methoxyamericanolide A | Amphilectosin B |
| Calamenene | 15-α-(Angeloyloxy)kaura-16-ene-18-oicacid | Amphilectolide |
| Calarene | (+)-Aristolone | Amphiphenalone |
| Caridiene | Amersiterol A | Colombiasin A |
| CMNPD12139 | Ameristerenol A | Cumbiasin A |
| CMNPD16184 | Americanolide C | Cumbiasin B |
| CMNPD16185 | Americanolide D | CMNPD12142 |
| Elisabethol | Americanolide E | CMNPD16186 |
| Elisabethin A acetate | Americanolide F | CMNPD16187 |
| Elisabethin B | Biformene | Elisabatin A |
| Elisabethin C | Bis-7-hydroxyerogorgiaene | Elisapterosin A |
| Elisabethin D acetate | (−)-β-Chamigrene | Elisapterosin B |
| Elisabethin E | (−)-Curcuquinone | Elisapterosin D |
| Elisabethin G | Curcuhydroquinone | Elisapterosin E |
| Elisabetholide | Cumbiasin C | Elisapterosin F |
| Furanogermacrene | Caribenol A | Ileabethin |
| Furanotriene | Caribenol B | Ileabethoxazole |
| γ-Maaliene | CMNPD5089 | Isoquercitrin |
| Germacrene D | CMNPD11416 | Kaempferol |
| Grandifloric acid | CMNPD12137 | Kaurenoicacid |
| Ichthyothereol acetate | CMNPD12138 | O-Methyl-nor-elisabethadione |
| Isofuranotriene | CMNPD12140 | Pseudopterosin K |
| Kaurane-16,18-diol18-acetate | CMNPD17188 | Pseudopterosin T |
| Pseudopterolide | Elisabanolide | Pseudopterosin U |
| Pseudopterosin D | Elisabatin B | Pseudopterosin V |
| Pseudopterosin G | Elisabatin C | Pseudopteroxazole |
| Pseudopterosin-MA | Elisabethadienol | Quercetin |
| Sandresolide A | Elisabethamine | Quercetin3-O-beta-d-arabinofuranoside |
| Elisabethatrienol | seco-Pseudopterosin E | |
| Elisabethin A | seco-Pseudopterosin F | |
| Elisabethin D | seco-Pseudopterosin G | |
| Elisabethin F | seco-Pseudopterosin H | |
| Elisabethin H | seco-Pseudopterosin I | |
| Elisapterosin C | seco-Pseudopterosin K | |
| ent-Kaur-16-en-19-ol | Sandresolide C | |
| Erogorgiaene | Taxifolin | |
| Furanoguaian-4-ene | ||
| Homopseudopteroxazole | ||
| Hyperoside | ||
| iso-Pseudopterosin E | ||
| Methoxyamericanolide A | ||
| Methoxyamericanolide B | ||
| Methoxyamericanolide E | ||
| Methoxyamericanolide G | ||
| Methoxyamericanolide H | ||
| Methoxyamericanolide I | ||
| O-Methylelisabethadione | ||
| Preclavulone A | ||
| Pseudopterosin A | ||
| Pseudopterosin B | ||
| Pseudopterosin C | ||
| Pseudopterosin E | ||
| Pseudopterosin F | ||
| Pseudopterosin H | ||
| Pseudopterosin I | ||
| Pseudopterosin J | ||
| Pseudopterosin L | ||
| Pseudopterosin M | ||
| Pseudopterosin N | ||
| Pseudopterosin O | ||
| Pseudopterosin P | ||
| Pseudopterosin Q | ||
| Pseudopterosin R | ||
| Pseudopterosin S | ||
| Pseudopterosin W | ||
| Pseudopterosin X | ||
| Pseudopterosin Y | ||
| Pseudopterosin Z | ||
| Pseudopterosin G-J aglycone | ||
| seco-Gorgosterol | ||
| seco-Pseudopterosin A | ||
| seco-Pseudopterosin B | ||
| seco-Pseudopterosin C | ||
| seco-Pseudopterosin D | ||
| seco-Pseudopterosin J | ||
| seco-Pseudopteroxazole | ||
| Sandresolide B | ||
3.1.3. nsp14 Docking
| Categorization of Ligands—nsp14 | ||
|---|---|---|
| Moderate | High | Very High |
| −6.5 to −7.9 kcal/mol | −8.0 to −9.9 kcal/mol | −10.0 kcal/mol and above |
| 1-Monoacetate | 1β,3β-Dihydroxy-5α,6α-epoxy-9-oxo-9,11-secogorgostan-11-ol | Amphilectosin A |
| 5-Hydroperoxyicosa-2,4,6,8-tetraenoicacid | 3β-Hydroxy-5α,6α-epoxy-9-oxo-9,11-secogorgostan-11-ol | Amphilectosin B |
| 8-epi-Methoxyamericanolide A | 3-O-Methylquercetin | Amersiterol A |
| 10-epi-Methoxyamericanolide A | 3-epi-Elisabanolide | Cumbiasin C |
| Americanolide E | 4-Acetylamphilectolide | CMNPD16186 |
| Americanolide F | 7-Hydroxyerogorgiaene | Elisabatin A |
| (+)-α-Terpineol | 7-Hydroxyerogorgiaenone | Elisabatin B |
| (+)-Aristolone | 7,14-erogorgiaenediol | Elisabatin C |
| (−)-β-Chamigrene | 8-epi-Americanolide C | Elisapterosin A |
| β-Eudesmol | 10-epi-Americanolide C | Homopseudopteroxazole |
| β-Selinene | 12-Acetoxypseudopterolide | Ileabethoxazole |
| β-Gorgonene | 15alpha-(Angeloyloxy)kaura-16-ene-18-oicacid | Iso-pseudopterosin E |
| Bicyclogermacrene | Aberrarone | Pseudopterosin A |
| Calarene | Ameristerenol A | Pseudopterosin D |
| Cyperene | Ameristerenol B | Pseudopterosin E |
| Curcuhydroquinone | Americanolide A | Pseudopterosin G |
| (−)-Curcuquinone | Americanolide B | Pseudopterosin I |
| ent-Kaur-16-en-19-ol | Americanolide C | Pseudopterosin J |
| Elisabethin C | Americanolide D | Pseudopterosin M |
| Elisabethin D | Amphilectolide | Pseudopterosin N |
| Elisabethin F | Amphiphenalone | Pseudopterosin O |
| Elisabethin G | Bis-7-hydroxyerogorgiaene | Pseudopterosin P |
| Elisabethin H | Biformene | Pseudopterosin Q |
| Furanogermacrene | Calamenene | Pseudopterosin R |
| Furanotriene | Caridiene | Pseudopterosin S |
| γ-Maaliene | Colombiasin A | Pseudopterosin T |
| Germacrene D | Cumbiasin A | Pseudopterosin U |
| Ichthyothereol acetate | Cumbiasin B | Pseudopterosin W |
| Isofuranotriene | Caribenol A | Pseudopterosin X |
| Kaurane-16,18-diol-18-acetate | Caribenol B | Pseudopterosin Y |
| Methoxyamericanolide A | CMNPD5089 | Pseudopterosin Z |
| Preclavulone A | CMNPD11416 | Pseudopteroxazole |
| CMNPD12137 | Pseudopterolide | |
| CMNPD12138 | seco-Pseudopterosin E | |
| CMNPD12139 | seco-Pseudopterosin F | |
| CMNPD12140 | seco-Pseudopterosin G | |
| CMNPD12142 | seco-Pseudopterosin H | |
| CMNPD16184 | Sandresolide C | |
| CMNPD16185 | ||
| CMNPD16187 | ||
| CMNPD17188 | ||
| Elisabethol | ||
| Elisabanolide | ||
| Elisabethadienol | ||
| Elisabethamine | ||
| Elisabethatrienol | ||
| Elisabethin A | ||
| Elisabethin A acetate | ||
| Elisabethin B | ||
| Elisabethin D acetate | ||
| Elisabethin E | ||
| Elisabetholide | ||
| Elisapterosin B | ||
| Elisapterosin C | ||
| Elisapterosin D | ||
| Elisapterosin E | ||
| Elisapterosin F | ||
| Erogorgiaene | ||
| Furanoguaian-4-ene | ||
| Grandifloric acid | ||
| Hyperoside | ||
| Isoquercitrin | ||
| Ileabethin | ||
| Kaempferol | ||
| Kaurenoicacid | ||
| Methoxyamericanolide B | ||
| Methoxyamericanolide E | ||
| Methoxyamericanolide G | ||
| Methoxyamericanolide H | ||
| Methoxyamericanolide I | ||
| O-Methylelisabethadione | ||
| O-Methyl-nor-elisabethadione | ||
| Pseudopterosin B | ||
| Pseudopterosin C | ||
| Pseudopterosin F | ||
| Pseudopterosin H | ||
| Pseudopterosin K | ||
| Pseudopterosin L | ||
| Pseudopterosin V | ||
| Pseudopterosin G-J aglycone | ||
| Pseudopterosin-MA | ||
| Quercetin | ||
| Quercetin-3-O-beta-d-arabinofuranoside | ||
| seco-Pseudopterosin A | ||
| seco-Pseudopterosin B | ||
| seco-Pseudopterosin C | ||
| seco-Pseudopterosin D | ||
| seco-Pseudopterosin I | ||
| seco-Pseudopterosin J | ||
| seco-Pseudopterosin K | ||
| Sandresolide A | ||
| Sandresolide B | ||
| seco-Gorgosterol | ||
| seco-pseudopteroxazole | ||
| Taxifolin | ||
3.1.4. Docking Protocol Validation—nsp14
3.2. Amino Acid Interactions
3.2.1. Selection Criteria to Filter Candidate Complexes
- The complex must interact with key catalytic residues
- It must interact by forming hydrogen and/or electrostatic bonds
- The bond distance for conventional hydrogen must not exceed (Å ≤ 3.00)
- The bond distance for electrostatic bonds must not exceed (Å ≤ 5.00)
3.2.2. Selected Docked Complexes
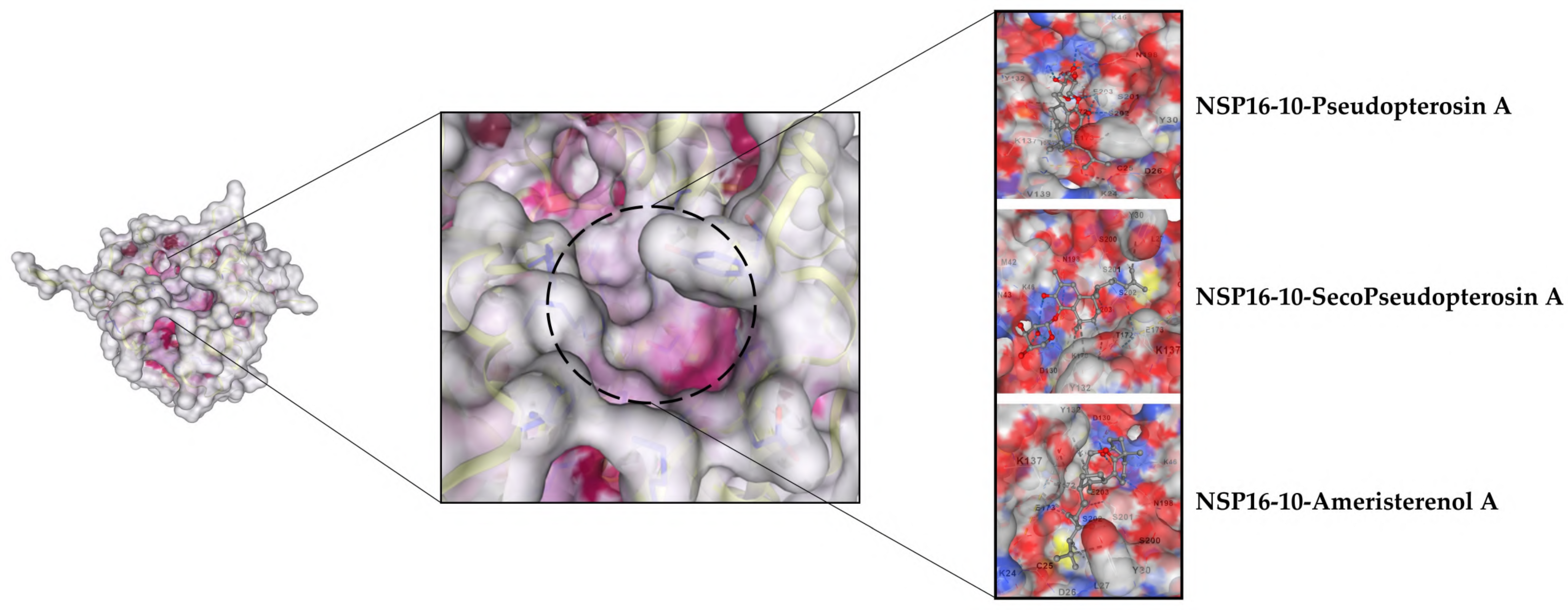
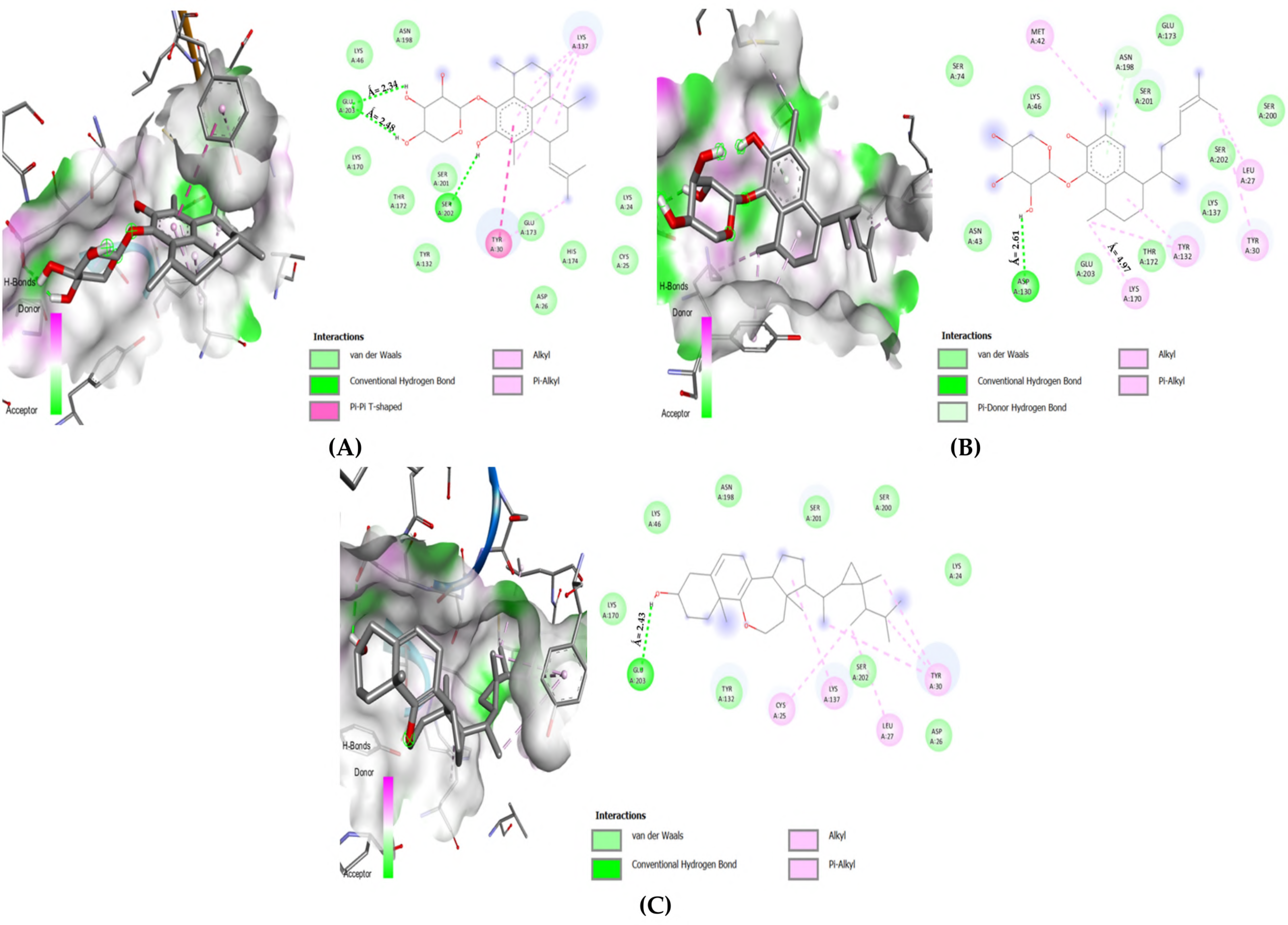
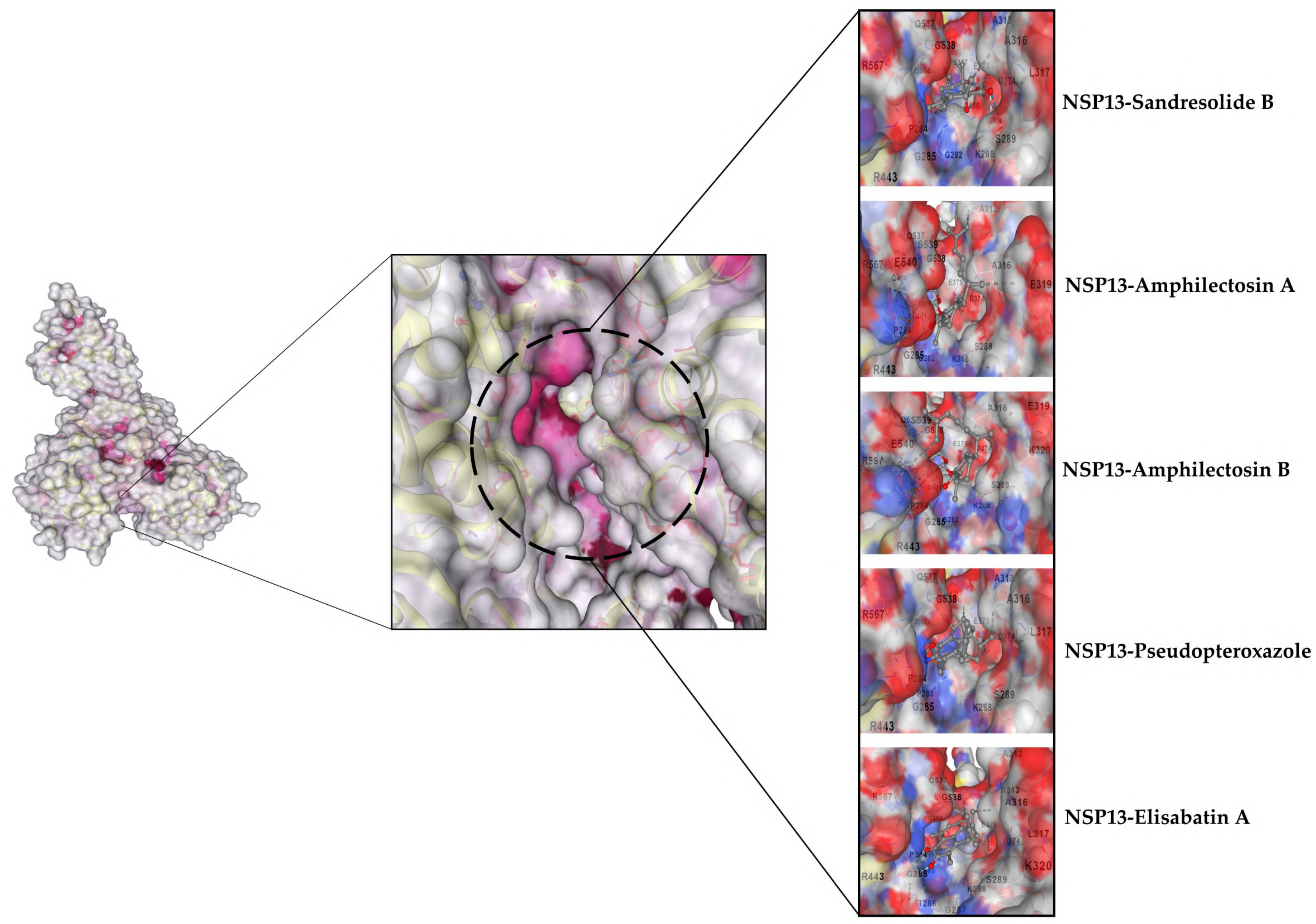

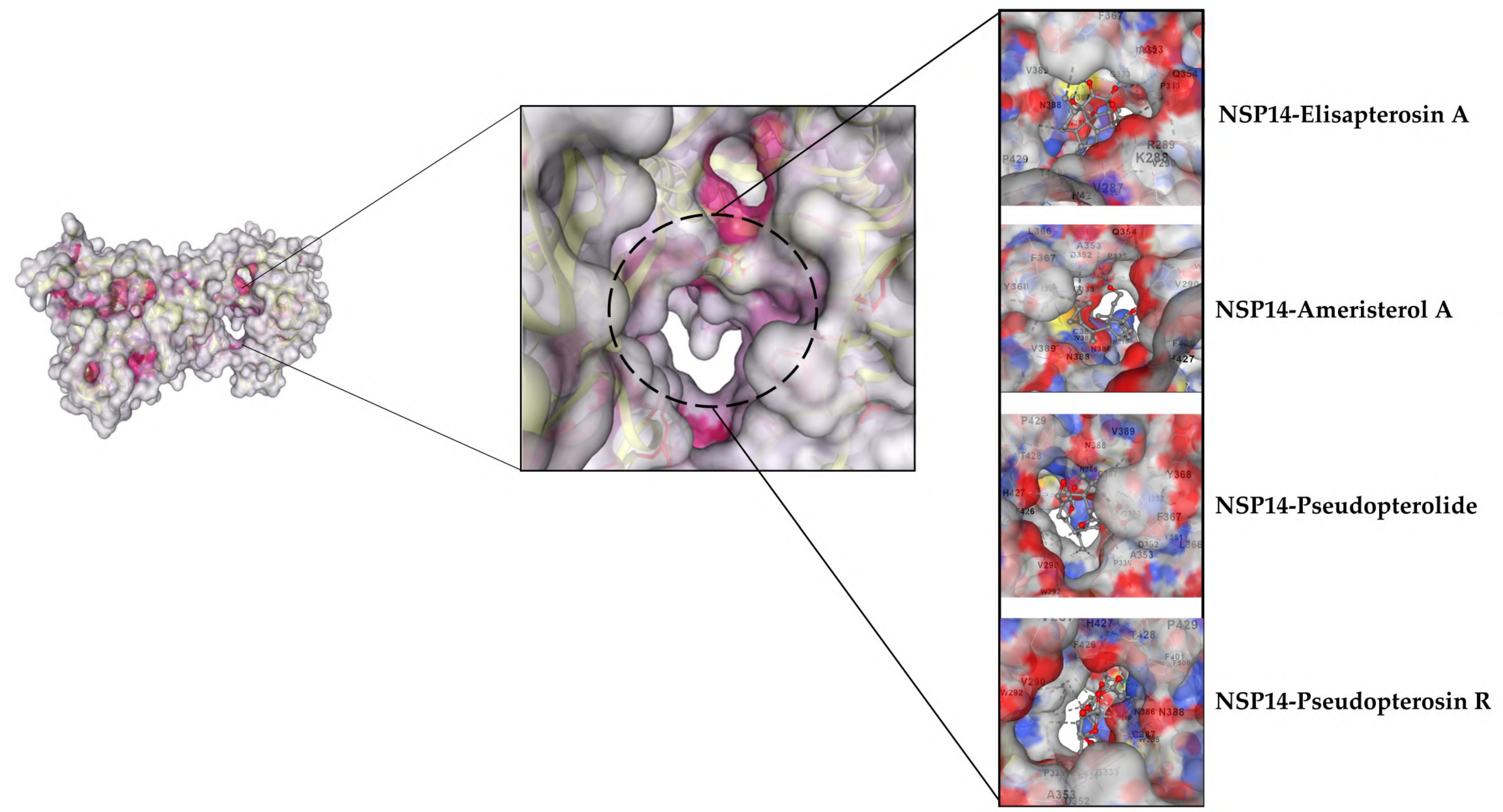
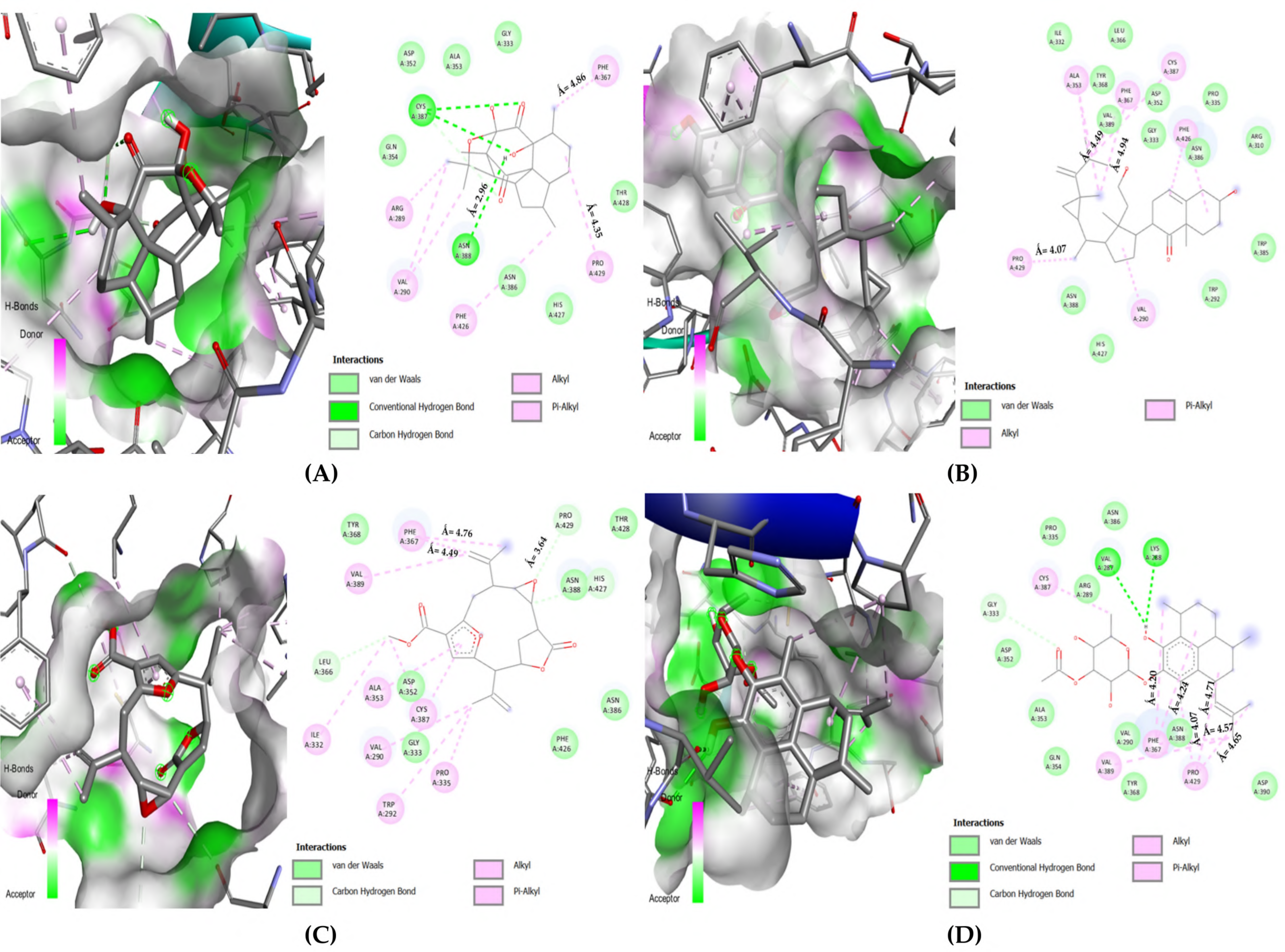
3.2.3. MDs Simulations
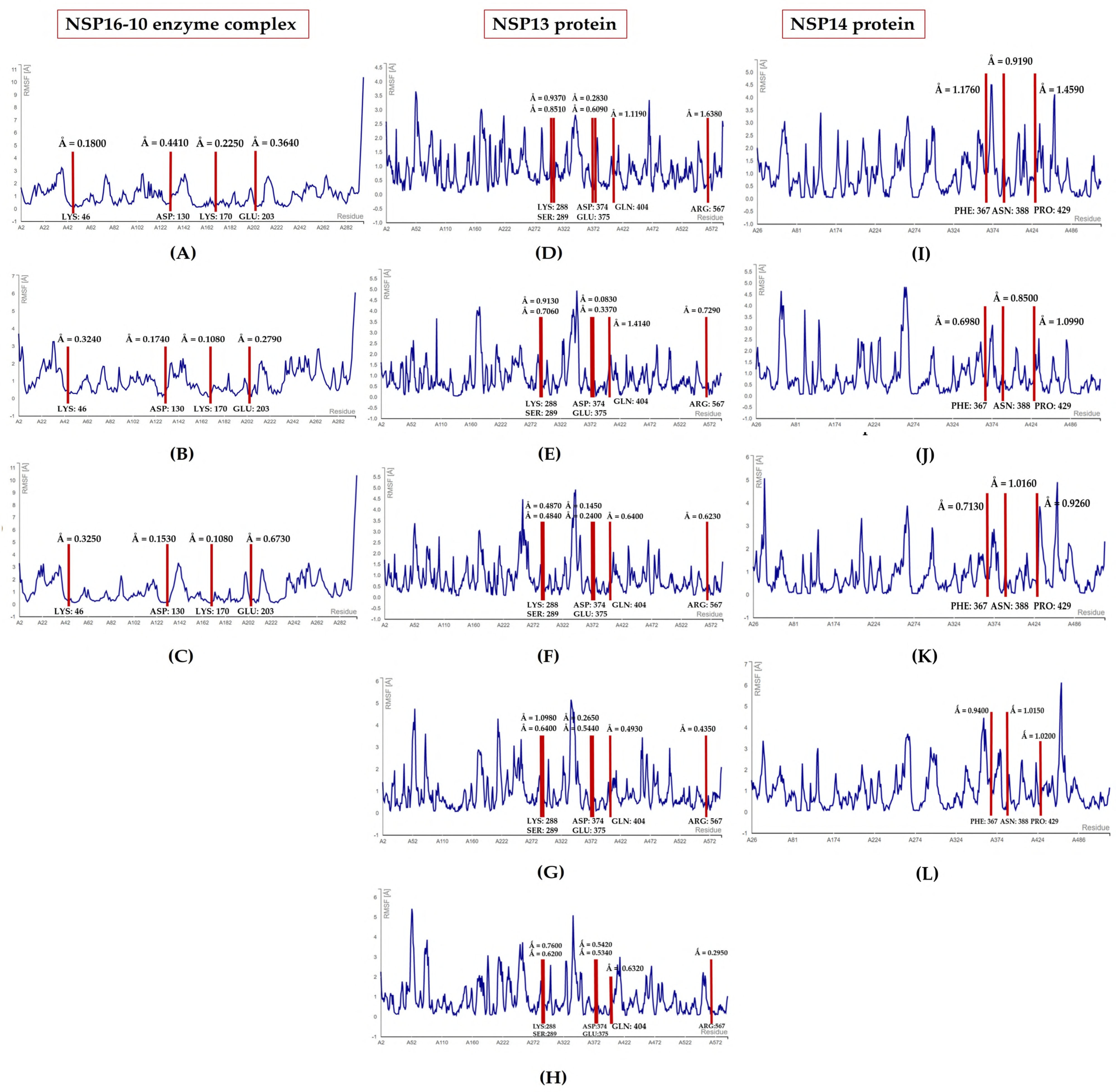
3.3. Toxicity Evaluation
| ProTox-II Toxicity Report | ||||||
|---|---|---|---|---|---|---|
| Top Ligands | Toxicity Values | Probability | ||||
| LD50 mg/kg | Toxicity Class | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | |
| Amphilectosin A | 3000 | 5 | Inactive | Inactive | Active | Inactive |
| Amphilectosin B | 3000 | 5 | Inactive | Inactive | Active | Inactive |
| Ameristerenol A | 2842 | 5 | Inactive | Inactive | Inactive | Inactive |
| Ameristerol A | 750 | 4 | Inactive | Inactive | Active | Inactive |
| Elisapterosin A | 50 | 2 | Inactive | Inactive | Active | Inactive |
| Elisabatin A | 220 | 3 | Inactive | Inactive | Active | Inactive |
| Pseudopterosin A | 3000 | 5 | Inactive | Inactive | Active | Inactive |
| Pseudopterosin R | 3000 | 5 | Inactive | Inactive | Active | Inactive |
| Pseudopterolide | 274 | 3 | Inactive | Inactive | Inactive | Active |
| Pseudopteroxazole | 1600 | 4 | Inactive | Inactive | Inactive | Inactive |
| Sandresolide B | 34 | 2 | Inactive | Active | Inactive | Inactive |
| seco-Pseudopterosin A | 3000 | 5 | Inactive | Inactive | Active | Inactive |
| StopTox Acute Toxicity Report | |||||
|---|---|---|---|---|---|
| Top Ligands | Endpoints | ||||
| Inhalation | Oral | Dermal | Irritation and Corrosion | Skin Sensitization | |
| Amphilectosin A | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-Sensitizer |
| Amphilectosin B | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-Sensitizer |
| Ameristerenol A | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-Sensitizer |
| Ameristerol A | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-Sensitizer |
| Elisapterosin A | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-Sensitizer |
| Elisabatin A | Toxic | Non-Toxic | Toxic | Eyes (−) Skin (−) | Sensitizer |
| Pseudopterosin A | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-Sensitizer |
| Pseudopterosin R | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-sensitizer |
| Pseudopterolide | Toxic | Toxic | Toxic | Eyes (+) Skin (−) | Non-Sensitizer |
| Pseudopteroxazole | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Sensitizer |
| Sandresolide B | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-Sensitizer |
| seco-Pseudopterosin A | Non-Toxic | Non-Toxic | Non-Toxic | Eyes (−) Skin (−) | Non-Sensitizer |
3.3.1. ProTox-II Results
3.3.2. StopTox Server Output
3.4. Drug-Likeness Evaluation
| Drug-Likeness Assessment | ||||||
|---|---|---|---|---|---|---|
| Top Ligands | Mol. Weight (MW ≤ 500) | Rotatable Bonds RB ≤ 10 | H Bond Acceptors HBA ≤ 10 | H Bond Donors HBD ≤ 5 | C Log p Log p ≤ 5 | TPSA (Å2) ≤ 140 |
| Amphilectosin A | 432.55 | 5 | 6 | 4 | 3.56 | 99.38 |
| Amphilectosin B | 432.55 | 5 | 6 | 4 | 3.56 | 99.38 |
| Ameristerenol A | 440.7 | 4 | 2 | 1 | 6.57 | 29.46 |
| Ameristerol A | 456.7 | 7 | 3 | 2 | 5.81 | 57.53 |
| Elisapterosin A | 348.43 | 0 | 5 | 2 | 2.08 | 83.83 |
| Elisabatin A | 310.39 | 1 | 3 | 1 | 3.71 | 54.37 |
| Pseudopterosin A | 432.55 | 3 | 6 | 4 | 3.34 | 99.38 |
| Pseudopterosin R | 488.61 | 5 | 7 | 3 | 4.06 | 105.45 |
| Pseudopterolide | 370.40 | 4 | 6 | 0 | 3.11 | 78.27 |
| Pseudopteroxazole | 309.45 | 1 | 2 | 0 | 5.31 | 26.03 |
| Sandresolide B | 320.42 | 1 | 4 | 2 | 2.91 | 66.76 |
| seco-Pseudopterosin A | 434.57 | 6 | 6 | 4 | 3.57 | 99.38 |
3.4.1. Lipinski Rule
3.4.2. Swiss-ADME
| Swiss-ADME Output | |||||
|---|---|---|---|---|---|
| Top Ligands | Water Solubility | Bioavailability | GI Absorption | Absorption (%) | BBB Permeant |
| Amphilectosin A | Soluble | 0.55 | High | 74.71 | No |
| Amphilectosin B | Soluble | 0.55 | High | 74.71 | No |
| Ameristerenol A | Poor | 0.55 | Low | 98.83 | No |
| Ameristerol A | Moderate | 0.55 | High | 89.15 | No |
| Elisapterosin A | Soluble | 0.55 | High | 80.07 | No |
| Elisabatin A | Moderate | 0.55 | High | 90.24 | Yes |
| Pseudopterosin A | Soluble | 0.55 | High | 74.71 | No |
| Pseudopterosin R | Moderate | 0.55 | High | 72.62 | No |
| Pseudopterolide | Soluble | 0.55 | High | 81.99 | Yes |
| Pseudopteroxazole | Moderate | 0.55 | High | 100.00 | No |
| Sandresolide B | Soluble | 0.55 | High | 85.96 | Yes |
| seco-Pseudopterosin A | Soluble | 0.55 | High | 74.71 | No |
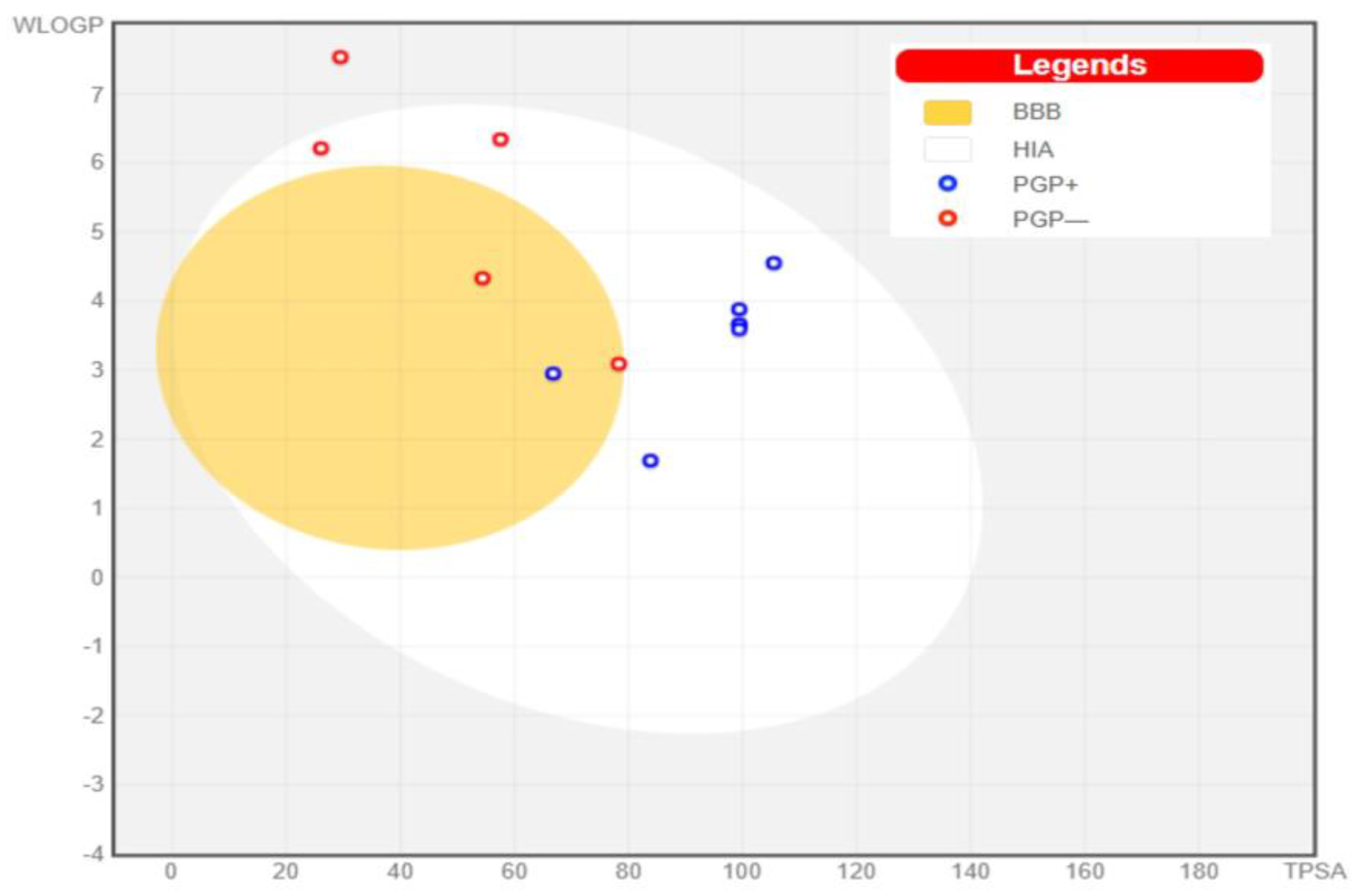
3.4.3. Bioactivity Prediction
| PASSonline Program | |
|---|---|
| Top Ligands | Predicted Relevant Bioactivity |
| Amphilectosin A | Antifungal, Antibiotic, Anti-diabetic, Anti-infective, Anti-viral, Antibacterial, Anti-parasitic, Anticancer, Antioxidant, Beta glucuronidase inhibitor, Histidine kinase inhibitor, 1,3-β-Glucan synthase inhibitor, Anti-inflammatory, Immunosuppressant, Immunomodulator, Interferon antagonist, Interferon gamma antagonist, Transcription factor NF kappa B stimulant, Wound healing agent, Free radical scavenger, Expectorant, and a Cytokine release inhibitor. |
| Amphilectosin B | Antifungal, Antibiotic, Anti-diabetic, Anti-infective, Anti-viral, Antibacterial, Anti-parasitic, Anticancer, Antioxidant, Beta glucuronidase inhibitor, Histidine kinase inhibitor, 1,3-β-Glucan synthase inhibitor, Anti-inflammatory, Immunosuppressant, Immunomodulator, Interferon antagonist, Interferon gamma antagonist, Transcription factor NF kappa B stimulant, Wound healing agent, Free radical scavenger, Expectorant, and a Cytokine release inhibitor. |
| Ameristerenol A | Antifungal, Anti-viral, Anti-inflammatory, Antineoplastic, 1,3-Beta-glucan synthase inhibitor, Transcription factor NF kappa B stimulant, Immunosuppressant, Respiratory analeptic, Antioxidant, Interferon antagonist, Interferon gamma antagonist, and an Interleukin 2 agonist. |
| Ameristerol A | Anti-viral, Antioxidant, Antifungal, Antibacterial, 1,3-Beta-glucan synthase inhibitor, Mucolytic, Respiratory analeptic, Transcription factor NF kappa B inhibitor, Expectorant, Anti-inflammatory steroid, Interferon gamma antagonist, Interleukin 2 agonist and an Immunosuppressant. |
| Elisapterosin A | Antiviral, Antifungal, Antioxidant, Histidine kinase inhibitor, Anti-parasitic, Antibacterial, JAK2 expression inhibitor, Interferon gamma antagonist, Immunosuppressant, Transcription factor NF kappa B stimulant, Tumour necrosis factor alpha release inhibitor, Cytokine release inhibitor, TNF expression inhibitor, Interleukin 2 agonist, Interleukin 1a and 10 antagonist, T cell inhibitor, RdRp Inhibitor, Respiratory analeptic, and an Anti-inflammatory steroid. |
| Elisabatin A | Antineoplastic, Anti-viral, Antifungal, histidine kinase inhibitor, β-Glucuronidase inhibitor, Anti-inflammatroy, Antibacterial, Anti-parasitic, Antimutagenic, hepatoprotectant, Immunosuppressant, JAK2 expression inhibitor, MMP9 expression inhibitor, TNF expression inhibitor, Cytokine release inhibitor, T cell inhibitor, Interleukin 10 antagonist, Interferon antagonist. |
| Pseudopterosin A | Anti-viral, Anti-infective, Antibiotic, Antineoplastic, Antioxidant, Anticancer, Free radical scavenger, Antibacterial, Antifungal, 1,3-β-Glucan synthase inhibitor, Beta glucuronidase inhibitor, Wound healing agent, Immunosuppressant, Anti-inflammatory, Transcription factor NF kappa B stimulant, Interleukin 2 agonist, Cytokine release inhibitor, Interferon antagonist, Interferon gamma antagonist, T cell inhibitor, Expectorant |
| Pseudopterosin R | Anti-bacterial, Anti-parasitic, Anti-infective, Anti-viral, Antifungal, Antifungal enhancer, β –Glucuronidase inhibitor, 1,3-β-Glucan synthase inhibitor, Antineoplastic, Anti-inflammatory, Immunosuppressant, Interleukin 6, 10 antagonist, Interferon antagonist, Interferon gamma antagonist, Cytokine release inhibitor, T cell inhibitor, GRP78 expression inhibitor, Expectorant |
| Pseudopterolide | Anti-inflammatory, Antioxidant, Antineoplastic, Antifungal, Antifungal enhancer, β-Glucuronidase inhibitor, Transcription factor NF kappa B stimulant, Antibacterial, Anti-parasitic, Histidine kinase inhibitor, Immunosuppressant, Antibiotic, Expectorant, Interleukin 10 antagonist, Severe acute respiratory syndrome treatment, Antineoplastic alkaloid, Interferon gamma antagonist, Cytokine release inhibitor, and a Viral entry inhibitor. |
| Pseudopteroxazole | Antibacterial, Antibiotic, Anti-parasitic, Antineoplastic alkaloid, Anti-inflammatory, Antioxidant, transcription factor NF kappa B stimulant, and an Immunosuppressant. |
| Sandresolide B | Antifungal, Antifungal enhancer, Mucolytic, Antibacterial, Antibiotic, Antineoplastic alkaloid, Anti-parasitic, Anti-infective, Immunosuppressant, Transcription factor NF kappa B stimulant, Interferon antagonist, Cytokine release inhibitor, Interferon gamma antagonist, T cell inhibitor, JAK2 expression inhibitor, TNF expression inhibitor, Macrophage colony stimulating factor agonist, Anti-inflammatory, Respiratory analeptic, Histidine kinase inhibitor, Beta glucuronidase inhibitor, Leukotriene agonist, and an Interleukin 2, 6, 10 antagonist. |
| Seco-Pseudopterosin A | Anti-viral, Anti-infective, Anti-tuberculosic, Anticancer, Antioxidant, Free radical scavenger, Antibiotic, Anti-diabetic, Antineoplastic, Antibacterial, Anti-parasitic, Antifungal, Mucolytic, Histidine kinase inhibitor, Immunosuppressant, Anti-inflammatory, Wound healing agent, Transcription factor NF kappa B stimulant, β –Glucuronidase inhibitor, 1,3-β-Glucan synthase inhibitor, Expectorant, Respiratory analeptic, Interleukin 2 and 12 agonist, and a Cytokine release inhibitor. |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, S. Timely development of vaccines against SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, G.; Chávez-Canales, M.; Espinosa, A.M.; Jordán-Ríos, A.; Malagon, D.A.; Murillo, M.F.M.; Araujo, L.V.T.; Campos, R.L.B.; Wong-Chew, R.M.; González, L.E.R.; et al. Intranasal dexamethasone: A new clinical trial for the control of inflammation and neuroinflammation in COVID-19 patients. Trials 2022, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Bouadma, L.; Mekontso-Dessap, A.; Burdet, C.; Merdji, H.; Poissy, J.; Dupuis, C.; Guitton, C.; Schwebel, C.; Cohen, Y.; Bruel, C.; et al. High-Dose Dexamethasone and Oxygen Support Strategies in Intensive Care Unit Patients With Severe COVID-19 Acute Hypoxemic Respiratory Failure: The COVIDICUS Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Maláska, J.; Stašek, J.; Duška, F.; Balík, M.; Máca, J.; Hruda, J.; Vymazal, T.; Klementová, O.; Zatloukal, J.; Gabrhelík, T.; et al. Effect of dexamethasone in patients with ARDS and COVID-19—Prospective, multi-centre, open-label, parallel-group, randomised controlled trial (REMED trial): A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 172. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Dyer, O. COVID-19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ 2020, 371, m4057. [Google Scholar] [CrossRef]
- Rogstam, A.; Nyblom, M.; Christensen, S.; Sele, C.; Talibov, V.O.; Lindvall, T.; Rasmussen, A.A.; André, I.; Fisher, Z.; Knecht, W.; et al. Crystal Structure of Nonstructural Protein 10 from Severe Acute Respiratory Syndrome Coronavirus-2. Int. J. Mol. Sci. 2020, 21, 7375. [Google Scholar] [CrossRef]
- Donaldson, E.F.; Sims, A.C.; Graham, R.L.; Denison, M.R.; Baric, R.S. Murine hepatitis virus replicase protein nsp10 is a critical regulator of viral RNA synthesis. J. Virol. 2007, 81, 6356–6368. [Google Scholar] [CrossRef] [PubMed]
- Decroly, E.; Ferron, F.; Lescar, J.; Canard, B. Conventional and unconventional mechanisms for capping viral mRNA. Nat. Rev. Microbiol. 2011, 10, 51–65. [Google Scholar] [CrossRef]
- Viswanathan, T.; Arya, S.; Chan, S.H.; Qi, S.; Dai, N.; Misra, A.; Park, J.G.; Oladunni, F.; Kovalskyy, D.; Hromas, R.A.; et al. Structural basis of RNA cap modification by SARS-CoV-2. Nat. Commun. 2020, 11, 3718. [Google Scholar] [CrossRef]
- Chang, L.J.; Chen, T.H. NSP16 2′-O-MTase in Coronavirus Pathogenesis: Possible Prevention and Treatments Strategies. Viruses 2021, 13, 538. [Google Scholar] [CrossRef]
- Chen, J.; Malone, B.; Llewellyn, E.; Grasso, M.; Shelton, P.M.M.; Olinares, P.D.B.; Maruthi, K.; Eng, E.T.; Vatandaslar, H.; Chait, B.T.; et al. Structural Basis for Helicase-Polymerase Coupling in the SARS-CoV-2 Replication-Transcription Complex. Cell 2020, 182, 1560–1573.e13. [Google Scholar] [CrossRef] [PubMed]
- Prajapat, M.; Sarma, P.; Shekhar, N.; Avti, P.; Sinha, S.; Kaur, H.; Kumar, S.; Bhattacharyya, A.; Kumar, H.; Bansal, S.; et al. Drug targets for corona virus: A systematic review. Indian J. Pharmacol. 2020, 52, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Rohaim, M.A.; El Naggar, R.F.; Clayton, E.; Munir, M. Structural and functional insights into nonstructural proteins of coronaviruses. Microb. Pathog. 2021, 150, 104641. [Google Scholar] [CrossRef]
- Spratt, A.N.; Gallazzi, F.; Quinn, T.P.; Lorson, C.L.; Sönnerborg, A.; Singh, K. Coronavirus helicases: Attractive and unique targets of anti-viral drug-development and therapeutic patents. Expert Opin. Ther. Pat. 2021, 31, 339–350. [Google Scholar] [CrossRef]
- Tanner, J.A.; Watt, R.M.; Chai, Y.B.; Lu, L.Y.; Lin, M.C.; Peiris, J.S.; Poon, L.L.; Kung, H.F.; Huang, J.D. The severe acute respiratory syndrome (SARS) coronavirus NTPase/helicase belongs to a distinct class of 5′ to 3′ viral helicases. J. Biol. Chem. 2003, 278, 39578–39582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, H.; Pan, J.; Xiang, N.; Tien, P.; Ahola, T.; Guo, D. Functional screen reveals SARS coronavirus nonstructural protein nsp14 as a novel cap N7 methyltransferase. Proc. Natl. Acad. Sci. USA 2009, 106, 3484–3489. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.; Selisko, B.; Le, N.T.; Huchting, J.; Touret, F.; Piorkowski, G.; Fattorini, V.; Ferron, F.; Decroly, E.; Meier, C.; et al. Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat. Commun. 2020, 11, 4682. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, L.; Shaw, N.; Gao, Y.; Wang, J.; Sun, Y.; Lou, Z.; Yan, L.; Zhang, R.; Rao, Z. Structural basis and functional analysis of the SARS coronavirus nsp14-nsp10 complex. Proc. Natl. Acad. Sci. USA 2015, 112, 9436–9441. [Google Scholar] [CrossRef] [PubMed]
- Becares, M.; Pascual-Iglesias, A.; Nogales, A.; Sola, I.; Enjuanes, L.; Zuñiga, S. Mutagenesis of Coronavirus nsp14 Reveals Its Potential Role in Modulation of the Innate Immune Response. J. Virol. 2016, 90, 5399–5414. [Google Scholar] [CrossRef]
- Hastie, K.M.; Kimberlin, C.R.; Zandonatti, M.A.; MacRae, I.J.; Saphire, E.O. Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3′ to 5′ exonuclease activity essential for immune suppression. Proc. Natl. Acad. Sci. USA 2011, 108, 2396–2401. [Google Scholar] [CrossRef]
- Hsu, J.C.; Laurent-Rolle, M.; Pawlak, J.B.; Wilen, C.B.; Cresswell, P. Translational shutdown and evasion of the innate immune response by SARS-CoV-2 NSP14 protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2101161118. [Google Scholar] [CrossRef]
- Santos, J.; Brierley, S.; Gandhi, M.J.; Cohen, M.A.; Moschella, P.C.; Declan, A.B.L. Repurposing Therapeutics for Potential Treatment of SARS-CoV-2: A Review. Viruses 2020, 12, 705. [Google Scholar] [CrossRef] [PubMed]
- Dotolo, S.; Marabotti, A.; Facchiano, A.; Tagliaferri, R. A review on drug repurposing applicable to COVID-19. Brief. Bioinform. 2021, 22, 726–741. [Google Scholar] [CrossRef]
- Mohamed, K.; Yazdanpanah, N.; Saghazadeh, A.; Rezaei, N. Computational drug discovery and repurposing for the treatment of COVID-19: A systematic review. Bioorg. Chem. 2021, 106, 104490. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, A.D.; Fatykhov, R.F.; Khalymbadzha, I.A.; Zyryanov, G.V.; Chupakhin, O.N.; Tsurkan, M.V. Plant Coumarins with Anti-HIV Activity: Isolation and Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 2839. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Albohy, A.; Abdulrazik, B.S.; Bayoumi, S.A.L.; Malak, L.G.; Khallaf, I.S.A.; Bringmann, G.; Farag, S.F. Natural coumarins as potential anti-SARS-CoV-2 agents supported by docking analysis. RSC Adv. 2021, 11, 16970–16979. [Google Scholar] [CrossRef]
- Pokharkar, O.; Lakshmanan, H.; Zyryanov, G.; Tsurkan, M. In Silico Evaluation of Antifungal Compounds from Marine Sponges against COVID-19-Associated Mucormycosis. Mar. Drugs 2022, 20, 215. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products: Metabolites of marine invertebrates. Nat. Prod. Rep. 1984, 1, 551–598. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1992, 9, 323–364. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1993, 10, 497–539. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1R–49R. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2003, 20, 1–48. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2004, 21, 1–49. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31–86. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2008, 25, 35–94. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.B.; Pérez, A.R.; Castro, H.V.; Argilagos, C.S.; Henriquez, R.D. Caridiene: A new sesquiterpene from Pseudoterogorgia Americana. Z. Naturforschung B 1990, 45, 1571–1572. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Rivera, J.; Boulanger, A. New polyhydroxydinostane sterols from the Caribbean gorgonian octocoral Pseudopterogorgia Americana. Tetrahedron Lett. 1998, 39, 7645–7648. [Google Scholar] [CrossRef]
- Naz, S.; Kerr, R.G.; Narayanan, R. New antiproliferative epoxysecosterols from Pseudopterogorgia americana. Tetrahedron Lett. 2000, 41, 6035–6040. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Boulanger, A. New guaiane metabolites from the Caribbean gorgonian coral, Pseudopterogorgia americana. J. Nat. Prod. 1997, 60, 207–211. [Google Scholar] [CrossRef]
- Chan, W.R.; Tinto, W.F.; Moore, R. New germacrane derivatives from gorgonian octocorals of the genus Pseudopterogorgia. Tetrahedron 1990, 46, 1499–1502. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Boulanger, A.; Martínez, J.R.; Huang, S.D. Sesquiterpene lactones from the Caribbean Sea plume Pseudopterogorgia Americana. J. Nat. Prod. 1998, 61, 451–455. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Boulanger, A. Americanolides A–C, new guaianolide sesquiterpenes from the Caribbean Sea plume Pseudopterogorgia americana. J. Nat. Prod. 1996, 59, 653–657. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Ramírez, C.; Rodríguez, I.I. Elisabatins A and B: New amphilectane-type diterpenes from the West Indian sea whip Pseudopterogorgia elisabethae. J. Nat. Prod. 1999, 62, 997–999. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Ramirez, C.; Medina, V.; Shi, Y.P. Novel lactones from Pseudopterogorgia elisabethae (Bayer). Tetrahedron Lett. 2000, 41, 5177–5180. [Google Scholar] [CrossRef]
- Rodriguez, A.D.; Ramirez, C.; Rodriguez, I.I.; Barnes, C.L. Novel terpenoids from the west Indian Sea Whip Pseudopterogorgia elisabethae (Bayer). Elisapterosins A and B: Rearranged diterpenes possessing an unprecedented cage like framework. J. Org. Chem. 2000, 65, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; González, E.; Huang, S.D. Unusual Terpenes with Novel Carbon Skeletons from the West Indian Sea Whip Pseudopterogorgia elisabethae (Octocorallia). J. Org. Chem. 1998, 63, 7083–7091. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; Ramírez, C. Serrulatane Diterpenes with Antimycobacterial Activity Isolated from the West Indian Sea Whip Pseudopterogorgia elisabethae. J. Nat. Prod. 2001, 64, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.I.; Rodríguez, A.D. Homopseudopteroxazole, a New Antimycobacterial Diterpene Alkaloid from Pseudopterogorgia elisabethae. J. Nat. Prod. 2003, 66, 855–857. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Ramírez, C.; Rodríguez, I.I.; González, E. Novel Antimycobacterial Benzoxazole Alkaloids, from the West Indian Sea Whip Pseudopterogorgia elisabethae. Org. Lett. 1999, 1, 527–530. [Google Scholar] [CrossRef]
- Coleman, A.C.; Kerr, R.G. Radioactivity-guided isolation and characterization of the bicyclic pseudopterosin diterpene cyclase product from Pseudopterogorgia elisabethae. Tetrahedron 2000, 56, 9569–9574. [Google Scholar] [CrossRef]
- Ata, A.; Kerr, R.G. Elisabethamine: A new diterpene alkaloid from Pseudopterogorgia elisabethae. Tetrahedron Lett. 2000, 41, 5821–5825. [Google Scholar] [CrossRef]
- Ata, A.; Kerr, R.G. 12-acetoxypseudopterolide: A new diterpene from Pseudopterogorgia elisabethae. Heterocycles 2000, 53, 717. [Google Scholar] [CrossRef]
- Shi, Y.P.; Rodríguez, I.I.; Rodríguez, A.D. Elisapterosins D and E: Complex polycyclic diterpenes of the rare elisapterane class of natural products from the Caribbean Sea whip Pseudopterogorgia elisabethae (Bayer). Tetrahedron Lett. 2003, 44, 3249–3253. [Google Scholar] [CrossRef]
- Ata, A.; Win, H.Y.; Holt, D.; Holloway, P.; Segstro, E.P.; Jayatilake, G.S. New antibacterial diterpenes from Pseudopterogorgia elisabethae. Helv. Chim. Acta 2004, 87, 1090–1098. [Google Scholar] [CrossRef]
- Rodríguez, I.I.; Shi, Y.P.; García, O.J.; Rodríguez, A.D.; Mayer, A.M.; Sánchez, J.A.; Ortega-Barria, E.; González, J. New pseudopterosin and seco-pseudopterosin diterpene glycosides from two Colombian isolates of Pseudopterogorgia elisabethae and their diverse biological activities. J. Nat. Prod. 2004, 67, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Correa, H.; Aristizabal, F.; Duque, C.; Kerr, R. Cytotoxic and Antimicrobial Activity of Pseudopterosins and seco-Pseudopterosins Isolated from the Octocoral Pseudopterogorgia elisabethae of San Andrés and Providencia Islands (Southwest Caribbean Sea). Mar. Drugs 2011, 9, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Duque, C.; Puyana, M.; Narváez, G.; Osorno, O.; Hara, N.; Fujimoto, Y. Pseudopterosins P–V, new compounds from the gorgonian octocoral Pseudopterogorgia elisabethae from Providencia island, Colombian Caribbean. Tetrahedron 2004, 60, 10627–10635. [Google Scholar] [CrossRef]
- Look, S.A.; Fenical, W.; Matsumoto, G.K.; Clardy, J. The pseudopterosins: A new class of anti-inflammatory and analgesic diterpene pentosides from the marine sea whip Pseudopterogorgia elisabethae (Octocorallia). J. Org. Chem. 1986, 51, 5140–5145. [Google Scholar] [CrossRef]
- Roussis, V.; Wu, Z.; Fenical, W.; Strobel, S.A.; Van Duyne, G.D.; Clardy, J. New anti-inflammatory pseudopterosins from the marine octocoral Pseudopterogorgia elisabethae. J. Org. Chem. 1990, 55, 4916–4922. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Ramírez, C.; Rodríguez, I.I. Sandresolides A and B: Novel nor-diterpenes from the sea whip Pseudopterogorgia elisabethae (Bayer). Tetrahedron Lett. 1999, 40, 7627–7631. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Shi, Y.P. Structurally diverse terpenoids from the sea whip Pseudopterogorgia elisabethae (Bayer). Tetrahedron 2000, 56, 9015–9023. [Google Scholar] [CrossRef]
- Duque, C.; Puyana, M.; Castellanos, L.; Arias, A.; Correa, H.; Osorno, O.; Asai, T.; Hara, N.; Fujimoto, Y. Further studies on the constituents of the gorgonian octocoral Pseudopterogorgia elisabethae collected in San Andrés and Providencia islands, Colombian Caribbean: Isolation of a putative biosynthetic intermediate leading to erogorgiaene. Tetrahedron 2006, 62, 4205–4213. [Google Scholar] [CrossRef]
- Shi, Y.P.; Wei, X.; Rodríguez, I.I.; Rodríguez, A.D.; Mayer, A.M. New terpenoid constituents of the southwestern Caribbean sea whip Pseudopterogorgia elisabethae (Bayer), including a unique pentanorditerpene. Eur. J. Org. Chem. 2009, 2009, 493–502. [Google Scholar] [CrossRef]
- Ata, A.; Kerr, R.G.; Moya, C.E.; Jacobs, R.S. Identification of anti-inflammatory diterpenes from the marine gorgonian Pseudopterogorgia elisabethae. Tetrahedron 2003, 59, 4215–4222. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Ramírez, C.; Shi, Y.P. The Cumbiasins, Structurally Novel Diterpenes Possessing Intricate Carbocyclic Skeletons from the West Indian Sea Whip Pseudopterogorgia elisabethae (Bayer). J. Org. Chem. 2000, 65, 6682–6687. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Ramírez, C. A Marine Diterpene with a Novel Tetracyclic Framework from the West Indian Gorgonian Octocoral Pseudopterogorgia elisabethae. Org. Lett. 2000, 2, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Home—Protein—NCBI. Available online: https://www.ncbi.nlm.nih.gov/protein/ (accessed on 5 December 2022).
- Rosas-Lemus, M.; Minasov, G.; Shuvalova, L.; Inniss, N.L.; Kiryukhina, O.; Brunzelle, J.; Satchell, K.J.F. High-resolution structures of the SARS-CoV-2 2′-O-methyltransferase reveal strategies for structure-based inhibitor design. Sci. Signal. 2020, 13, eabe1202. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.A.; Douangamath, A.; Yadzani, S.; Yosaatmadja, Y.; Aimon, A.; Brandão-Neto, J.; Dunnett, L.; Gorrie-Stone, T.; Skyner, R.; Fearon, D.; et al. Structure, mechanism and crystallographic fragment screening of the SARS-CoV-2 NSP13 helicase. Nat. Commun. 2021, 12, 4848. [Google Scholar] [CrossRef] [PubMed]
- Czarna, A.; Plewka, J.; Kresik, L.; Matsuda, A.; Karim, A.; Robinson, C.; O’Byrne, S.; Cunningham, F.; Georgiou, I.; Wilk, P.; et al. Refolding of lid subdomain of SARS-CoV-2 nsp14 upon nsp10 interaction releases exonuclease activity. Structure 2022, 30, 1050–1054.e2. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202. [Google Scholar] [CrossRef]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Lyu, C.; Chen, T.; Qiang, B.; Liu, N.; Wang, H.; Zhang, L.; Liu, Z. CMNPD: A comprehensive marine natural products database towards facilitating drug discovery from the ocean. Nucleic Acids Res. 2021, 49, D509–D515. [Google Scholar] [CrossRef]
- BIOVIA DS, Discovery, Studio. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 15 December 2022).
- Avogadro: An Open-Source Molecular Builder and Visualization Tool. Version 1.95. Available online: http://avogadro.cc/ (accessed on 18 October 2022).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Kuriata, A.; Gierut, A.M.; Oleniecki, T.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. CABS-flex 2.0: A web server for fast simulations of flexibility of protein structures. Nucleic Acids Res. 2018, 46, W338–W343. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257. [Google Scholar] [CrossRef]
- Borba, J.; Alves, V.; Overdahl, K.; Silva, A.; Hall, S.; Overdahl, E.; Braga, R.; Kleinstreuer, N.; Strickland, J.; Allen, D.; et al. STopTox: An In-Silico Alternative to Animal Testing for Acute Systemic and TOPical TOXicity. Environ. Health Perspect. 2022, 130, 27012. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 71, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Le, J.; Hersey, A.; Luscombe, C.N.; Beck, G.; Sherborne, B.; Cooper, I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Ahmad, A.; Khan, S.A.; Asif, M.; Bhutani, R.; Al-Abbasi, F.A. Synthesis, molecular properties, toxicity and biological evaluation of some new substituted imidazolidine derivatives in search of potent anti-inflammatory agents. Saudi Pharm. J. 2016, 24, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Cron, R.Q.; Caricchio, R.; Chatham, W.W. Calming the cytokine storm in COVID-19. Nat. Med. 2021, 27, 1674–1675. [Google Scholar] [CrossRef]
- Worldometer Coronavirus Statistics. Available online: https://www.worldometers.info/coronavirus/ (accessed on 25 March 2023).
- Yan, W.; Zheng, Y.; Zeng, X.; He, B.; Cheng, W. Structural biology of SARS-CoV-2: Open the door for novel therapies. Signal Transduct. Target. Ther. 2022, 7, 26. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sharma, S.; Singh, S.K. Molecular Docking Studies to Identify Promising Natural Inhibitors Targeting SARS-CoV-2 Nsp10-Nsp16 Protein Complex. Turk. J. Pharm. Sci. 2022, 19, 93–100. [Google Scholar] [CrossRef]
- Piplani, S.; Singh, P.; Winkler, D.A.; Petrovsky, N. Potential COVID-19 Therapies from Computational Repurposing of Drugs and Natural Products against the SARS-CoV-2 Helicase. Int. J. Mol. Sci. 2022, 23, 7704. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Belkacem, R.; Hausdorff, M.; Delpal, A.; Sutto-Ortiz, P.; Colmant, A.M.G.; Touret, F.; Ogando, N.S.; Snijder, E.J.; Canard, B.; Coutard, B.; et al. Potent Inhibition of SARS-CoV-2 nsp14 N7-Methyltransferase by Sulfonamide-Based Bisubstrate Analogues. J. Med. Chem. 2022, 65, 6231–6249. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokharkar, O.; Lakshmanan, H.; Zyryanov, G.V.; Tsurkan, M.V. Antiviral Potential of Antillogorgia americana and elisabethae Natural Products against nsp16–nsp10 Complex, nsp13, and nsp14 Proteins of SARS-CoV-2: An In Silico Investigation. Microbiol. Res. 2023, 14, 993-1019. https://doi.org/10.3390/microbiolres14030068
Pokharkar O, Lakshmanan H, Zyryanov GV, Tsurkan MV. Antiviral Potential of Antillogorgia americana and elisabethae Natural Products against nsp16–nsp10 Complex, nsp13, and nsp14 Proteins of SARS-CoV-2: An In Silico Investigation. Microbiology Research. 2023; 14(3):993-1019. https://doi.org/10.3390/microbiolres14030068
Chicago/Turabian StylePokharkar, Omkar, Hariharan Lakshmanan, Grigory V. Zyryanov, and Mikhail V. Tsurkan. 2023. "Antiviral Potential of Antillogorgia americana and elisabethae Natural Products against nsp16–nsp10 Complex, nsp13, and nsp14 Proteins of SARS-CoV-2: An In Silico Investigation" Microbiology Research 14, no. 3: 993-1019. https://doi.org/10.3390/microbiolres14030068
APA StylePokharkar, O., Lakshmanan, H., Zyryanov, G. V., & Tsurkan, M. V. (2023). Antiviral Potential of Antillogorgia americana and elisabethae Natural Products against nsp16–nsp10 Complex, nsp13, and nsp14 Proteins of SARS-CoV-2: An In Silico Investigation. Microbiology Research, 14(3), 993-1019. https://doi.org/10.3390/microbiolres14030068








