Abstract
The effect of climate change on flora and fauna has been widely discussed for years. However, its consequences on microorganisms are generally poorly considered. The main effect of climate change on microbiota is related to biodiversity changes in different regions of the planet, mainly due to variations in temperature. These alterations are resulting in a worldwide (re)distribution of pathogens, which was not considered a few years ago. They mainly affect different food chain sectors (such as agriculture, livestock and fishing), as well as human health. Hence, the spread of numerous animal and plant pathogens has been observed in recent years from south to north (especially in America, Europe and Asia), leading to the spread of numerous plant and animal diseases, which results in economic and ecological losses. In addition, global warming that accompanies climate change could also be related to emerging antibiotic resistance. However, the mitigation of climate change goes hand in hand with microorganisms, which can help us through different natural and industrial processes. Thus, this manuscript presents the direct and indirect effects of climate change on microorganisms described up to date and how they act on this worldwide phenomenon.
1. Introduction
Climate change is probably the main concern of the 21st century, which not only affects the weather people experience but also the air they breathe, the water they drink, the food they eat and even where they are able to live. In the last decades, extreme weather events have become frequent, and records are continuously being broken. Thus, the last few years have been the hottest on average as long as records have existed, and more than 400 weather stations all around the World have beaten their heat records in 2021 (reaching up to 48.8 °C in Italy on 20 July, 49.6 °C in Canada on 29 June or even 54.4 °C in the US on 9 July) [1]. The global annual temperature has increased by 0.08 °C on average per decade between 1880 and 1981, culminating in 2021 with a temperature of 0.84 °C above the average of the 20th century, according to the “2021 Annual Climate Report” of the NOAA (National Oceanic and Atmospheric Administration of the US Department of Commerce; https://www.ncdc.noaa.gov/sotc/briefings/20220113.pdf, accessed on 19 July 2023). In addition, the mean annual precipitation is expected to be lower in southern Europe and higher in northern Europe over the next few years, leading to warmer and drier climates, particularly in southern and central Europe during spring and summer. In short, an increase in the risk of extreme weather events, such as heatwaves, extreme rainfall and extended drought periods, in the years to come due to climate change is strongly suggested [2].
Climate change seems to be the result of both natural (Earth’s magnetic field changes) and anthropogenic (e.g., methane and carbon emissions) factors [3,4]. It was first reported in 1824 by the French physicist Joseph Fourier [5], but it was not until 1975 that Wallace Broecker introduced the term “global warming” into the public domain [6]. Sixteen years later, the Intergovernmental Panel on Climate Change (IPCC) was formed to collate and assess evidence. Thus, climate change can be defined as the long-term alteration of temperature and typical weather patterns (including, but not only, temperature, rainfall and wind). These changes can cause an unprecedented loss of biodiversity and endangered life on Earth. However, although the loss of plant and animal species has been reasonably well studied and documented, the effects of climate change on microorganisms are generally overlooked [2].
Microorganisms date back to Earth’s origin, at least 3.5 billion years ago [7], and they will undoubtedly exist well beyond any future extinction events. Their abundance and diversity (~1030 bacteria and archaea cells and ~1012 microbial species) are the life support of the biosphere and the basis for maintaining healthy ecosystems [2]. They play a critical role in nutritional cycles, such as carbon and nitrogen, as well as in animal (including humans) and plant health, agricultural yields and food security. Thus, as climate change can intensify seasonal disturbances and lead to an increase in extreme events [8], it is reasonable to consider a possible effect on microbial biodiversity and its subsequent consequences [2,9,10]. Nowadays, the most studied effect of climate change on microorganisms refers to the increase in animal and plant pathogens due to the geographical expansion of numerous marine and terrestrial vectors, mainly because of global warming [2], as detailed throughout this work. However, the relationship between microorganisms and climate change is a two-way road, since microorganisms can directly affect climate change due to their involvement in greenhouse gas (GHG) synthesis and consumption, but they are part of the solution by acting as mitigation agents, and also their biodiversity is being affected by environmental modifications.
2. Impact of Climate Change on Microbial Diversity
Ecology aims to understand the generation and maintenance of biodiversity over time and space, where climate strongly conditions the structure and interactions inside communities, as well as their functionalities [11]. Thus, the impact of climate change on the ecosystems’ inhabitants, including microorganisms, is expected to be significant and complex, although its quantification seems challenging [12]. Among all the environmental factors affected by climate change (e.g., alterations in precipitation patterns, drought stress and changes in radiation), global warming has a pervasive influence on the variation in soil microbial diversity, playing a primary role in shaping microbial diversity compared to other proposed environmental drivers by increasing the temperature in the surface soil and decreasing its moisture [13,14].
It is important to note from the outset that the effects of climate change on microbial communities in both terrestrial and aquatic environments are complex and, at times, even contradictory, with both deterministic and stochastic influences. On one hand, it has been widely reported that changes in temperature have an impact on microbial biodiversity at various levels (e.g., geographical range, phenology, distribution or abundance) [13]. Microbial metabolism, population growth rate and species number increase exponentially with increasing temperature. In fact, bacterial and fungal diversity tends to decrease with increasing global latitude as temperatures decline [14]. However, variations in temperature can affect microbial biodiversity through several mechanisms. First, the most commonly reported mechanism is that increasing temperature leads to higher rates of metabolism, resulting in increased population doubling times [14] as well as the rates of ecological and evolutionary processes, including mutation, speciation and interactions (e.g., parasitism, predation, competition or mutualism among others) [12,14,15]. Therefore, if temperature drives the increase in microbial diversity, it can be expected that warming ecosystems (from tropical to alpine forests) will become more diverse and active, potentially enhancing processes such as decomposition, nutrient cycling and carbon sequestration [14]. However, higher temperatures can also act as a deterministic filter, selecting more adapted microorganisms and constraining the stochastic drift and dispersal of species, ultimately leading to a decrease in the temporal scaling rates of soil microbial communities. In addition, microbial community responses to climate change greatly depend on microbial lineages and functional groups [12,15].
On the other hand, higher temperatures lead to an increase in the number of plant species, and this higher plant diversity allocates nutrients to belowground ecosystems, as there is a well-known correlation between microbial taxa richness and plant species diversity [14]. This shift in nutrient allocation could lead to changes in plant–microbe interactions, particularly affecting populations of plant growth-promoting rhizobacteria (PGPR) that rely on rhizodeposition [16,17]. In addition, the alterations in precipitation patterns and drought stress associated with climate change negatively impact the formation of a mycorrhizal mycelium in plant roots [16,18]. These changes in climatic conditions contribute to an increased incidence of environmental stresses, which can also enhance the activities of pathogens and heterotrophic microorganisms, leading to the redistribution of beneficial microbes across different ecological niches [16,17].
Despite the reported potential impact of climate change on microbial composition and interactions, most of the conducted studies have focused on specific regions and conditions (especially on terrestrial ecosystems). Therefore, given the complex interplay between microorganisms and their biotic and abiotic surroundings, information obtained from a single point in time provides only a snapshot of the microbial community, and it is unsuitable for ecosystem model simulation and interpretation. Further research is needed to fully comprehend this dynamic relationship.
3. Microorganism Involvement in Production/Consumption of Greenhouse Gases
The appearance of oxygenic photosynthetic cyanobacteria, 2.3 billion years ago, altered the course of evolution by allowing aerobic respiration and the development of complex multicellular life [19]. Since then, microorganisms have been involved in some of the most relevant events in recent history, such as the 1918 influenza pandemic (with a third of the world’s population infected and 50 to 100 million deaths); the discovery of penicillin by Alexander Fleming in 1928 [20], allowing the beginning of the golden era of antibiotics; or the recent COVID-19 outbreak due to the SARS-CoV-2 coronavirus.
Microorganisms are simultaneously involved in the production and consumption of three main GHGs responsible for 98% of the increase in global warming: carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) [21]. First, microorganisms are part of a large global carbon cycle. They extract carbon from non-living sources and make it available to other living organisms, mostly from atmospheric carbon dioxide (carbon fixation). The best-known example of carbon fixation is photosynthesis, and, despite this process being mainly attributed to plants, half of the global CO2 photosynthetic incorporation is performed by marine phytoplankton (e.g., Vicicitus spp. or Emiliania huxleyi) even though they only represent 1% of the photosynthetic biomass of the entire biosphere [2,19].
Secondly, methane is a simple hydrocarbon that is ~30 times more impactful than CO2 as a GHG. While microbes are sources of CH4 (between 70–90% of the total methane produced) as part of natural processes, some of their recent increase is due to changes in human activities. Methane is mostly produced “anthropogenically” (as a result of human activity) during agricultural practices (e.g., feed digestion in ruminants due to methanogenic archaea, rice paddies), as well as methanogenic microorganisms in landfills, coal mines and natural gas production. However, natural sources of methane also exist, such as anoxic sediments (oceans and lakes), wetlands, termite nests and soils [22]. In contrast, methane-consuming microorganisms are crucial for maintaining a worldwide balance, as these methanotroph species (which literally means “methane eaters”) are able to use this methane as an energy source [1,2,19,23]. Examples of well-known methanotrophs are the members of the genus Methylococcus or Methylobacter [22].
Finally, nitrogen is one of the most important gases in the atmosphere (~78%), which exists in numerous forms, such as NH3, NO and N2O, although N2 is its most habitual state. However, it is not directly available to plants and animals, and it enters the biosphere via biological fixation through different bacteria (such as Azotobacter, Beijerinckia, Clostridium or Rhizobium) or blue-green algae. Hence, marine fixation is 30% higher than terrestrial nitrogen fixation [19,23].
This brief introduction of microbial relevance in the GHG worldwide balance presents microorganisms as relevant players in climate change, but they also play several roles outside atmospheric gas control. Thus, the relationship between climate change and microorganisms (including bacteria, fungi, unicellular algae and protozoa) will be addressed point-by-point along different human sectors (e.g., industrial and health).
4. Microorganism-Mediated Effect on Productive Sectors
Climate variations (e.g., temperature increase, rainfall instability, glacial retreat, extreme weather events, etc.) have foreseeable impacts on certain industries. These impacts encompass technical concerns (e.g., power supply) or extend to more severe problems in sectors such as agriculture, forestry, livestock and commercial fishing, which are primary sectors of great economic relevance, whose global value grew by 73% between 2000 and 2019, reaching USD 3.5 trillion in 2018 [24]. Nowadays, farmers and scientists are already both turning their eyes to the use of biofertilizers and eco-friendly tillage practices in order to protect soil health, reduce GHG emissions and increase carbon sequestration [25]. However, the increase in outbreaks of several diseases, as well as the geographical expansion of different pathogens, is compromising the balance between eco-friendly activities and the way to face these new climate-change-derived risks [2] (Figure 1). Thus, the microorganisms involved in these processes play a crucial role as detailed below.
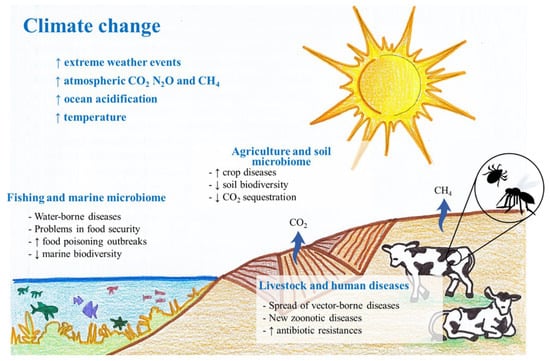
Figure 1.
Main effects of climate change on microorganisms: (i) expansion and increase in waterborne diseases affecting fish and shellfish and causing an increase in food poisoning outbreaks and loss of marine biodiversity; (ii) expansion and intensification of pathogenic fungal infections in crops all around the world; and (iii) outbreaks of livestock diseases, emergence of new zoonotic diseases and increase in antibiotic resistance.
4.1. Agriculture and Soil Microbiome: Eternal Feedback
One of the most important worldwide primary sectors is agriculture, which relies on food production. The total production of crops increased by 53% between 2000 and 2019, yielding 9.4 billion tons in 2019 [26]. However, the variation in soil microbial communities influenced by climatic change affects the physiology, temperature sensitivity or plant growth rate, which jeopardizes the final yields and compromises the future of this sector [27]. The relationships among microorganisms, atmospheric gases and crops showcase an intricate feedback system. Discrepancies have been observed between short-term laboratory studies and long-term field experiments on how climate change affects soil communities and nutritional balances. These differences may be caused by several factors, including variations in environmental conditions, differences in experimental designs and the complexity of soil ecosystems [27,28,29,30,31].
Thus, increased temperatures accelerate microbial decomposition activities, leading to faster CO2 emissions. As a result, soils will become a carbon dioxide source rather than a sink [27]. However, an increase in atmospheric CO2 has been reported to stimulate rhizosphere-colonizing bacteria such as Burkholderia and Pseudomonas as well as plant-growth-promoting fungi, while non-rhizospheric species such as Bacillus were not stimulated. This CO2 enrichment enhances the development of rhizobial populations and the increase in N-fixing microorganisms in controlled environments, although multiple resource limitations dampen rhizobial responses in natural systems. In contrast, high temperatures lead to drought, which reduces colonization by arbuscular mycorrhizal fungi [27,28].
In addition, temperature and soil humidity play a critical role in microbial-soil abundance, diversity and metabolic functionality, since climatic factors greatly modify the type and quantity of some plant species that predominate in a landscape. Thus, owing to the relationship between soil microbial biodiversity and the presence of certain plant species, this vegetal redistribution also affects soil microbial communities in a continuous feedback system [29,30]. Furthermore, the consequences of climate change on soil communities and nutritional balances depend on the specific soil type, vegetation cover and management techniques. As a result, the total effects of climate change on soil communities and nutritional balances seem logical but unclear [27,28,32].
4.2. Plant Pests’ and Diseases’ Effect on Agriculture and Forest: The Uncomfortable Travelers
The prediction of climate change effects on crop yields is being analyzed by means of several models, which, despite not being consistent with the final balances, predict multi-million dollar losses in numerous crops throughout the world as a result of pest and pathogen movement to new geographic locations [33,34,35,36,37,38]. In 2020, Delgado-Baquerizo and co-workers demonstrated that warming temperatures increase the presence of soil-borne fungal pathogens [39], and Bebber and co-workers’ study in 2013 reported that the poleward movement of numerous pathogens and pests of around 3 km per year has been underway since 1960 [40]. However, although there are certain difficulties in accurately quantifying the potential impacts of climate change on plant pests, crop production and risks to natural biodiversity [41,42], numerous bacterial and fungal diseases have already begun their geographical expansion, although most of them have not actually been related to climate change (Table 1).
Fungi annually destroy one-third of all food crops, including rice, wheat, maize, potatoes and soybean. The mitigation of this loss would have been enough to feed around 8.5% of the world population of seven billion in 2011 [38]. According to Chaloner and co-workers [43], Europe and North America, followed by some regions of Asia, are expected to be the most affected areas by the spread of non-commonly detected fungi as a result of the temperature increase, where the most economically devastating is Magnaporthe oryzae affecting rice and wheat [38]. Thus, some models create a devastating scenario for 2050, with a large increase in fungal infections throughout Europe, such as the following:
- Brown rust (mainly caused by Puccinia recondita), in the case of wheat, is forecast to increase its pressure on the crop by 20–100%, and yellow rust (caused by Puccinia striiformis) will increase by 5–20% in cold regions.
- Rice pathogens such as Pyricularia oryzae (the main cause of blast) and Bipolaris oryzae (or brown spot) are favored in all European rice districts, with the most critical situation in northern Italy (with an increase of close to 100%).
- In the case of grape, Plasmopara viticola (also called downy mildew) will increase by 5–20% across Europe, while Botrytis cinerea (or bunch rot) will have diverse impacts, ranging from a 20% decrease to a 100% increase in infection events [44].
Indeed, the spread of several agricultural diseases toward northern regions of Europe has already been reported:
- An increase in cases of diseases in leafy vegetable and cereal crops has already been reported in Italy, as a result of several pathogens’ effect, such as Plectosphaerella cucumerina, Alternaria sp., Fusarium equiseti, Myrothecium verrucaria, Myrothecium roridum, Phoma valerianellae, Pleospora betae, Peronospora belbahrii and Pythium ultimum, as well as the appearance of new pathogens like different species of Pythium (Pythium aphanidermatum, Pythium irregulare, Pythium dissotocum, Pythium coloratum, Pythium diclinum or Pythium lutarium) and new species causing yellow rust or stem rust [44,45,46].
- Among the new infections that have been reported in recent years in southern Europe and the Mediterranean coast, different species of the fungus Diaporthe have been found infecting several citruses, and the bacterium Xylella fastidiosa has also been discovered in olive trees [47].
On the other hand, fungal infections of invertebrate hosts also require attention, not only because they are an important route of transmission of pathogens to new areas but also because they can boost agricultural crises due to ecological imbalance. Thus, a dramatic example is bee broods, which are susceptible to some fungal infections, like those caused by Ascosphaera and Aspergillus, and viruses, such as Deformed wing virus (DWV) or Varroa destructor virus-1 (VDV1). Thus, agricultural production is highly dependent on bee pollination, and infections may lead to unprecedented disasters (see below) [29,41,48].

Table 1.
Geographical expansion of agricultural diseases reported in recent years.
Table 1.
Geographical expansion of agricultural diseases reported in recent years.
| Disease | Pathogen | Host | Origin | Spread and Development | Refs. |
|---|---|---|---|---|---|
| Ash dieback | Hymenoscyphus fraxineus | Ash trees | Asia | Asia, Europe and Africa | [49,50] |
| Bacterial blight or Bacterial leaf blight | Xanthomonas oryzae | Rice | Japan | Worldwide (especially Asia and Africa) | [51,52] |
| Bacterial canker | Pseudomonas syringae | Fruit trees | Depends on pathovar | Worldwide | [53,54] |
| Brown rust or Leaf rust | Puccinia recondita | Cereals (wheat, rye and barley) | Eastern Australia | Worldwide | [55,56] |
| Brown spot | Bipolaris oryzae | Rice | USA | Asia, Europe and South America | [44,57] |
| Bunch rot or Gray mold | Botrytis cinerea | Wide range | Unknown | Worldwide | [58] |
| Chestnut canker | Cryphonectria parasitic | Chestnut tree | Asia | North America | [59] |
| Disease dependent on the Diaporthe species | Diaporthe spp. | Wide range | Germany | Europe, Australia and Asia | [47,60] |
| Disease dependent on the X. fastidious subspecies | Xylella fastidiosa | Wide range | USA | South and North America and Europe | [47,61] |
| Downy mildew | Plasmopara viticola | Grape | North America | Worldwide | [62,63,64] |
| Dry root rot | Rhizoctonia bataticola (also Macrophomina phaseolina) | Chickpea | India | North America, Asia and Africa | [65] |
| Dutch Elm disease | Ceratocystis ulmi (also Ophistoma ulmi) | Elm | Asia | Worldwide | [66] |
| Fire blight | Erwinia amylovora | Apple, Pearl and some Rosaceae | North America | Europe and Asia | [67] |
| Rice blast | Magnaporthe oryzae (anamorph Pyricularia oryzae) | Rice | Brazil | South America, Asia, Africa and Europe | [44,68] |
| Stewart’s wilt | Pantoea stewartii (formerly Erwinia stewartii) | Corn | USA | Italy, Malaysia | [69,70] |
| Yellow rust or Stripe rust | Puccinia striiformis | Wheat | Transcaucasia (Armenia, Georgia and Azerbaijan) | Worldwide | [55,71] |
| Wheat blast | Pyricularia graminis-tritici | Wheat | Brazil | North and South America and Asia | [72] |
The forest industry accounted for USD 244 billion in derivatives such as paper and paperboard, agglomerates or pellets in 2020 (https://www.fao.org/forestry/statistics/80938/en/, accessed on 19 July 2023). In the same way as agronomic soils, global warming is affecting the microbial communities of forest soils where a variation of just 5 °C unbalanced the relative abundance of soil microbiota in a field experiment (mainly fungi and some actinobacteria) [73]. However, the most visible problem today is the increase and expansion of pests and pathogens into new areas, which yield severe environmental and economic impacts, including the near-extinction of certain tree species, such as the following:
- Chestnut blight, caused by the pathogenic fungus Cryphonectria parasitica, native to Southeast Asia, which has killed more than 4 billion trees in the US to date.
- Ash dieback, caused by the newly identified Hymenoscyphus fraxineus, first detected in Asia, which has been recently detected in the UK and Northern Ireland [59].
- Dutch Elm disease, caused by Ceratocystis ulmi, a fungus responsible for its spread from Asia to Europe in 1918 that has killed millions of elms in Europe, western Asia and North America all throughout the 20th century [74].
- Needle blight of Pinus contora in north-western British Columbia, Canada, caused by outbreaks of Dothistroma septosporum as a result of a summer rainfall increase [75].
In summary, the causative agents of plant diseases are troublesome travelers that result in huge economic losses for the agricultural and forestry sectors, although obtaining realistic analyses seems complex.
4.3. Livestock and Climate Change: An Arthropoda Matter
The livestock sector is a capital pillar of the global food system, which, according to the FAO [24], represents 40% of the global agricultural output value and supports the livelihood, food and nutrition security of almost 1.3 billion people [24]. Although the increase in productivity efficiency helps to minimize the detrimental environmental impact of livestock, global farm-gate GHG emissions increased by 11% between 2000 and 2019, and around 55% of them are related to the livestock industry [76,77].
As in the agricultural sector, climate change could directly and indirectly influence livestock production in many ways: (i) growth performance, (ii) the yield and quality of the product, (iii) reproductive performance and (iv) health status. However, the main risk of climate change, as far as microorganisms are concerned, is an increase in fungal and bacterial diseases [76]. In addition, changes in temperature may trigger the secretion of stress hormones such as cortisol, which suppresses the immune system and favors pathogen infections, which also increases their transmission and expansion [78].
Pathogens’ geographical extension is sometimes attributed to both anthropogenic and natural effects, including climatic factors and fauna and flora spread. Hence, the increased temperatures in the northern regions favor the expansion of numerous pathogen vectors (e.g., insects) that increase their chance to be established in new regions [79], promoting the geographic spread and increasing the incidence of some infections and diseases [80] (Table 2). The most climate-sensitive diseases are those transmitted by arthropods. Thus, the prevalence of tick-borne diseases has increased throughout Europe during the last decades [79]. In fact, 50% of tick species have the potential to expand their range worldwide, many of them being vectors of important animal diseases. These tick vectors that are already spreading north include the following:

Table 2.
Geographical expansion of livestock diseases reported in recent years.
Table 2.
Geographical expansion of livestock diseases reported in recent years.
| Disease | Pathogen | Vector | Host | Origin | Spread and Development | Refs. |
|---|---|---|---|---|---|---|
| Anaplasmosis | Anaplasma phagocytophilum | Ixodes scapularis, Ixodes pacificus | Sheep and cattle | Scotland | Worldwide | [81,82] |
| Babesiosis | Babesia microti, Babesia venatorum and Babesia divergens | Ixodes ricinus, I. scapularis | Mammals | Romania | Europe and North America | [83] |
| Babesia bovis and Babesia bigemina | Rhipicephalus microplus | Mammals | Asia, Africa, South and Central America | Europe and North America | [84] | |
| Bluetongue Virus | Orbivirus | Culicoides imicola | Ruminants | South Africa | USA, Canada, Australia, South and Central Europe | [85] |
| Canine Babesiosis | Babesia spp. | Dermacentor reticulatus | Mammals (especially cattle) and birds | Romania | Worldwide | [26] |
| Colorado tick fever | Coltivirus | Dermacentor andersoni | Mammals | Western US | Europe and North America | [86,87] |
| Ehrlichiosis | Ehrlichia chaffeensis and Ehrlichia ewingi | Amblyomma americanum | Mammals | Canada | North America and Europe | [88,89] |
| Leptospirosis | Leptospira spp. | Environmental transmission | Mammals | Japan and Europe | Asia, Australia, America and South Europe | [90,91] |
| Lyme disease | Borrelia burgdorferi | Ixodes scapularis | Rodents | USA | North America and Eurasia | [92,93,94] |
| Powassan virus disease | Powassan virus | Several tick species | Mammals | Unknown | North America | [95] |
| Q fever | Coxiella burnetii | D. reticulatus | Ruminants (cattle, goat and sheep) | Australia | Europe and North America | [96] |
| Rocky Mountain spotted fever | Rickettsia rickettsii | D. andersoni | Mammals | South and Central America | North America | [97] |
| Rickettsia parkeri | A. maculatum | Central and North America |
- Ixodes scapularis and Ixodes pacificus, which transmit anaplasmosis disease, mainly caused by the bacterium Anaplasma phagocytophilum (formerly Erilichia phagocytophilum), which has also been reported in new regions worldwide [98]. In addition, they can also transmit the pathogen bacteria Borrelia miyamotoi, which has recently been reported in new areas of North America [81,82,99].
- Dermacentor reticulatus, which can transmit canine babesiosis, tularemia or Q fever [100,101]. Canine babesiosis is caused by the intracellular protozoan Babesia spp., and the number of reported cases has increased in northern countries, including Canada, Germany, Hungary, Switzerland and the Netherlands [26,78,95]. Q fever, meanwhile, is an important zoonotic disease caused by the bacterium Coxiella burnetiid. It was originally described in Australia in 1933, although it began to spread across North America and Europe only two decades ago. In Europe, some cases were initially reported in southern countries such as France, Spain and Germany, although in recent years, significant outbreaks have been reported even in countries further north like the Netherlands [96].
- Ixodes ricinus, which transmits encephalitis and Lyme disease (borreliosis).
Another important group of vectors spreading to the north are mosquitoes, such as Culicoides imicola, which transmits bluetongue virus (BTV). It has spread northward from Africa and Cyprus causing BTV outbreaks (with high mortality and morbidity rates) in the Netherlands, Germany, Belgium, Luxembourg, Great Britain and other northern European countries since 2007 [80,85]. BTV is an infectious disease caused by an Orbivirus that mainly affects ruminants (especially sheep), but other non-ruminants can also be infected (e.g., shrews or dogs) [102,103]. In addition, changes in air currents associated with climate change can alter the dispersal of mosquitoes, leading to outbreaks of infectious diseases, such as leptospirosis and foot rot, although no confirmatory studies have been conducted to prove this [78].
On the other hand, although vector-borne diseases are prone to be impacted by global warming, other kinds of weather events (such as abundant rains, extreme drought or changes in air currents) may play an important role in their geographical expansion. These events have been extensively studied in processes such as El Niño events. After these extreme weather events, an increase in diseases, like Rift Valley fever, has been observed. This acute viral hemorrhagic fever is caused by a member of the genus Phlebovirus. It triggers high livestock morbidity and mortality and has traditionally been identified in sub-Sahara regions. However, there have recently been outbreaks as far north as the Arabian Peninsula and Asia, with the potential to spread further into Europe in the future [13,78,104].
In addition to the geographical expansion of vector-borne diseases, the ability of microbial pathogens to mutate and adapt to environmental changes is a significant factor in understanding the potential impact of climate change on livestock. For instance, in the case of Venezuelan equine encephalitis virus (VEEV), a single amino acid substitution in the envelope glycoprotein was observed (Ser → Asn), enabling the virus to adapt to a new efficient epizootic vector. This change occurred after a shift in the representative mosquito population, where the mosquito species Ochlerotatus taeniorhynchus became the most abundant after deforestation eliminated the habitats of Culex taeniopus, the previous principal mosquito vector. It serves as an example of viruses’ rapid response to changes in their vector populations [80]. In addition, RNA viruses have no proof-reading, leading to a high evolutionary rate, mainly those with segmented genomes. The conservation degree of host receptors used by the pathogen is also important, and, although it is not directly affected by climate conditions, climate change could reduce the species barrier and facilitate contagion to new species through the invasion of new habitats [80].
Finally, apiculture is a livestock sector often forgotten when it comes to climate change. Apis mellifera is the most common species of honeybees in Europe and is the most valuable pollinator of agricultural crops worldwide [105,106,107]. However, they suffer from a wide variety of bacterial, fungal and viral pathogens, as well as microsporidial or ectoparasitic mites. Among its most common diseases, European foulbrood (EFB), American foulbrood (AFB), chronic bee paralysis (CBP), sacral brood, calcareous brood and varroosis [108] are worth mentioning. Thus, different model predictions have described a positive relationship between temperature and the incidence of some of these important diseases [109], although they are not usually the focus of numerous analyses. Two of the most studied ones are chronic bee paralysis (caused by an unclassified bipartite RNA virus usually called chronic bee paralysis virus) [110] and American foulbrood (caused by the Gram-positive bacteria Paenibacillus larvae), whose expansion and intensification have already been reported despite the absence of models linking it to climate change [108,111].
4.4. Fishing and Marine Microbiome
Fishing and shellfish are relevant food industrial sectors. On average, a person eats approximately 20.5 kg of fish and shellfish every year. The 179 million metric tons of total seafood from 2018 landings had a farm-gate production value exceeding USD 401 billion, and global fish consumption has continued to increase at an average annual rate of 3.1% since 1961 (a higher rate than all other animal protein foods) [24,112]. However, marine animals are mainly ectotherms, and, as a result, they are particularly sensitive to changes in water temperature, which highlights the effect of global warming on this fauna. Over the past few decades, there has been a significant increase in temperature in the European seas, with warming rates four to seven times higher than the global average. This accelerated warming has important implications for marine life because it can disrupt migration patterns, reproductive cycles, food availability and species distribution [113].
Climate change may also affect other environmental factors such as seawater acidification, hypoxia, CO2 accumulation, salinity or sea level modifications [114] (Figure 2). These changes disturb marine ecosystems, including microbial biodiversity, which has been under-researched for decades. Marine microorganisms account for 90% of marine biomass. They form the basis of marine food webs and are responsible for important biogeochemical cycles [115]. However, most of the ocean observation programs and studies do not target microbes [2,116], and the few reported just point to changes in the ocean’s microbial biodiversity mainly due to the increase in water temperatures and sea acidification [117,118]. Therefore, Wang and co-workers [119] observed that warmer oceans will alter microbial communities, especially in thermally stable regions, and Hutchins and Fu [120] reported that ocean warming causes losses in microbial populations, leading to the predominance of a few earlier insignificant taxa.
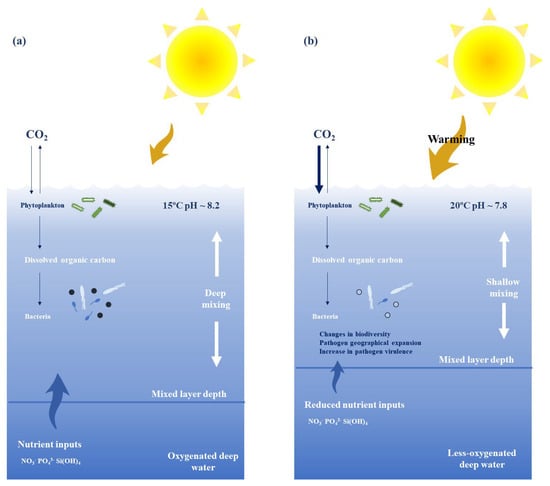
Figure 2.
Main effects of climate change on key chemical and physical factors influencing the community composition of marine planktonic microorganisms. The current ocean (a) vs. the future one (b) after 2100. Among the changes that will have the greatest impact are (i) increases in atmospheric CO2 uptake and, therefore, water acidification, (ii) increase in temperature, (iii) shallowing of the surface mixed layer, (iv) intensified stratification, leading to lower vertical fluxes of nutrients, and (v) reduction in oxygen both at the surface and in the underlying deep ocean. All these changes lead to changes in microbial biodiversity and expansion of both pathogen geographic ranges and pathogen virulence, inducing an increase in disease outbreaks and fauna and flora mortality.
To date, 25 viruses, 33 bacteria, 23 protists and 21 metazoans have been reported as the main causes of marine diseases in plants, corals, mollusks, crustaceans, echinoderms, fishes, turtles and mammals [114], although it is estimated that a greater part of the diversity in marine microbial biodiversity remains undiscovered, with less than 0.1% being described [121]. Thus, some studies have illustrated how marine warming alters host–pathogen dynamics, which can be described as follows:
- First, environmental changes like those caused by climate change may lead to stress in both fish and shellfish species, leading to lower immune responses against various pathogens and diseases [2]. Urchins are a clear example of this. Both tank and real-world experiments showed a strong correlation between mass mortality events and long-term elevated temperatures. While urchins experience thermal stress, leading to a decrease in the immune response (increasing infection rates) and fertility (minimizing population recovery) at higher temperatures, pathogens increase their replication and transmission rates [122].
- Second, a rise in temperatures leads to an increase in several marine pathogens’ virulence by increasing their metabolism and inducing higher rates of transmission [122]. Such as the case of the host–pathogen interaction between Pocillopora damicornis and Vibrio coralliilyticus. At temperatures above 27 °C, pathogen virulence increases because multiple virulence factors are upregulated, including extracellular proteases that cause lysis and mortality in P. damicornis [123].
- Third, pathogen geographical expansion comes with increasing temperatures. A shining example is Perkinsus marinus, a protist parasite of the eastern oyster Crassostrea virginica that has expanded its range from the mid-Atlantic to the northeast in recent decades, primarily due to increased winter water temperatures, since both P. marinus infection patterns and C. virginica immune response are dependent on the temperature [124,125]. In addition, P. marinus infection intensity increases above 20 °C, especially at temperatures close to 25 °C, and some models predict longer periods of sustained higher temperatures after 2100, which may allow the geographical spread of P. marinus and its establishment farther north along the coast [122]. Other cases come from the Vibrio genus, which includes more than 110 different species. Some of them are well-known animal and human pathogens, like Vibrio cholerae, responsible for cholera disease, which causes between 100,000 and 120,000 deaths every year globally. Zooplankton is one of the main environmental reservoirs of vibrios in aquatic environments, and some species can be found infecting molluscan and crustacean shellfishes, as in the case of Vibrio parahaemolyticus and Vibrio vulnificus. Both species are related to typical human infections associated with seafood consumption, and the risk area for both vibrio infections has greatly increased during warmer water temperature episodes in the last years [113,126]. A similar effect is expected in other aquaculture species due to Vibrio harveyi, which poses a great risk to some of the most important species in the fish market, such as rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar L.), Senegalese sole (Solea senegalensis), Japanese seabass (Lateolabrax japonicus), cobia (Rachycentron canadum) and common dentex (Dentex dentex), among others [127].
- Last, habitat expansion is likely to cause novel contact among populations, pathogens and vectors, potentially increasing interspecies infections. For example, Brucella, a group of Gram-negative bacteria and the causative agents of brucellosis. Novel species of Brucella named Brucella ceti and Brucella pinnipedialis have been reported to cause infection in marine mammals like cetaceans and seals, respectively [122].
On the one hand, although temperature is presented as the dominant climate change effect disrupting microbial marine communities, there are physical and chemical factors that may also affect infection and disease transmission in aquatic environments. A reduction in water salinity has the potential to significantly impact the immune response of host organisms. For instance, oysters exposed to low-salinity stress exhibited increased susceptibility to infection by Vibrio alginolyticus, resulting in higher mortality rates [128]. Similarly, ocean acidification can affect the immunological defenses of diverse organisms, such as urchins, mussels, oysters, finfishes, corals, bivalves and seagrasses. In addition, different studies have reported that Vibrio tubiashii grows better and increases its infectivity against mussels (Mytilus edulis) and hemocytes when exposed to low-pH conditions [114,129]. On the other hand, elevated partial pressures of CO2 (usually known as hypercarbia) may impact the immune response by activating the complement system, activating the inflammatory response and down-regulating IgM expression. Thus, the joint effect of hypercarbia and temperature changes can alter the first and second lines of finfish defense, affecting their susceptibility to infections and diseases [114].
5. Human Health and Climate Change
Last but not least, human health is also affected by climate change effects. According to the World Health Organization, the epidemiology of infectious diseases constantly fluctuates in response to environmental changes and interactions among pathogens, hosts, reservoirs and vectors. The report of the IPCC (Intergovernmental Panel on Climate Change; https://www.ipcc.ch/report/sixth-assessment-report-working-group-ii/, accessed on 19 July 2023) indicates that an intensification in infectious disease outbreaks due to climate change will be observed because several pathogens will spread to new regions and emerge in areas where they were previously under control. In addition, diseases that have never previously affected humans may “spill over” from animals, through a process known as zoonosis [130]. It properly fits the concept of One Health which recognizes the close connection between human and animal health and our shared environment (https://www.cdc.gov/onehealth/index.html, accessed on 19 July 2023). Thus, climate variation and its effect on human health can be considered directly due to the evolution and expansion of different disease vectors or indirectly through antibiotic resistance, which hampers microbial disease control.
5.1. Human Infective Diseases: Evolution and Expansion
Climate change can modify the relationship between humans and pathogens, thereby increasing their probability of contracting infections and diseases. Rising temperatures would modify the host immune system and boost the growth rate of pathogens, which supports their perpetuation and transfer [2,131] in a similar manner previously described for livestock. The most positively affected pathogens are those transmitted by vectors, food, air, water and other environmental agents [2]. Thus, the forecast for the near future is an increase in the infection transference and spread, as well as a change in the patterns of infectious pathologies due to rising temperatures, early changes of season and fluctuations in rainfall. For example, the spread of mosquito-borne diseases like dengue fever or Zika virus will be the result of increasing temperatures and changes in rainfall patterns, which lead to more breeding sites. In addition, infectious disease-causing pathogens are able to adapt to new climatic conditions that enhance their transmission and prevalence [132,133,134].
The impact of rising temperatures and the adaptation of pathogens to new conditions poses a significant threat, leading to the emergence of previously unrecorded human infectious diseases. A notable example is the Chikungunya virus, transmitted by mosquitoes, which causes fever and severe joint pain. Although it was initially identified in Tanzania in the 1950s, it has since spread to other parts of the world, including America and Asia [135,136]. Pathogens may develop thermotolerance as a survival mechanism in response to changing environmental conditions and their adaptation to new hosts. The acquisition of this thermotolerance involves various molecular and cellular mechanisms, such as (i) the activation of heat shock response genes encoding heat shock proteins (HSPs), (ii) DNA repair mechanisms, metabolic adaptations to optimize energy production and utilization at higher temperatures or (iii) gene regulatory network modulation and signaling pathways to coordinate the expression of heat-responsive genes [137]. As these pathogens adapt to higher temperatures, they acquire the ability to cross the mammalian endothermic barrier (which creates a zone of thermal inhibition to prevent infection), thereby adapting to the internal environment of the host. This, together with the increased population density of pathogens, poses a greater threat to human health and increases the likelihood of infection. For instance, certain strains of Vibrio, commonly found in coastal waters, have become more virulent in response to escalating ocean temperatures, leading to outbreaks of infections among individuals exposed to contaminated water or seafood. Additionally, pathogens preserved in Arctic permafrost may also resurface, causing diseases such as tularemia or anthrax, as old variants long forgotten by the immune system regain activity [138].
The effect of climate change on the expansion and proliferation of some human diseases is presented in this section based on their transmission manners (vector-borne, foodborne or waterborne diseases) (see Table 3 and Figure 3).

Figure 3.
Summary of the main causes and effects of climate change on human health due to microbial global variations. White font: (up) factors that contribute to the worldwide increase in antibiotic resistance; (down) different sources of borne diseases. Black font: result on human health of the different factors presented in the manuscript.
5.1.1. Human Vector-Borne Diseases
The most important vector-borne diseases in Europe are caused by pathogens sensitive to climatic conditions because they are spread mostly by cold-blooded arthropods, which expand their geographic ranges owing to the effects of climate change (global warming, rainfall variations and extreme weather events) [2,130,138]. Vector-borne diseases account for more than 17% of all infectious diseases, causing 700,000 deaths every year worldwide. Although mosquitoes are the best-known disease vector in humans and animals, other arthropods, such as ticks, black flies, sandflies, midges, fleas and triatomine bugs, also act as relevant vectors and should be kept in mind [2,139,140,141].
The effects of climatic variations can be observed with the spread of some tropical diseases, such as dengue and Zika, both transmitted by mosquitoes of the Aedes family. Dengue transmission has increased since 1950 by 8.9% in the case of Aedes aegypti and by 15.0% when considering Aedes albopictus, reaching almost 4 billion people in over 128 countries at risk of contracting dengue [130,139,140,142]. In addition, there was a 50% increase between 2005 and 2015 in the number of deaths caused by dengue, which has become the most prevalent disease among travelers in Southeast Asia, surpassing malaria.

Table 3.
Effect of climate change on the expansion and development of different diseases.
Table 3.
Effect of climate change on the expansion and development of different diseases.
| Disease | Microorganism | Vector | Host | Origin | Spread and Development | Effects of Climate Change | Refs. | |
|---|---|---|---|---|---|---|---|---|
| Vector-borne diseases | Chikungunya | Chikungunya virus | Aedes albopictus | Human | Tanzania | Asia and Africa | Increase in temperatures, changes in rainfall and increase in the number and severity of extreme weather events multiply the risk and the ranges of infection and the spread of diseases | [139] |
| Dengue | Dengue virus (DENV) | Aedes aegypti | Human | Africa | Tropical and subtropical areas and Europe | [133,142] | ||
| Aedes albopictus | ||||||||
| Leishmaniasis | Leishmania spp. | Female sand-fly | Human | Africa | Southern countries of the European continent | [138,139] | ||
| Zika | Zika virus (ZIKV) | Aedes albopictus | Human | Africa | North America and South America | [138,139] | ||
| Foodborne diseases | Diarrheal disease | Campylobacter spp. | Contaminated food | Human | America | Worldwide | Temperatures rise favors the contamination of food by pathogens that cause diseases | [138,139] |
| Salmonellosis | Salmonella spp. | Contaminated food | Human | America | Worldwide | [139,143] | ||
| Waterborne diseases | Cholera | Vibrio cholerae | Contaminated water | Human | Asia and Africa | Africa, Asia and North America | Increased precipitation can wash pathogens into waterways, while rising sea temperatures activate impulses for pathogen spread and development | [1,111,112,117,119,120] |
| Leptospirosis | Leptospira spp. | Contaminated water | Human | Germany | Tropical and subtropical areas | Warmer temperatures and extreme weather events can create favorable conditions for the survival and persistence of Leptospira bacteria in the environment. Alterations in land use and deforestation can also increase human contact with animal reservoirs of leptospirosis | [2] |
Thus, a 30-fold increase in dengue incidence over the last 50 years, as well as an extension in the geographic area of this disease, is a clear warning sign. Hence, forecasts estimate the future expansion of dengue, which will increase the risk of infection in the global population [138,144]. Currently, the greatest concern is the mosquito A. albopictus, which can spread from temperate regions to higher altitudes. This mosquito species is known to transmit diseases such as chikungunya and Zika viruses, making its presence a cause for heightened attention and concern. Some studies have related the spread of outbreaks to an increase in total viral load with an increase in the number and range of vectors, which is directly related to climate change [139,144,145,146].
Leishmaniasis, which is transmitted in warm environments by protozoa of the Leishmania genus, is moving into regions that are now temperate as a result of climate change, including areas of southern Europe such as Italy, Greece and Spain, as well as regions in South America, such as Argentina and Brazil [147,148,149]. In this way, the reproduction and survival of protozoa are advantaged because although the number of protozoa per cell does not increase, the concentration of these protozoa in the environment rises, since warm temperatures enhance their reproductive cycle. This has resulted in an alarming growth of cutaneous leishmaniasis in areas where this protozoa was not previously detected (because temperatures were much lower before), such as the southern countries of Europe, especially in Mediterranean countries such as Spain, Italy, France and Greece [138,139,150].
The effectiveness of disease transmission by a vector depends on the time taken for the vector to become infectious after encountering an infected host. Higher temperatures play a significant role in boosting efficacy. When temperatures are higher, the time between contact with the infected host and the vector becoming infectious is shortened. This shorter timeframe results in higher vector concentrations. As a result, there are more opportunities for the vector to transmit the disease within its lifespan, thereby increasing the overall transmission potential. Hence, the spread of vector-borne diseases is favored by rising temperatures, lengthening transmission seasons, increasing pathogen replication in the vector and increasing geographic range [2].
5.1.2. Foodborne Diseases
Nowadays, more than 200 types of foodborne diseases have been reported, most of which could be affected by climate change [139,143]. Approximately 420,000 deaths per year are caused by foodborne diseases, and an increase is expected because rising temperatures favor food contamination and pathogen establishment in new temperate regions [139,143]. In addition, the more humid the environment, the greater the survival of pathogens (such as Trichinella spp. and Toxoplasma gondii) and parasite eggs, larvae and cysts. The rainfall rises and its intensity, as well as the increase in floods, favor crop and food contamination because the air bacterial concentration is increased after a drizzle. Additionally, after a flood, wastewater is more likely to overflow, causing contamination of fresh produce [134,151].
Several examples of climate change effects on foodborne diseases have been described, such as an increase in Campylobacter infections due to rising temperatures and intense rainfalls [152]. According to a study carried out by Kuhn and collaborators in 2020 [152], there is a possible increase of between 3 and 20% in cases of Campylobacter infections if climate change is not delayed. In addition, salmonellosis, transmitted by Salmonella species, which activates reproduction at high temperatures, is another relevant concern. Global warming has been linked to an increase in Salmonella infections, and it is estimated that every degree of temperature rise would cause a 5% to 10% increase in Salmonella infections [139,143,153]. Morgado and co-workers, in 2021 [154], showed a direct relationship between warm temperatures, increased rainfall (especially during extreme weather events) and a rise in diagnosed cases of salmonellosis. In this way, higher temperatures can lead to increased bacterial concentrations, potentially resulting in more severe cattle infections. This has significant implications for the food industry, as contaminated meat can contribute to outbreaks of salmonellosis in humans [128].
5.1.3. Waterborne Diseases
Increased rainfall can drive pathogens into waterways and overwhelm water treatment systems, causing different disease outbreaks. In addition, as presented in previous sections, the increase in sea temperature and salinity changes can cause the development and proliferation of numerous Vibrio species, such as Vibrio cholerae. Thus, it should be kept in mind that these temperature changes, no matter how small, can lead to significant alterations in host–pathogen relationships [133,139,153,155]. In addition, it must be considered that the spread and development of some pathogens (Vibrio cholerae, Cryptosporidium, Leptospira spp., etc.) can lead to the contamination of urban water, which can result in an increase in several diseases [156,157]. Most of these pathogens are transmitted by the fecal–oral route or indirectly through contaminated water, where they can survive for long periods, even though their nutrient access can be scarce [143,144]. An example is cholera disease, whose clinical impact has increased in recent years, mainly due to temperature and rainfall variations. In fact, it has been observed that each increased degree raises the risk of cholera infections by around 15–29% [144,158].
Other cases of waterborne infections are those caused by the toxic microalgae Vicicitus globosus (formerly Chattonella globosa), which contaminates the food chain through shellfish consumption. Hence, climate change has intensified the proliferation of this algae because of the increase in the concentration of CO2 in water, driving an upsurge in food poisoning outbreaks [2,159]. Ocean acidification has also been linked to the formation of toxic algal blooms. Some species of algae, including Emiliania huxleyi, produce toxins when their environment changes due to acidification. These blooms can lead to the accumulation of toxins in the food chain, causing harm to marine organisms and potentially even to humans who consume seafood [159].
5.2. Antibiotic Resistance
The World Health Organization indicates antibiotic resistance as “one of the greatest threats to global health, food security and development today” (https://www.who.int/en/news-room/fact-sheets/detail/antibiotic-resistance, accessed on 19 July 2023). In recent years, antibiotic resistance has risen sharply to become a public health problem, with 23,000 people dying annually as a result of antibiotic-resistant infections. This health threat has been due to the wide variety of antibiotic applications (e.g., human and animal health, growth promotion in food-producing animals, disease prevention, crop-disease control as pesticides) in the last 80 years [160]. Worldwide, antibiotic consumption rose by approximately 39% between 2000 and 2015, and it is estimated that it will continue to rise up to 200% in a few years. These data, which are worrying for public health, were reviewed by Jim O’Neill (UK Prime Minister commissioned the Review on Antimicrobial Resistance) in 2014 [161], who reported that deaths from untreatable infections could reach 10 million annually after 2050. In addition, there was a reminder a few years later in 2019 from the IACG (Interagency Coordinating Group on Antimicrobial Resistance) hosted by the WHO (World Health Organization) with contributions from FAO and OIE (Organization for Animal Health) [162].
Latest studies relate antibiotic resistance spread to temperature increase because bacterial duplication time is accelerated, multiplying the chance of mutations, the horizontal transfer of genes (some of them related to antibiotic resistance) and infectivity [2,161,163,164] (Figure 3). Particularly, this increase in resistance has been notably higher in southern Europe, where climate change has led to a rise in minimum temperatures. As a consequence of this temperature increase in these southern countries, the intensification of antibiotic resistance has increased from 0.33% to 1.2% per year [163]. Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae are examples of this peculiar connection between temperature and antibiotic resistance, which is currently causing serious sequelae because some strains are hard to tackle with current antibiotic formulations [165,166]. In addition, the increase in deaths due to antibiotic-resistant strains of tuberculosis causes more than 2000 deaths annually [167].
Furthermore, it has been observed that the higher the temperature, the faster the new instances of resistance upsurge [163,168]. Thus, salmonellosis could increase both burden and morbidity due to global warming [169].
Another particular case is Candida auris, a new drug-resistant yeast species [167]. Its most enigmatic aspect is that it emerged simultaneously as a human pathogen on three continents in 2009, exhibiting antifungal agent resistance. One hypothesis is that the increase in environmental temperature increased the concentration of C. auris and favored the appearance of new mutations, including those for antibiotic resistance. In addition, seabirds acted as intermediate hosts and reservoirs, and it became a zoonotic disease after breaking the mammalian thermal barrier through adaptation to climate change. According to Casadevall’s conclusions [135], C. auris was the first human pathogen to appear as a result of climate change [135,167].
The increase in temperature leads to higher growth rates and enhanced survival of pathogens, resulting in higher population densities. This, in turn, leads to an increased number of pathogens, which subsequently contributes to an increased occurrence of random mutations [138,170]. It is important to note that resistance to one stressor, such as higher temperatures, can provide protection against other stressors (e.g., antibiotics) [171]. This phenomenon is known as collateral resistance or cross-protection, which was studied by Rodríguez-Verdugo et al. in 2013 [172]. Interestingly, certain generations of E. coli have acquired antibiotic resistance despite never being directly exposed to them. Instead, resistance emerged owing to exposure to elevated temperatures. Both temperature and antibiotics influence the transcription of RNA polymerase, leading to the acquisition of mutations as a survival mechanism against heat stress, conferring also resistance to antibiotics [167,172].
Additionally, warmer environmental temperatures promote biofilm formation, mainly because of the decrease in oxygen solubility with increasing temperature. In response to these changing conditions, bacteria adapt their growth and behavior, transitioning from planktonic bacteria to biofilms. Within biofilms, bacteria enter a dormant state, enabling them to better tolerate the presence of antibiotics. Consequently, biofilms exhibit increased resistance to antibiotics. In the context of reduced oxygen availability, certain bacterial species can adapt their metabolism and form biofilms to enhance their survival and persistence. Biofilms provide protection and access to nutrients, allowing bacteria to thrive in oxygen-depleted environments [171]. In summary, microorganisms have the ability to adapt and persist by undergoing genetic mutations and selecting strains that are better suited to their environment. This includes developing resistance to antibiotics as a means of survival in the presence of these drugs [167].
6. Microbial Mitigation of Climate Change
Hitherto, this review has presented microbes as actors and recipients of climate change (e.g., GHG producers, crop destroyers or players in human and animal health concerns). However, although there are still a limited number of non-model studies focused on using microorganisms as a counterpart (mitigation agents) of global climate variation, some hope glimmers appear on the horizon. Thus, microbial support can be considered in a direct manner (e.g., plant biostimulants) or in an indirect way (e.g., helping in the transition to the circular bioeconomy (a way to maximize resource efficiency, reduce waste generation and promote the regeneration of natural systems) [173]).
The direct commitment of microorganisms to decreasing or slowing down climate change can be considered if those microbes are relevant players acting straightly against the causes or effects, such as the following:
- Inoculants: one of the main strategies to deal with the inevitable effects of climate change on crops is the use of biostimulants, which recover plant resistance and resilience to biotic and abiotic stresses showing low toxicity and avoiding the appearance of new resistant strains of pests and pathogens. Hence, the biofertilizer market has steadily increased in the last years, with Europe being the world industry leader [174]. Several microorganisms can act as biostimulants providing crop-limiting nutrients: (i) by nitrogen fixation (Azospirillum, Azotobacter or Rhizobium), (ii) mineral solubilization like phosphorus, potassium or zinc (Pseudomonas, Bacillus or arbuscular mycorrhiza) or (iii) siderophore production (Pseudomonas and Acinetobacter) [174,175].
- Biofertilizers tackle climate change concerns in different positions:
- (i)
- Some microbial species have shown crop protection from warmer temperatures, such as Paraburkholderia phytofirmans in potato crops, Bacillus and Azospirillum species in wheat and soy or Pseudomonas species in wheat [174],
- (ii)
- Biofertilization minimizes N2O (GHG) emissions from soybean root nodules. Thus, bacterial strains with higher N2O reductase activities (such as Azospira sp. or Bradyrhizobium diazoefficiens) may provide avenues for reducing N2O emissions in such crops [176,177],
- (iii)
- Methanotrophic bacteria are gaining attention as biofertilizers, since it has been estimated that they are able to consume approximately 40–60% of the methane produced in wetland environments. Thus, methanotrophs can be used in landfills and agricultural soils, ultimately helping to reduce atmospheric methane levels (e.g., Methylococcus or Methylococcus species) [22,178,179].
- Microorganisms are leaders in carbon sequestration:
- (i)
- Several soil microbes contribute to carbon sequestration through different mechanisms, although certain microbes possess faster metabolic rates and, therefore, sequester carbon faster. It is possible to enrich soils with these species of interest through the introduction of microbial formulations respecting the environment, as well as by enhancing the capacity to collect carbon in agricultural soil [180].
- (ii)
- In addition, studies on the production of biofuels at an industrial level by means of algae, which are one of the most powerful microorganisms for carbon fixation (1.83 kg of CO2 is needed for each kg of dry algae biomass production), connect two relevant concepts of the circular bioeconomy. On the one hand, CO2 sequestration is closely connected to the carbon credits that pollute the air, and on the other hand, the biomass produced by some of these algae genera (e.g., Botryococcus sp., Scenedesmus, Neochloris) is ideal for energy fuel production and multiple value-added products (e.g., feeds). Therefore, it would be a particularly interesting cycle, since concerns about the depletion of fossil fuels have increased general interest in recent years, and this process could be interesting not only from an ecological point of view for the biological sequestration of carbon from punctual sources but also from an economic one [181].
- Livestock rumen: manipulation of rumen microbiota and breeding programs has been proposed as a suitable solution to reduce methane emissions from cattle. The objective would be to obtain cattle lines producing less methane without affecting the health and productivity of animals [2].
On the other hand, the indirect involvement of microorganisms in the fight against climate change can be considered if those microbes work in the transition to the circular bioeconomy by helping to reintegrate residual materials or by-products in the production chains, as well as by supporting the degradation of recalcitrant material to their elementary components or biomolecules (e.g., as biomass to the environment), such as the following:
- The Rs concept is aimed at the transition to a circular bioeconomy by means of different wording that collects the spirit of the materials’ reinsertion into the production system. Initially, three Rs were considered (Reduce, Reuse and Recycle); later, it was increased to six Rs (the three Rs plus Rethink, Refuse, Repair), and, nowadays, it continues increasing the number of Rs (the six Rs plus Refurbish, Remanufacture, Repurpose, Recover). In this context, the recent isolation of the poly(ethylene terephthalate) (PET)-degrading bacterium Ideonella sakaiensis [182], the description of epoxy-degrader microorganisms, such as Pseudomonas putida [183], or the description of the wax moth caterpillars (Galleria mellonella) as polyethylene degraders [184] have boosted the research on the biotechnological degradation of plastic. Excessive use of these materials increases plastic accumulation on land and sea. Microbes are the predominant organisms able to face this problem, the so-called plastic biodegradation [185]. The degradation to these basic molecular components presents the microorganisms or their enzymatic activities as relevant players in environmental sustainability.
- New protein sources: the search for new sources of protein to cover the increasing demand for meat protein has led to single-cell protein (SCP) development. In the first place, efforts have been made to convert plant protein into meat protein, getting low yields. Thus, the production of SCP was initiated, which are dried microbial biomass or the total amount of protein extracted from bacteria, yeasts, fungi or algae cultures [186]. A clear example is the production of burger patties from the fungal protein of Aspergillus oryzae, with similar organoleptic properties to meat burgers and rich nutritional content [187].
- Bioremediation: oil spills are one example of pollution that can have devastating effects on ecosystems and habitats. The bacterium Alcanivorax borkumensis thrives in hydrocarbon-rich environments and has been shown to play a crucial role in the degradation of oil spills [188]. This microorganism has been used in several bioremediation efforts, including the clean-up of the Deepwater Horizon oil spill in the Gulf of Mexico in 2010 [189].
- Liquid-3: Pollution is growing steadily around major urban centers. In 2021, Dr. Ivan Spasojević from the University of Belgrade launched Liquid-3 on the market, a 600-L tank filled with microalgae design to remove CO2. The tank effect is able to replace two 10-year-old trees or 200 square meters of lawn (https://liquid3.rs/, accessed on 19 July 2023).
7. Conclusions
Doomsday scenarios have been a constant concern throughout the second half of the XX century and the beginning of the XXI. Thus, the “unsustainable” worldwide overcrowding in 60 s that could compromise food supplies (±3.0 billion people in 1960 compared to ±7.9 billion today) or the nuclear arms race of the Cold War between the United States and the Soviet Union, which kept the Doomsday Clock a few minutes before the midnight for several years (nowadays, it is 90 s to midnight (https://thebulletin.org/doomsday-clock/, accessed on 19 July 2023), are two examples of theoretical, but fortunately non-fulfilled, risks for the humankind. Currently, climate change is a highly relevant concern at different levels all around the world that could be presented as the next doomsday scenario due to social controversy, which also affects the scientific community, with both pros and cons to climate change. However, what is sure is that microorganisms are rarely the focus of the analyses. They have not been considered in policy development, probably because of their immense diversity and varied responses to environmental changes, which make it a challenge to determine their role in the ecosystem. Nevertheless, based on scientific evidence, as it has been reviewed throughout this manuscript, numerous alarms have been raised in recent years due to the rise in the appearance and spread of several diseases throughout the world. Several pathogenic fungi and bacteria have already extended their geographical area of influence northward and are advancing at a great speed through Europe and North America, with the immense economic losses that this entails. These pathogens not only affect the three most important food production sectors—agriculture, livestock and fishing—but human diseases (especially tropical) are also targeted, and the appearance of new ones (zoonoses) is relevant. The mitigation of these illnesses is complicated since they are climate-conditioned, and the appearance of new antibiotic resistance further complicates the resolution.
Nowadays, microorganisms can be used to bring some light to climate change concerns, and they should play a more relevant role. On the one hand, they can be used as reporters of the weather variations tracing the appearance, evolution or impact of non-common diseases at novel locations. On the other hand, their ability to decrease greenhouse gases, due to their involvement in the carbon (fixing atmospheric CO2) and nitrogen cycles, is also remarkable and should be valued at its full potential. In addition, microbial involvement in the worldwide degradative tasks of recalcitrant compounds also presents them as relevant players in climate change mitigation.
The evolution of climate change, as well as the truthfulness and accuracy of the model predictions, highly condition the future results of climate variations and the role of microorganisms. However, although the hands of the Doomsday Clock point to midnight, the microorganisms will continue living here.
Author Contributions
Conceptualization and writing (original draft preparation, review and editing), A.I., S.G.-C. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
Ana Ibañez (A.I.) is funded by a “Margarita Salas” modality postdoctoral grant (Reference no.: UP2021-025) through the University of León awarded by the Spanish Ministry of Universities within the Recovery, Transformation and Resilience Plan (Modernization and digitalization of the Educational System), with funding from the European Recovery Instrument European Union-NextGenerationEU.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Special thanks to (i) the ESTELLA project (“DESign of bio-based Thermoset polymer with recycling capability by dynamic bonds for bio-composite manufacturing”) (Project no.: 101058371) funded by the European Union through the Horizon Europe Framework Programme (call: HORIZON-CL4-2021-RESILIENCE-01-11) and (ii) the BioPAC project (Development of bioactive and lifespan-controlled bioplastics) (Ref. no. TED2021-131864B-C21) funded by the MCIN (Ministerio de Ciencia e Innovación)/AEI (Agencia Estatal de Investigación)/10.13039/501100011033 (Digital Object Identifier) and the European Union “NextGenerationEU”/PRTR (Recovery, Transformation and Resilience Plan).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sobel, A.H.; Tippett, M.K. Extreme Events: Trends and Risk Assessment Methodologies. In Resilience: The Science of Adaptation to Climate Change; Zommers, Z., Alverson, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–12. ISBN 9780128118917. [Google Scholar]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Buis, A. Flip Flop: Why Variations in Earth’s Magnetic Field Aren’t Causing Today’s Climate Change. Available online: https://climate.nasa.gov/explore/ask-nasa-climate/3104/flip-flop-why-variations-in-earths-magnetic-field-arent-causing-todays-climate-change/#:~:text=AirIsn’tFerrous,%3Aairisn’tferrous (accessed on 19 July 2023).
- Mikhaylov, A.; Moiseev, N.; Aleshin, K.; Burkhardt, T. Global climate change and greenhouse effect. Entrep. Sustain. Issues 2020, 7, 2897–2913. [Google Scholar] [CrossRef] [PubMed]
- Fourier, J. Remarques générales sur les températures du globe terrestre et des espaces planétaires. Ann. Chim. Phys. 1824, 27, 136–167. [Google Scholar]
- Broecker, W.S. Climatic Change: Are We on the Brink of a Pronounced Global Warming? Science 1975, 189, 460–463. [Google Scholar] [CrossRef]
- Knoll, A.H. Paleobiological Perspectives on Early Microbial Evolution. Cold Spring Harb. Perspect. Biol. 2015, 7, a018093. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, S. Soil Biodiversity and Climate Change. Adv. Plants Agric. Res. 2016, 3, 166–168. [Google Scholar] [CrossRef][Green Version]
- Griffith, G.W. Do we need a global strategy for microbial conservation? Trends Ecol. Evol. 2012, 27, 1–2. [Google Scholar] [CrossRef]
- Banerjee, A.; Cornejo, J.; Bandopadhyay, R. Emergent climate change impact throughout the world: Call for “Microbiome Conservation” before it’s too late. Biodivers. Conserv. 2020, 29, 345–348. [Google Scholar] [CrossRef]
- Jain, P.K.; Purkayastha, S.D.; De Mandal, S.; Passari, A.K.; Govindarajan, R.K. Effect of climate change on microbial diversity and its functional attributes. In Recent Advancements in Microbial Diversity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 315–351. [Google Scholar]
- Guo, X.; Zhou, X.; Hale, L.; Yuan, M.; Ning, D.; Feng, J.; Shi, Z.; Li, Z.; Feng, B.; Gao, Q.; et al. Climate warming accelerates temporal scaling of grassland soil microbial biodiversity. Nat. Ecol. Evol. 2019, 3, 612–619. [Google Scholar] [CrossRef]
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Change 2018, 8, 813–818. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature mediates continental-scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Singh, V.K.; Shukla, A.K.; Singh, A.K. Impact of Climate Change on Plant–Microbe Interactions under Agroecosystems. In Climate Change and Agricultural Ecosystems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–179. [Google Scholar]
- Sharma, B.; Singh, B.N.; Dwivedi, P.; Rajawat, M.V.S. Interference of Climate Change on Plant-Microbe Interaction: Present and Future Prospects. Front. Agron. 2022, 3, 113. [Google Scholar] [CrossRef]
- Zhang, C.; Xi, N. Precipitation Changes Regulate Plant and Soil Microbial Biomass Via Plasticity in Plant Biomass Allocation in Grasslands: A Meta-Analysis. Front. Plant Sci. 2021, 12, 614968. [Google Scholar] [CrossRef]
- Vicuña, R.; González, B. The microbial world in a changing environment. Rev. Chil. Hist. Nat. 2021, 94, 2. [Google Scholar] [CrossRef]
- Fleming, A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Bruns, M.A.; Casadevall, A.; Criddle, C.S.; Eloe-Fadrosh, E.; Karl, D.M.; Nguyen, N.K.; Zhou, J. Microbes and Climate Change: A Research Prospectus for the Future. mBio 2022, 13, e00800-22. [Google Scholar] [CrossRef]
- Masood, F.; Ahmad, S.; Malik, A. Role of Methanotrophs in Mitigating Global Warming. In Microbiomes and the Global Climate Change; Springer: Singapore, 2021; pp. 43–60. [Google Scholar]
- Mangodo, C.; Adeyemi, T.O.A.; Bakpolor, V.R.; Adegboyega, D.A. Impact of microorganisms on climate change: A review. World News Nat. Sci. 2020, 31, 36–47. [Google Scholar]
- FAO. Statistical Yearbook 2021: World Food and Agriculture; FAO (Food and Agriculture Organization): Rome, Italy, 2021. [Google Scholar]
- Mangalassery, S.; Sjögersten, S.; Sparkes, D.L.; Sturrock, C.J.; Craigon, J.; Mooney, S.J. To what extent can zero tillage lead to a reduction in greenhouse gas emissions from temperate soils? Sci. Rep. 2015, 4, 4586. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasites Vectors 2016, 9, 336. [Google Scholar] [CrossRef]
- Kannojia, P.; Sharma, P.K.; Sharma, K. Climate Change and Soil Dynamics: Effects on Soil Microbes and Fertility of Soil. In Climate Change and Agricultural Ecosystems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–64. [Google Scholar]
- Mekala, S.; Polepongu, S. Impact of Climate Change on Soil Microbial Community. In Plant Biotic Interactions; Springer International Publishing: Cham, Switzerland, 2019; pp. 31–41. ISBN 978-3-030-26657-8. [Google Scholar]
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Wardle, D.A.; van der Putten, W.H. Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci. Adv. 2019, 5, eaaz1834. [Google Scholar] [CrossRef] [PubMed]
- Sheik, C.S.; Beasley, W.H.; Elshahed, M.S.; Zhou, X.; Luo, Y.; Krumholz, L.R. Effect of warming and drought on grassland microbial communities. ISME J. 2011, 5, 1692–1700. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: What lies ahead? Ecosphere 2015, 6, art130. [Google Scholar] [CrossRef]
- Lobell, D.B.; Asseng, S. Comparing estimates of climate change impacts from process-based and statistical crop models. Environ. Res. Lett. 2017, 12, 015001. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat. Clim. Change 2016, 6, 1130–1136. [Google Scholar] [CrossRef]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Matiu, M.; Ankerst, D.P.; Menzel, A. Interactions between temperature and drought in global and regional crop yield variability during 1961–2014. PLoS ONE 2017, 12, e0178339. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Chen, M. Economic impacts of climate change on agriculture: The importance of additional climatic variables other than temperature and precipitation. J. Environ. Econ. Manag. 2017, 83, 8–31. [Google Scholar] [CrossRef]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Change 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Change 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe. Theor. Appl. Genet. 2021, 134, 1771–1785. [Google Scholar] [CrossRef]
- FAO. Plant Pests and Diseases in the Context of Climate Change and Climate Variability: Food Security and Biodiversity Risks; FAO (Food and Agriculture Organization): Budapest, Hungary, 2019; Volume 41. [Google Scholar]
- Chaloner, T.M.; Gurr, S.J.; Bebber, D.P. Plant pathogen infection risk tracks global crop yields under climate change. Nat. Clim. Change 2021, 11, 710–715. [Google Scholar] [CrossRef]
- Bregaglio, S.; Donatelli, M.; Confalonieri, R. Fungal infections of rice, wheat, and grape in Europe in 2030–2050. Agron. Sustain. Dev. 2013, 33, 767–776. [Google Scholar] [CrossRef]
- Bhattacharya, S. Deadly new wheat disease threatens Europe’s crops. Nature 2017, 542, 145–146. [Google Scholar] [CrossRef]
- Gilardi, G.; Gullino, M.L.; Garibaldi, A. Emerging foliar and soil-borne pathogens of leafy vegetable crops: A possible threat to Europe. EPPO Bull. 2018, 48, 116–127. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by species of Diaporthe. IMA Fungus 2017, 8, 317–334. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Childers, A.K.; Chen, Y.; Madella, S.; Nessa, A.; VanEngelsdorp, D.; Evans, J.D. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Sci. Rep. 2017, 7, 17447. [Google Scholar] [CrossRef]
- Wallen-Russell, C.; Wallen-Russell, S. Topical Probiotics Do Not Satisfy New Criteria for Effective Use Due to Insufficient Skin Microbiome Knowledge. Cosmetics 2021, 8, 90. [Google Scholar] [CrossRef]
- Europe’s Changing Woods and Forests: From Wildwood to Managed Landscapes; Kirby, K.J., Watkins, C., Eds.; CABI: Wallingford, UK, 2015; ISBN 9781780643373. [Google Scholar]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.I.; Zubair, M.; Iqbal, M. Biocontrol of Bacterial Leaf Blight of Rice and Profiling of Secondary Metabolites Produced by Rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar] [CrossRef]
- Banerjee, A.; Roy, S.; Bag, M.K.; Bhagat, S.; Kar, M.K.; Mandal, N.P.; Mukherjee, A.K.; Maiti, D. A survey of bacterial blight (Xanthomonas oryzae pv. oryzae) resistance in rice germplasm from eastern and northeastern India using molecular markers. Crop Prot. 2018, 112, 168–176. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; He, S.; Liu, Y.; Wang, M.; Jiang, G. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China. PLoS ONE 2018, 13, e0192153. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, K.; Łochyńska, M. Identification and characterization of Pseudomonas syringae pv. mori affecting white mulberry (Morus alba) in Poland. Eur. J. Plant Pathol. 2020, 158, 281–291. [Google Scholar] [CrossRef]
- Park, R.F.; Burdon, J.J.; McIntosh, R.A. Studies on the origin, spread, and evolution of an important group of Puccinia recondita f. sp. tritici pathotypes in Australasia. Eur. J. Plant Pathol. 1995, 101, 613–622. [Google Scholar] [CrossRef]
- Peksa, K.; Bankina, B. Characterization of Puccinia recondita, the causal agent of brown rust: A review. Res. Rural Dev. 2019, 2, 70–76. [Google Scholar]
- Schwanck, A.A.; Meneses, P.R.; Farias, C.R.J.; Funck, G.R.D.; Maia, A.H.N.; Del Ponte, E.M. Bipolaris oryzae seed borne inoculum and brown spot epidemics in the subtropical lowland rice-growing region of Brazil. Eur. J. Plant Pathol. 2015, 142, 875–885. [Google Scholar] [CrossRef][Green Version]
- Rosslenbroich, H.-J.; Stuebler, D. Botrytis cinerea—History of chemical control and novel fungicides for its management. Crop Prot. 2000, 19, 557–561. [Google Scholar] [CrossRef]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security; FAO (Food and Agriculture Organization): Rome, Italy, 2021. [Google Scholar]
- Thompson, S.M.; Tan, Y.P.; Shivas, R.G.; Neate, S.M.; Morin, L.; Bissett, A.; Aitken, E.A.B. Green and brown bridges between weeds and crops reveal novel Diaporthe species in Australia. Persoonia Mol. Phylogeny Evol. Fungi 2015, 35, 39–49. [Google Scholar] [CrossRef]
- Rapicavoli, J.; Ingel, B.; Blanco-Ulate, B.; Cantu, D.; Roper, C. Xylella fastidiosa: An examination of a re-emerging plant pathogen. Mol. Plant Pathol. 2018, 19, 786–800. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.S.; Choi, Y.-J. Plasmopara viticola Causing Downy Mildew on Vitis davidii in Korea. Plant Dis. 2021, 105, 3309. [Google Scholar] [CrossRef]
- Williams, M.G.; Magarey, P.A.; Sivasithamparam, K. Effect of temperature and light intensity on early infection behaviour of a Western Australian isolate of Plasmopara viticola, the downy mildew pathogen of grapevine. Australas. Plant Pathol. 2007, 36, 325. [Google Scholar] [CrossRef]
- Thines, M. Recent outbreaks of downy mildew on grape ivy (Parthenocissus tricuspidata, Vitaceae) in Germany are caused by a new species of Plasmopara. Mycol. Prog. 2011, 10, 415–422. [Google Scholar] [CrossRef]
- Sharma, M.; Ghosh, R.; Pande, S. Dry root rot (Rhizoctonia bataticola (Taub.) Butler): An emerging disease of chickpea—Where do we stand? Arch. Phytopathol. Plant Prot. 2015, 48, 797–812. [Google Scholar] [CrossRef]
- Hantula, J.; Vainio, E.J. Viruses as components of forest microbiome. In Forest Microbiology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 371–382. [Google Scholar]
- Gaganidze, D.; Sadunishvili, T.; Aznarashvili, M.; Abashidze, E.; Gurielidze, M.; Carnal, S.; Rezzonico, F.; Zubadalashvili, M. Fire blight distribution in Georgia and characterization of selected Erwinia amylovora isolates. J. Plant Pathol. 2021, 103, 121–129. [Google Scholar] [CrossRef]
- Singh, P.K.; Gahtyari, N.C.; Roy, C.; Roy, K.K.; He, X.; Tembo, B.; Xu, K.; Juliana, P.; Sonder, K.; Kabir, M.R.; et al. Wheat Blast: A Disease Spreading by Intercontinental Jumps and Its Management Strategies. Front. Plant Sci. 2021, 12, 1467. [Google Scholar] [CrossRef]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; Milonas, P.; et al. Risk assessment of the entry of Pantoea stewartii subsp. stewartii on maize seed imported by the EU from the USA. EFSA J. 2019, 17, e05851. [Google Scholar] [CrossRef]
- Abidin, N.; Ismail, S.I.; Vadamalai, G.; Yusof, M.T.; Hakiman, M.; Karam, D.S.; Ismail-Suhaimy, N.W.; Ibrahim, R.; Zulperi, D. Genetic diversity of Pantoea stewartii subspecies stewartii causing jackfruit-bronzing disease in Malaysia. PLoS ONE 2020, 15, e0234350. [Google Scholar] [CrossRef]
- Ali, S.; Gladieux, P.; Leconte, M.; Gautier, A.; Justesen, A.F.; Hovmøller, M.S.; Enjalbert, J.; de Vallavieille-Pope, C. Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen Puccinia striiformis f.sp. tritici. PLoS Pathog. 2014, 10, e1003903. [Google Scholar] [CrossRef]
- McDonald, B.A.; Stukenbrock, E.H. Rapid emergence of pathogens in agro-ecosystems: Global threats to agricultural sustainability and food security. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160026. [Google Scholar] [CrossRef]
- DeAngelis, K.M.; Pold, G.; Topçuoğlu, B.D.; van Diepen, L.T.A.; Varney, R.M.; Blanchard, J.L.; Melillo, J.; Frey, S.D. Long-term forest soil warming alters microbial communities in temperate forest soils. Front. Microbiol. 2015, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Karnosky, D.F. Dutch Elm Disease: A Review of the History, Environmental Implications, Control, and Research Needs. Environ. Conserv. 1979, 6, 311–322. [Google Scholar] [CrossRef]
- Sukumar Chakraborty, S.C. Impacts of global change on diseases of agricultural crops and forest trees. CABI Rev. 2008, 3, 1–15. [Google Scholar] [CrossRef]
- Ali, M.Z.; Carlile, G.; Giasuddin, M. Impact of global climate change on livestock health: Bangladesh perspective. Open Vet. J. 2020, 10, 178–188. [Google Scholar] [CrossRef]
- FAO. Meat Market Review; FAO (Food and Agriculture Organization): Rome, Italy, 2019. [Google Scholar]
- Bett, B.; Kiunga, P.; Gachohi, J.; Sindato, C.; Mbotha, D.; Robinson, T.; Lindahl, J.; Grace, D. Effects of climate change on the occurrence and distribution of livestock diseases. Prev. Vet. Med. 2017, 137, 119–129. [Google Scholar] [CrossRef]
- Voyiatzaki, C.; Papailia, S.I.; Venetikou, M.S.; Pouris, J.; Tsoumani, M.E.; Papageorgiou, E.G. Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. Int. J. Environ. Res. Public Health 2022, 19, 6516. [Google Scholar] [CrossRef]
- Gale, P.; Drew, T.; Phipps, L.P.; David, G.; Wooldridge, M. The effect of climate change on the occurrence and prevalence of livestock diseases in Great Britain: A review. J. Appl. Microbiol. 2009, 106, 1409–1423. [Google Scholar] [CrossRef]
- Woldehiwet, Z. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef]
- Uminski, K.; Kadkhoda, K.; Houston, B.L.; Lopez, A.; MacKenzie, L.J.; Lindsay, R.; Walkty, A.; Embil, J.; Zarychanski, R. Anaplasmosis: An emerging tick-borne disease of importance in Canada. IDCases 2018, 14, e00472. [Google Scholar] [CrossRef]
- Gray, J.S.; Ogden, N.H. Ticks, Human Babesiosis and Climate Change. Pathogens 2021, 10, 1430. [Google Scholar] [CrossRef]
- Marques, R.; Krüger, R.F.; Peterson, A.T.; de Melo, L.F.; Vicenzi, N.; Jiménez-García, D. Climate change implications for the distribution of the babesiosis and anaplasmosis tick vector, Rhipicephalus (Boophilus) microplus. Vet. Res. 2020, 51, 81. [Google Scholar] [CrossRef]
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2669–2681. [Google Scholar] [CrossRef]
- Hughes, H.R.; Velez, J.O.; Fitzpatrick, K.; Davis, E.H.; Russell, B.J.; Lambert, A.J.; Staples, J.E.; Brault, A.C. Genomic Evaluation of the Genus Coltivirus Indicates Genetic Diversity among Colorado Tick Fever Virus Strains and Demarcation of a New Species. Diseases 2021, 9, 92. [Google Scholar] [CrossRef]
- Viral CNS Infections. In Hunter’s Tropical Medicine and Emerging Infectious Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 344–379.
- Paddock, C.D.; Childs, J.E. Ehrlichia chaffeensis: A Prototypical Emerging Pathogen. Clin. Microbiol. Rev. 2003, 16, 37–64. [Google Scholar] [CrossRef]
- Marcdante, K.J.; Kliegman, R.M. Zoonoses and Vector Borne Infections. In Nelson Essentials of Pediatrics; Marcdante, K.J., Kliegman, R.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 439–447. ISBN 0323511457. [Google Scholar]
- Adler, B. History of leptospirosis and leptospira. Curr. Top. Microbiol. Immunol. 2015, 387, 1–9. [Google Scholar] [CrossRef]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef]
- Howard, S.; Ginsberg, P.; Jannelle Couret, P.M.; Jason Garrett, B.M.; Thomas, N.; Mather, P.; Roger, A.; Lebrun, P. Potential effects of climate change on tick-borne diseases in Rhode Island. Rhode Isl. Med. J. 2021, 104, 29–33. [Google Scholar]
- Steinbrink, A.; Brugger, K.; Margos, G.; Kraiczy, P.; Klimpel, S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 2022, 121, 781–803. [Google Scholar] [CrossRef]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Effect of Climate Change on Lyme Disease Risk in North America. Ecohealth 2005, 2, 38–46. [Google Scholar] [CrossRef]
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.; Lindsay, L. Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019, 45, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, B. Dutch Q Fever Epidemic in a ‘One Health’ Context: Outbreaks, Seroprevalence and Occupational Risks. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 2018. [Google Scholar]
- Alkishe, A.; Peterson, A.T. Climate change influences on the geographic distributional potential of the spotted fever vectors Amblyomma maculatum and Dermacentor andersoni. PeerJ 2022, 10, e13279. [Google Scholar] [CrossRef] [PubMed]
- Guzman, N.; Yarrarapu, S.N.S.; Beidas, S.O. Anaplasma Phagocytophilum; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zinck, C.B.; Lloyd, V.K. Borrelia burgdorferi and Borrelia miyamotoi in Atlantic Canadian wildlife. PLoS ONE 2022, 17, e0262229. [Google Scholar] [CrossRef]
- Cameron, B.; Bierl, C.; Davenport, T.; Vollmer-Conna, U.; Hickie, I.; Wakefield, D.; Lloyd, A. Drought and Q fever: The association between trends in the incidence of infection and rainfall in rural Australia. In The Principles and Practice of Q Fever: The One Health Paradigm; Caetano Simões, J.C., Ferreira Anastácio, S., Jorge da Silva, G., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2017; ISBN 978-1-53610-851-4. [Google Scholar]
- Tan, T.S.; Hernandez-Jover, M.; Hayes, L.M.; Wiethoelter, A.K.; Firestone, S.M.; Stevenson, M.A.; Heller, J. Identifying scenarios and risk factors for Q fever outbreaks using qualitative analysis of expert opinion. Zoonoses Public Health 2022, 69, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, K.; MacLachlan, N.J.; Frank, L.G.; Sawyer, M.; Osburn, B.I.; Mills, M.G.L.; Lerche, N.W.; Laurenson, M.K.; McNutt, J.W.; Alexander, K.A.; et al. Evidence of Natural Bluetongue Virus Infection among African Carnivores. Am. J. Trop. Med. Hyg. 1994, 51, 568–576. [Google Scholar] [CrossRef]
- More, S.; Bicout, D.; Bøtner, A.; Butterworth, A.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Gortázar Schmidt, C.; Michel, V.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Bluetongue. EFSA J. 2017, 15, e04957. [Google Scholar] [CrossRef]
- Flahault, A.; de Castaneda, R.R.; Bolon, I. Climate change and infectious diseases. Public Health Rev. 2016, 37, 21. [Google Scholar] [CrossRef]
- Le Conte, Y.; Navajas, M. Climate change: Impact on honey bee populations and diseases. Rev. Sci. Tech. 2008, 27, 499–510. [Google Scholar]
- Etxegarai-Legarreta, O.; Sanchez-Famoso, V. The Role of Beekeeping in the Generation of Goods and Services: The Interrelation between Environmental, Socioeconomic, and Sociocultural Utilities. Agriculture 2022, 12, 551. [Google Scholar] [CrossRef]
- Giliba, R.A.; Mpinga, I.H.; Ndimuligo, S.A.; Mpanda, M.M. Changing climate patterns risk the spread of Varroa destructor infestation of African honey bees in Tanzania. Ecol. Process. 2020, 9, 48. [Google Scholar] [CrossRef]
- Rowland, B.W.; Rushton, S.P.; Shirley, M.D.F.; Brown, M.A.; Budge, G.E. Identifying the climatic drivers of honey bee disease in England and Wales. Sci. Rep. 2021, 11, 21953. [Google Scholar] [CrossRef]
- Veen, N.; Yadav, A.S. Seasonal Incidence of European Foul Brood and Sac Brood Disease in Apis mellifera L. and their Correlation with Colony and Weather Parameters. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 13–24. [Google Scholar] [CrossRef]
- Budge, G.E.; Simcock, N.K.; Holder, P.J.; Shirley, M.D.F.; Brown, M.A.; Van Weymers, P.S.M.; Evans, D.J.; Rushton, S.P. Chronic bee paralysis as a serious emerging threat to honey bees. Nat. Commun. 2020, 11, 2164. [Google Scholar] [CrossRef]
- Stephan, J.G.; de Miranda, J.R.; Forsgren, E. American foulbrood in a honeybee colony: Spore-symptom relationship and feedbacks between disease and colony development. BMC Ecol. 2020, 20, 16. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO (Food and Agriculture Organization): Budapest, Hungary, 2020. [Google Scholar]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef]
- Lafferty, K.D. Marine Infectious Disease Ecology. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 473–496. [Google Scholar] [CrossRef]
- Hillebrand, H.; Brey, T.; Gutt, J.; Hagen, W.; Metfies, K.; Meyer, B.; Lewandowska, A. Climate Change: Warming Impacts on Marine Biodiversity. In Handbook on Marine Environment Protection; Springer International Publishing: Cham, Swizterland, 2018; pp. 353–373. [Google Scholar]
- Canonico, G.; Buttigieg, P.L.; Montes, E.; Muller-Karger, F.E.; Stepien, C.; Wright, D.; Benson, A.; Helmuth, B.; Costello, M.; Sousa-Pinto, I.; et al. Global Observational Needs and Resources for Marine Biodiversity. Front. Mar. Sci. 2019, 6, 367. [Google Scholar] [CrossRef]
- Bunse, C.; Lundin, D.; Karlsson, C.M.G.; Akram, N.; Vila-Costa, M.; Palovaara, J.; Svensson, L.; Holmfeldt, K.; González, J.M.; Calvo, E.; et al. Response of marine bacterioplankton pH homeostasis gene expression to elevated CO2. Nat. Clim. Change 2016, 6, 483–487. [Google Scholar] [CrossRef]
- Kling, J.D.; Lee, M.D.; Fu, F.; Phan, M.D.; Wang, X.; Qu, P.; Hutchins, D.A. Transient exposure to novel high temperatures reshapes coastal phytoplankton communities. ISME J. 2020, 14, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tsementzi, D.; Williams, T.C.; Juarez, D.L.; Blinebry, S.K.; Garcia, N.S.; Sienkiewicz, B.K.; Konstantinidis, K.T.; Johnson, Z.I.; Hunt, D.E. Environmental stability impacts the differential sensitivity of marine microbiomes to increases in temperature and acidity. ISME J. 2021, 15, 19–28. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Fu, F. Microorganisms and ocean global change. Nat. Microbiol. 2017, 2, 17058. [Google Scholar] [CrossRef]
- Abirami, B.; Radhakrishnan, M.; Kumaran, S.; Wilson, A. Impacts of global warming on marine microbial communities. Sci. Total Environ. 2021, 791, 147905. [Google Scholar] [CrossRef]
- Cohen, R.; James, C.; Lee, A.; Martinelli, M.; Muraoka, W.; Ortega, M.; Sadowski, R.; Starkey, L.; Szesciorka, A.; Timko, S.; et al. Marine Host-Pathogen Dynamics: Influences of Global Climate Change. Oceanography 2018, 31, 182–193. [Google Scholar] [CrossRef]
- Ben-Haim, Y.; Zicherman-Keren, M.; Rosenberg, E. Temperature-Regulated Bleaching and Lysis of the Coral Pocillopora damicornis by the Novel Pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 2003, 69, 4236–4242. [Google Scholar] [CrossRef]
- Malek, J.C.; Byers, J.E. Responses of an oyster host (Crassostrea virginica) and its protozoan parasite (Perkinsus marinus) to increasing air temperature. PeerJ 2018, 6, e5046. [Google Scholar] [CrossRef]
- Marcos-López, M.; Gale, P.; Oidtmann, B.C.; Peeler, E.J. Assessing the Impact of Climate Change on Disease Emergence in Freshwater Fish in the United Kingdom. Transbound. Emerg. Dis. 2010, 57, 293–304. [Google Scholar] [CrossRef]
- De Souza Valente, C.; Wan, A.H.L. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef]
- Firmino, J.; Furones, M.D.; Andree, K.B.; Sarasquete, C.; Ortiz-Delgado, J.B.; Asencio-Alcudia, G.; Gisbert, E. Contrasting outcomes of Vibrio harveyi pathogenicity in gilthead seabream, Sparus aurata and European seabass, Dicentrachus labrax. Aquaculture 2019, 511, 734210. [Google Scholar] [CrossRef]
- Li, X.; Yang, B.; Shi, C.; Wang, H.; Yu, R.; Li, Q.; Liu, S. Synergistic Interaction of Low Salinity Stress With Vibrio Infection Causes Mass Mortalities in the Oyster by Inducing Host Microflora Imbalance and Immune Dysregulation. Front. Immunol. 2022, 13, 859975. [Google Scholar] [CrossRef]
- Dorfmeier, E.M. Ocean Acidification and Disease: How Will a Changing Climate Impact Vibrio tubiashii Growth and Pathogenicity to Pacific Oyster Larvae? Master’s Thesis, University of Washington, Seattle, WA, USA, 2012. [Google Scholar]
- Bartlow, A.W.; Manore, C.; Xu, C.; Kaufeld, K.A.; Del Valle, S.; Ziemann, A.; Fairchild, G.; Fair, J.M. Forecasting Zoonotic Infectious Disease Response to Climate Change: Mosquito Vectors and a Changing Environment. Vet. Sci. 2019, 6, 40. [Google Scholar] [CrossRef]
- Raffel, T.R.; Romansic, J.M.; Halstead, N.T.; McMahon, T.A.; Venesky, M.D.; Rohr, J.R. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 2013, 3, 146–151. [Google Scholar] [CrossRef]
- Tong, S.; Ebi, K.; Olsen, J. Infectious disease, the climate, and the future. Environ. Epidemiol. 2021, 5, e133. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B. Epidemics on the move: Climate change and infectious disease. PLoS Biol. 2020, 18, e3001013. [Google Scholar] [CrossRef] [PubMed]
- Duchenne-Moutien, R.A.; Neetoo, H. Climate Change and Emerging Food Safety Issues: A Review. J. Food Prot. 2021, 84, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio 2019, 10, e01397-19. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I. The effect of global warming on infectious diseases. Osong Public Health Res. Perspect. 2010, 1, 4–9. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Coates, S.J.; Norton, S.A. The effects of climate change on infectious diseases with cutaneous manifestations. Int. J. Women’s Dermatol. 2021, 7, 8–16. [Google Scholar] [CrossRef]
- Semenza, J.C.; Paz, S. Climate change and infectious disease in Europe: Impact, projection and adaptation. Lancet Reg. Health Eur. 2021, 9, 100230. [Google Scholar] [CrossRef]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef]
- The Lancet Microbe. Climate change: Fires, floods, and infectious diseases. Lancet Microbe 2021, 2, e415. [Google Scholar] [CrossRef]
- Fouladkhah, A. Changing climate: A ‘threat multiplier’ for foodborne and waterborne infectious diseases and antibiotic resistance. Res. Outreach 2020, 114, 130–133. [Google Scholar] [CrossRef]
- Williams, P.C.M.; Bartlett, A.W.; Howard-Jones, A.; McMullan, B.; Khatami, A.; Britton, P.N.; Marais, B.J. Impact of climate change and biodiversity collapse on the global emergence and spread of infectious diseases. J. Paediatr. Child Health 2021, 57, 1811–1818. [Google Scholar] [CrossRef]
- Koch, L.K.; Kochmann, J.; Klimpel, S.; Cunze, S. Modeling the climatic suitability of leishmaniasis vector species in Europe. Sci. Rep. 2017, 7, 13325. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Ashford, R.W. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 2000, 30, 1269–1281. [Google Scholar] [CrossRef]
- Hotez, P.J.; Bottazzi, M.E.; Franco-Paredes, C.; Ault, S.K.; Periago, M.R. The Neglected Tropical Diseases of Latin America and the Caribbean: A Review of Disease Burden and Distribution and a Roadmap for Control and Elimination. PLoS Negl. Trop. Dis. 2008, 2, e300. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M. den Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Impactos del Cambio Climático en la Salud; Ministerio De Sanidad Igualdad y Servicios: Madrid, Spain, 2013.
- Pozio, E. How globalization and climate change could affect foodborne parasites. Exp. Parasitol. 2020, 208, 107807. [Google Scholar] [CrossRef]
- Kuhn, K.G.; Nygård, K.M.; Guzman-Herrador, B.; Sunde, L.S.; Rimhanen-Finne, R.; Trönnberg, L.; Jepsen, M.R.; Ruuhela, R.; Wong, W.K.; Ethelberg, S. Campylobacter infections expected to increase due to climate change in Northern Europe. Sci. Rep. 2020, 10, 13874. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Herbst, S.; Rechenburg, A.; Suk, J.E.; Höser, C.; Schreiber, C.; Kistemann, T. Climate Change Impact Assessment of Food and Waterborne Diseases. Crit. Rev. Environ. Sci. Technol. 2012, 42, 857–890. [Google Scholar] [CrossRef]
- Morgado, M.E.; Jiang, C.; Zambrana, J.; Upperman, C.R.; Mitchell, C.; Boyle, M.; Sapkota, A.R.; Sapkota, A. Climate change, extreme events, and increased risk of salmonellosis: Foodborne diseases active surveillance network (FoodNet), 2004–2014. Environ. Health 2021, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C. Cascading risks of waterborne diseases from climate change. Nat. Immunol. 2020, 21, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Chala, B.; Hamde, F. Emerging and Re-emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef]
- Trærup, S.L.M.; Ortiz, R.A.; Markandya, A. The costs of climate change: A study of cholera in Tanzania. Int. J. Environ. Res. Public Health 2011, 8, 4386–4405. [Google Scholar] [CrossRef]
- Riebesell, U.; Aberle-Malzahn, N.; Achterberg, E.P.; Algueró-Muñiz, M.; Alvarez-Fernandez, S.; Arístegui, J.; Bach, L.T.; Boersma, M.; Boxhammer, T.; Guan, W.; et al. Toxic algal bloom induced by ocean acidification disrupts the pelagic food web. Nat. Clim. Change 2018, 8, 1082–1086. [Google Scholar] [CrossRef]
- Barreiro, C.; Barredo, J.L. Worldwide Clinical Demand for Antibiotics: Is It a Real Countdown? Methods Mol. Biol. 2021, 2296, 3–15. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of nations. Rev. Antimicrob. Resist. 2014, 1–18. [Google Scholar]
- IACG. No Time to Wait: Securing the Future from Drug-Resistant Infections; WHO (World Health Organization): New York, NY, USA, 2019. [Google Scholar]
- McGough, S.F.; MacFadden, D.R.; Hattab, M.W.; Mølbak, K.; Santillana, M. Rates of increase of antibiotic resistance and ambient temperature in Europe: A cross-national analysis of 28 countries between 2000 and 2016. Eurosurveillance 2020, 25, 1900414. [Google Scholar] [CrossRef]
- Barreiro, C.; Gutiérrez, S.; Olivera, E.R. Fungal Horizontal Gene Transfer: A History Beyond the Phylogenetic Kingdoms. In Horizontal Gene Transfer; Villa, T., Viñas, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 315–336. ISBN 978-3-030-21861-4. [Google Scholar]
- Souli, M.; Galani, I.; Giamarellou, H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Eurosurveillance 2008, 13, 19045. [Google Scholar] [CrossRef]
- Gifford, D.R.; Moss, E.; MacLean, R.C. Environmental variation alters the fitness effects of rifampicin resistance mutations in Pseudomonas aeruginosa. Evolution 2016, 70, 725–730. [Google Scholar] [CrossRef]
- Rodríguez-Verdugo, A.; Lozano-Huntelman, N.; Cruz-Loya, M.; Savage, V.; Yeh, P. Compounding Effects of Climate Warming and Antibiotic Resistance. iScience 2020, 23, 101024. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic Resistance Increases with Local Temperature. Nat. Clim. Change 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Burnham, J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021, 8, 2049936121991374. [Google Scholar] [CrossRef]
- Waits, A.; Emelyanova, A.; Oksanen, A.; Abass, K.; Rautio, A. Human infectious diseases and the changing climate in the Arctic. Environ. Int. 2018, 121, 703–713. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Rodríguez-Verdugo, A.; Gaut, B.S.; Tenaillon, O. Evolution of Escherichia coli rifampicin resistance in an antibiotic-free environment during thermal stress. BMC Evol. Biol. 2013, 13, 50. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Ibáñez, A.; Diez-Galán, A.; Cobos, R.; Calvo-Peña, C.; Barreiro, C.; Medina-Turienzo, J.; Sánchez-García, M.; Coque, J.J.R. Using Rhizosphere Phosphate Solubilizing Bacteria to Improve Barley (Hordeum vulgare) Plant Productivity. Microorganisms 2021, 9, 1619. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhou, Y.; Suenaga, T.; Oba, K.; Lu, J.; Wang, G.; Zhang, L.; Yoon, S.; Terada, A. Organic carbon determines nitrous oxide consumption activity of clade I and II nosZ bacteria: Genomic and biokinetic insights. Water Res. 2022, 209, 117910. [Google Scholar] [CrossRef] [PubMed]
- Bueno, E.; Mania, D.; Mesa, S.; Bedmar, E.J.; Frostegård, Å.; Bakken, L.R.; Delgado, M.J. Regulation of the Emissions of the Greenhouse Gas Nitrous Oxide by the Soybean Endosymbiont Bradyrhizobium diazoefficiens. Int. J. Mol. Sci. 2022, 23, 1486. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Cruz, S.; Vaksmaa, A.; Horn, M.A.; Niemann, H.; Pijuan, M.; Ho, A. Methanotrophs: Discoveries, Environmental Relevance, and a Perspective on Current and Future Applications. Front. Microbiol. 2021, 12, 678057. [Google Scholar] [CrossRef]
- Aimen, H.; Khan, A.S.; Kanwal, N. Methanotrophs: The Natural Way to Tackle Greenhouse Effect. J. Bioremediat. Biodegrad. 2018, 9, 432. [Google Scholar] [CrossRef]
- Gayathri, R.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Vodovnik, M.; Vijayalakshmi, S. A review on biological carbon sequestration: A sustainable solution for a cleaner air environment, less pollution and lower health risks. J. King Saud Univ. 2021, 33, 101282. [Google Scholar] [CrossRef]
- Paul, V.; Chandra Shekharaiah, P.S.; Kushwaha, S.; Sapre, A.; Dasgupta, S.; Sanyal, D. Role of Algae in CO2 Sequestration Addressing Climate Change: A Review. In Renewable Energy and Climate Change; Deb, D., Dixit, A., Chandra, L., Eds.; Springer: Singapore, 2020; pp. 257–265. [Google Scholar]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Wang, G.; Chai, K.; Wu, J.; Liu, F. Effect of Pseudomonas putida on the degradation of epoxy resin varnish coating in seawater. Int. Biodeterior. Biodegrad. 2016, 115, 156–163. [Google Scholar] [CrossRef]
- Sanluis-Verdes, A.; Colomer-Vidal, P.; Rodriguez-Ventura, F.; Bello-Villarino, M.; Spinola-Amilibia, M.; Ruiz-Lopez, E.; Illanes-Vicioso, R.; Castroviejo, P.; Cigliano, R.A.; Montoya, M.; et al. Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. Nat. Commun. 2022, 13, 5568. [Google Scholar] [CrossRef]
- Zeenat; Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Junaid, F.; Khawaja, L.A.; Ali, S. Single cell protein as a potential meat substitute: A review. World J. Pharm. Res. 2020, 9, 141–161. [Google Scholar]
- Gamarra-Castillo, O.; Echeverry-Montaña, N.; Marbello-Santrich, A.; Hernández-Carrión, M.; Restrepo, S. Meat Substitute Development from Fungal Protein (Aspergillus oryzae). Foods 2022, 11, 2940. [Google Scholar] [CrossRef]
- Yakimov, M.M.; Golyshin, P.N.; Lang, S.; Moore, E.R.B.; Abraham, W.-R.; Lünsdorf, H.; Timmis, K.N. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int. J. Syst. Evol. Microbiol. 1998, 48, 339–348. [Google Scholar] [CrossRef]
- Hazen, T.C.; Dubinsky, E.A.; DeSantis, T.Z.; Andersen, G.L.; Piceno, Y.M.; Singh, N.; Jansson, J.K.; Probst, A.; Borglin, S.E.; Fortney, J.L.; et al. Deep-Sea Oil Plume Enriches Indigenous Oil-Degrading Bacteria. Science 2010, 330, 204–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).