Clostridium butyricum Strain MIYAIRI 588 (CBM588) as a Precision Probiotic Therapy in the Ketogenic Diet: A Possible Application?
Abstract
1. Introduction
2. Gut Microbiota and Nutrition: An Ecosystem View
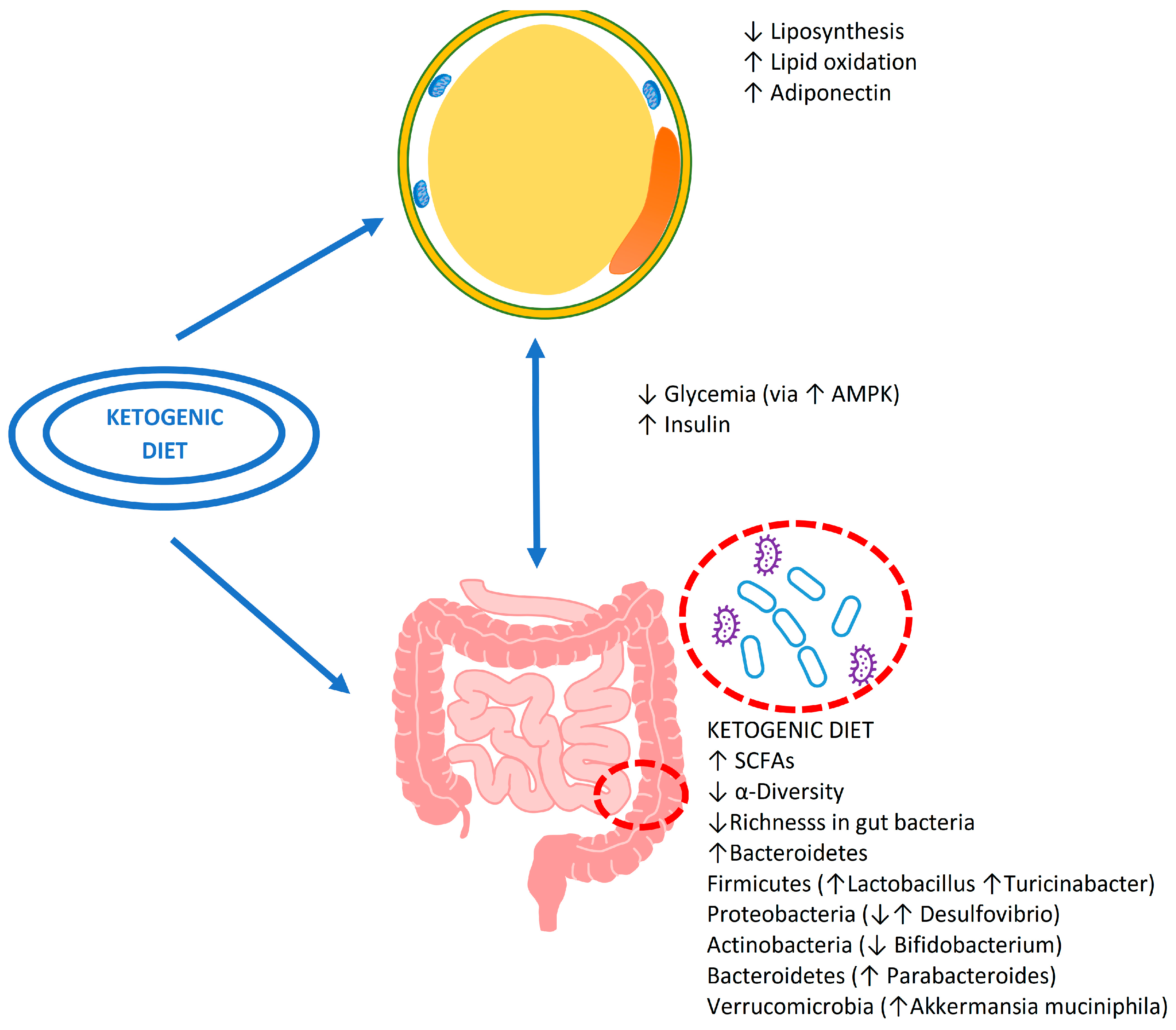
3. Ketogenic Diet and Microbiota
3.1. The Effect of Nutrients during the Ketogenic Diet
3.2. The Possible Consequences on Intestinal Health
4. Clostridium butyricum CBM588
5. Functional Characteristics of Clostridium butyricum CBM588
5.1. Effects of CBM588 on Digestive Disease Models
5.2. Accessory Metabolic Effects of Clostridium butyricum Potentially Functional to Diet Therapy
5.3. Clinical Evaluation of Clostridium butyricum CBM588
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooder, H.R. Epilepsy in Children: With Particular Reference to the Ketogenic Diet. Calif. West. Med. 1933, 39, 169–173. [Google Scholar]
- Pérez-Guisado, J. Ketogenic diets: Additional benefits to the weight loss and unfounded secondary effects. Arch. Latinoam. Nutr. 2008, 58, 323–329. [Google Scholar] [PubMed]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-Related Disorders: What Is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Bertuccioli, A.; Ninfali, P. The Mediterranean Diet in the Era of Globalization: The Need to Support Knowledge of Healthy Dietary Factors in the New Socio-Economical Framework. Mediterr. J. Nutr. Metab. 2014, 7, 75–86. [Google Scholar] [CrossRef]
- Veech, R.L. The Therapeutic Implications of Ketone Bodies: The Effects of Ketone Bodies in Pathological Conditions: Ketosis, Ketogenic Diet, Redox States, Insulin Resistance, and Mitochondrial Metabolism. Prostaglandins Leukot Essent Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Klepper, J. GLUT1 Deficiency Syndrome in Clinical Practice. Epilepsy Res. 2012, 100, 272–277. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-Term Impact of a Classical Ketogenic Diet on Gut Microbiota in GLUT1 Deficiency Syndrome: A 3-Month Prospective Observational Study. Clin. Nutr. ESPEN 2017, 17, 33–37. [Google Scholar] [CrossRef]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic Diet Enhances Neurovascular Function with Altered Gut Microbiome in Young Healthy Mice. Sci. Rep. 2018, 8, 6670. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Göktas, Ö.; Reißhauer, A.; Neuhaus, J.; Weylandt, K.-H.; Guschin, A.; Bock, M. Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Andoh, A. The gut microbiota is a new organ in our body. Nihon Shokakibyo Gakkai Zasshi 2015, 112, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Lundin, A.; Bok, C.M.; Aronsson, L.; Björkholm, B.; Gustafsson, J.-A.; Pott, S.; Arulampalam, V.; Hibberd, M.; Rafter, J.; Pettersson, S. Gut Flora, Toll-like Receptors and Nuclear Receptors: A Tripartite Communication That Tunes Innate Immunity in Large Intestine. Cell Microbiol. 2008, 10, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as Vitamin Suppliers to Their Host: A Gut Microbiota Perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Vrieze, A.; Holleman, F.; Zoetendal, E.G.; de Vos, W.M.; Hoekstra, J.B.L.; Nieuwdorp, M. The Environment within: How Gut Microbiota May Influence Metabolism and Body Composition. Diabetologia 2010, 53, 606–613. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The Role of GABA in the Pathophysiology and Treatment of Anxiety Disorders. Psychopharmacol. Bull. 2003, 37, 133–146. [Google Scholar]
- Cryan, J.F.; Kaupmann, K. Don’t Worry “B” Happy!: A Role for GABA(B) Receptors in Anxiety and Depression. Trends Pharmacol. Sci. 2005, 26, 36–43. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium Adolescentis as a Key Member of the Human Gut Microbiota in the Production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome Responses to an Uncontrolled Short-Term Diet Intervention in the Frame of the Citizen Science Project. Nutrients 2018, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Whitfield, J.B. Gamma Glutamyl Transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef]
- Pica, A.; Chi, M.-C.; Chen, Y.-Y.; d’Ischia, M.; Lin, L.-L.; Merlino, A. The Maturation Mechanism of γ-Glutamyl Transpeptidases: Insights from the Crystal Structure of a Precursor Mimic of the Enzyme from Bacillus Licheniformis and from Site-Directed Mutagenesis Studies. Biochim. Biophys. Acta 2016, 1864, 195–203. [Google Scholar] [CrossRef]
- Hertz, L. The Glutamate-Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front. Endocrinol. 2013, 4, 59. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic Diet Modifies the Gut Microbiota in a Murine Model of Autism Spectrum Disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, Q.; Qiu, C.-Z.; Dai, W.-K.; Wang, H.-P.; Li, Y.-H.; Liao, J.-X.; Lu, X.-G.; Lin, S.-F.; Ye, J.-H.; et al. Ketogenic Diet Poses a Significant Effect on Imbalanced Gut Microbiota in Infants with Refractory Epilepsy. World J. Gastroenterol. 2017, 23, 6164–6171. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered Gut Microbiome Composition in Children with Refractory Epilepsy after Ketogenic Diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10-/- Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Naso, M.; Perna, S.; Bazire, P.; Sajuox, I.; Maugeri, R.; Rigon, C. The Potential Roles of Very Low Calorie, Very Low Calorie Ketogenic Diets and Very Low Carbohydrate Diets on the Gut Microbiota Composition. Front. Endocrinol. 2021, 12, 662591. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human Colonic Microbiota Associated with Diet, Obesity and Weight Loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-Protein, Reduced-Carbohydrate Weight-Loss Diets Promote Metabolite Profiles Likely to Be Detrimental to Colonic Health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Alemán, J.O.; Bokulich, N.A.; Swann, J.R.; Walker, J.M.; De Rosa, J.C.; Battaglia, T.; Costabile, A.; Pechlivanis, A.; Liang, Y.; Breslow, J.L.; et al. Fecal Microbiota and Bile Acid Interactions with Systemic and Adipose Tissue Metabolism in Diet-Induced Weight Loss of Obese Postmenopausal Women. J. Transl. Med. 2018, 16, 244. [Google Scholar] [CrossRef]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary Fat, the Gut Microbiota, and Metabolic Health—A Systematic Review Conducted within the MyNewGut Project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of Dietary Fat on Gut Microbiota and Faecal Metabolites, and Their Relationship with Cardiometabolic Risk Factors: A 6-Month Randomised Controlled-Feeding Trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef]
- Nakatani, A.; Li, X.; Miyamoto, J.; Igarashi, M.; Watanabe, H.; Sutou, A.; Watanabe, K.; Motoyama, T.; Tachibana, N.; Kohno, M.; et al. Dietary Mung Bean Protein Reduces High-Fat Diet-Induced Weight Gain by Modulating Host Bile Acid Metabolism in a Gut Microbiota-Dependent Manner. Biochem. Biophys. Res. Commun. 2018, 501, 955–961. [Google Scholar] [CrossRef]
- Meddah, A.T.; Yazourh, A.; Desmet, I.; Risbourg, B.; Verstraete, W.; Romond, M.B. The Regulatory Effects of Whey Retentate from Bifidobacteria Fermented Milk on the Microbiota of the Simulator of the Human Intestinal Microbial Ecosystem (SHIME). J. Appl. Microbiol. 2001, 91, 1110–1117. [Google Scholar] [CrossRef]
- Świątecka, D.; Narbad, A.; Ridgway, K.P.; Kostyra, H. The Study on the Impact of Glycated Pea Proteins on Human Intestinal Bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The Colonic Microbiome and Epithelial Transcriptome Are Altered in Rats Fed a High-Protein Diet Compared with a Normal-Protein Diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Li, C.; Zhou, G. Meat, Dairy and Plant Proteins Alter Bacterial Composition of Rat Gut Bacteria. Sci. Rep. 2015, 5, 15220. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets with Whey, Vegetable, or Animal Protein in Patients with Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, dgaa336. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Pastor-Villaescusa, B.; Rueda-Robles, A.; Abadia-Molina, F.; Ruiz-Ojeda, F.J. Plausible Biological Interactions of Low- and Non-Calorie Sweeteners with the Intestinal Microbiota: An Update of Recent Studies. Nutrients 2020, 12, 1153. [Google Scholar] [CrossRef]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Wölwer-Rieck, U.; Jeppesen, P.B.; Rogers, P.J.; Rowland, I.; Mathews, R. Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhuge, A.; Wang, K.; Lv, L.; Bian, X.; Yang, L.; Xia, J.; Jiang, X.; Wu, W.; Wang, S.; et al. Ketogenic Diet Aggravates Colitis, Impairs Intestinal Barrier and Alters Gut Microbiota and Metabolism in DSS-Induced Mice. Food Funct. 2021, 12, 10210–10225. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-Producing Human Gut Symbiont, Clostridium Butyricum, and Its Role in Health and Disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Sutter, V.L.; Mathisen, G.E. Normal Indigenous Intestinal Flora. In Human Intestinal Microflora in Health and Disease; Academic Press: Cambridge, MA, USA, 1983; Chapter 1; pp. 3–31. ISBN 9780123412805. [Google Scholar] [CrossRef]
- Tipton, L.; Darcy, J.L.; Hynson, N.A. A Developing Symbiosis: Enabling Cross-Talk Between Ecologists and Microbiome Scientists. Front. Microbiol. 2019, 10, 292. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Seki, H.; Shiohara, M.; Matsumura, T.; Miyagawa, N.; Tanaka, M.; Komiyama, A.; Kurata, S. Prevention of Antibiotic-Associated Diarrhea in Children by Clostridium Butyricum MIYAIRI. Pediatr. Int. 2003, 45, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Miyarisan Pharmaceutical CO., LTD. Clostridium Butyricum MIYAIRI Strain. Available online: http://www.miyarisan.com/english_index.htm (accessed on 1 September 2020).
- Imase, K.; Takahashi, M.; Tanaka, A.; Tokunaga, K.; Sugano, H.; Tanaka, M.; Ishida, H.; Kamiya, S.; Takahashi, S. Efficacy of Clostridium Butyricum Preparation Concomitantly with Helicobacter Pylori Eradication Therapy in Relation to Changes in the Intestinal Microbiota. Microbiol. Immunol. 2008, 52, 156–161. [Google Scholar] [CrossRef]
- Isa, K.; Oka, K.; Beauchamp, N.; Sato, M.; Wada, K.; Ohtani, K.; Nakanishi, S.; McCartney, E.; Tanaka, M.; Shimizu, T.; et al. Safety Assessment of the Clostridium Butyricum MIYAIRI 588 ® Probiotic Strain Including Evaluation of Antimicrobial Sensitivity and Presence of Clostridium Toxin Genes in Vitro and Teratogenicity in Vivo. Hum. Exp. Toxicol. 2016, 35, 818–832. [Google Scholar] [CrossRef] [PubMed]
- The European Commission. Commission Implementing Decision of 11 December 2014 authorising the placing on the market of Clostridium butyricum (CBM 588) as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (notified under document C(2014) 9345). OJEU 2014, 57, 153. [Google Scholar]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Sato, R.; Tanaka, M. Intestinal Distribution and Intraluminal Localization of Orally Administered Clostridium Butyricum in Rats. Microbiol. Immunol. 1997, 41, 665–671. [Google Scholar] [CrossRef]
- Okamoto, T.; Sasaki, M.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T.; Kusunoki, M. Preventive Efficacy of Butyrate Enemas and Oral Administration of Clostridium Butyricum M588 in Dextran Sodium Sulfate-Induced Colitis in Rats. J. Gastroenterol. 2000, 35, 341–346. [Google Scholar] [CrossRef]
- Hagihara, M.; Kuroki, Y.; Ariyoshi, T.; Higashi, S.; Fukuda, K.; Yamashita, R.; Matsumoto, A.; Mori, T.; Mimura, K.; Yamaguchi, N.; et al. Clostridium Butyricum Modulates the Microbiome to Protect Intestinal Barrier Function in Mice with Antibiotic-Induced Dysbiosis. iScience 2020, 23, 100772. [Google Scholar] [CrossRef] [PubMed]
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate Specifically Modulates MUC Gene Expression in Intestinal Epithelial Goblet Cells Deprived of Glucose. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 287, G1168–G1174. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The Regulation of Intestinal Mucin MUC2 Expression by Short-Chain Fatty Acids: Implications for Epithelial Protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]
- Pan, L.; Niu, W.; Fang, X.; Liang, W.; Li, H.; Chen, W.; Zhang, H.; Bhatia, M.; Sun, J. Clostridium Butyricum Strains Suppress Experimental Acute Pancreatitis by Maintaining Intestinal Homeostasis. Mol. Nutr. Food Res. 2019, 63, 1801419. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Du, J.; Wang, F.; Fang, R.; Yu, C.; Xiong, J.; Chen, W.; Lu, Z.; Liu, J. Clostridium Butyricum Exerts a Neuroprotective Effect in a Mouse Model of Traumatic Brain Injury via the Gut-Brain Axis. Neurogastroenterol. Motil. 2018, 30, e13260. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Tato, C.M.; Joyce-Shaikh, B.; Gulen, M.F.; Cayatte, C.; Chen, Y.; Blumenschein, W.M.; Judo, M.; Ayanoglu, G.; McClanahan, T.K.; et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity 2015, 43, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, T.; Hagihara, M.; Eguchi, S.; Fukuda, A.; Iwasaki, K.; Oka, K.; Takahashi, M.; Yamagishi, Y.; Mikamo, H. Clostridium Butyricum MIYAIRI 588-Induced Protectin D1 Has an Anti-Inflammatory Effect on Antibiotic-Induced Intestinal Disorder. Front. Microbiol. 2020, 11, 587725. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, T.; Dalli, J.; Colas, R.A.; Federici Canova, D.; Aursnes, M.; Bonnet, D.; Alric, L.; Vergnolle, N.; Deraison, C.; Hansen, T.V.; et al. Protectin D1 n-3 DPA and Resolvin D5 n-3 DPA Are Effectors of Intestinal Protection. Proc. Natl. Acad. Sci. USA 2017, 114, 3963–3968. [Google Scholar] [CrossRef]
- Araki, Y.; Andoh, A.; Takizawa, J.; Takizawa, W.; Fujiyama, Y. Clostridium Butyricum, a Probiotic Derivative, Suppresses Dextran Sulfate Sodium-Induced Experimental Colitis in Rats. Int. J. Mol. Med. 2004, 13, 577–580. [Google Scholar] [CrossRef]
- Hayashi, A.; Sato, T.; Kamada, N.; Mikami, Y.; Matsuoka, K.; Hisamatsu, T.; Hibi, T.; Roers, A.; Yagita, H.; Ohteki, T.; et al. A Single Strain of Clostridium Butyricum Induces Intestinal IL-10-Producing Macrophages to Suppress Acute Experimental Colitis in Mice. Cell Host Microbe 2013, 13, 711–722. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, W.-R.; Wang, X.-H.; Li, G.-Q.; Xu, L.-Q.; Cui, X.; Liu, Y.; Zuo, X.-L. Clostridium Butyricum Alleviates Intestinal Low-Grade Inflammation in TNBS-Induced Irritable Bowel Syndrome in Mice by Regulating Functional Status of Lamina Propria Dendritic Cells. World J. Gastroenterol. 2019, 25, 5469–5482. [Google Scholar] [CrossRef]
- Wang, F.-Y. Potential Protective Effects of Clostridium Butyricum on Experimental Gastric Ulcers in Mice. World J. Gastroenterol. 2015, 21, 8340. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chen, J.; Xia, J.; Wang, B.; Liu, H.; Yang, L.; Wang, Y.; Ling, Z. Role of Probiotics in the Treatment of Minimal Hepatic Encephalopathy in Patients with HBV-Induced Liver Cirrhosis. J. Int. Med. Res. 2018, 46, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-Diabetic Effects of Clostridium Butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-Producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017, 7, 7046. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Weng, D.; Ju, Z.; Zhao, Y.; Liu, S.; Liu, Y.; Song, M.; Cui, L.; Sun, S.; et al. Clostridium Butyricum Inhibits Fat Deposition via Increasing the Frequency of Adipose Tissue-Resident Regulatory T Cells. Mol. Nutr. Food Res. 2022, 66, 2100884. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Yu, M.; Darby, T.M.; Vaccaro, C.; Li, J.-Y.; Owens, J.A.; Hsu, E.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131.e7. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, T.C.; MacDougald, O.A. Wnt/β-Catenin Signaling in Adipogenesis and Metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Kennell, J.A.; MacDougald, O.A. Wnt Signaling Inhibits Adipogenesis through β-Catenin-Dependent and -Independent Mechanisms. J. Biol. Chem. 2005, 280, 24004–24010. [Google Scholar] [CrossRef]
- Seo, M.; Inoue, I.; Tanaka, M.; Matsuda, N.; Nakano, T.; Awata, T.; Katayama, S.; Alpers, D.H.; Komoda, T. Clostridium Butyricum MIYAIRI 588 Improves High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Rats. Dig. Dis. Sci. 2013, 58, 3534–3544. [Google Scholar] [CrossRef]
- Obanda, D.N.; Husseneder, C.; Raggio, A.M.; Page, R.; Marx, B.; Stout, R.W.; Guice, J.; Coulon, D.; Keenan, M.J. Abundance of the Species Clostridium Butyricum in the Gut Microbiota Contributes to Differences in Obesity Phenotype in Outbred Sprague-Dawley CD Rats. Nutrition 2020, 78, 110893. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Fang, W.; Xue, H.; Chen, X.; Chen, K.; Ling, W. Supplementation with Sodium Butyrate Modulates the Composition of the Gut Microbiota and Ameliorates High-Fat Diet-Induced Obesity in Mice. J. Nutr. 2019, 149, 747–754. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.-Z.; Zhang, R.-N.; He, C.-X.; Chen, G.-Y.; Liu, C.; Chen, Y.-W.; Fan, J.-G. Sodium Butyrate Attenuates High-Fat Diet-Induced Steatohepatitis in Mice by Improving Gut Microbiota and Gastrointestinal Barrier. World J. Gastroenterol. 2017, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Yasueda, A.; Mizushima, T.; Nezu, R.; Sumi, R.; Tanaka, M.; Nishimura, J.; Kai, Y.; Hirota, M.; Osawa, H.; Nakajima, K.; et al. The Effect of Clostridium Butyricum MIYAIRI on the Prevention of Pouchitis and Alteration of the Microbiota Profile in Patients with Ulcerative Colitis. Surg. Today 2016, 46, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Lee, W.-J.; Tsou, J.-J.; Liu, T.-P.; Tsai, P.-L. Effect of Probiotics on Postoperative Quality of Gastric Bypass Surgeries: A Prospective Randomized Trial. Surg. Obes. Relat. Dis. 2016, 12, 57–61. [Google Scholar] [CrossRef]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium Butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharm. 2018, 41, 151–155. [Google Scholar] [CrossRef]
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus Ipilimumab with or without Live Bacterial Supplementation in Metastatic Renal Cell Carcinoma: A Randomized Phase 1 Trial. Nat. Med. 2022, 28, 704–712. [Google Scholar] [CrossRef]
- Fasano, A.; Shea-Donohue, T. Mechanisms of Disease: The Role of Intestinal Barrier Function in the Pathogenesis of Gastrointestinal Autoimmune Diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sorrell, M.F.; Batra, S.K.; Dhawan, P.; Singh, A.B. Gut Permeability and Mucosal Inflammation: Bad, Good or Context Dependent. Mucosal Immunol. 2017, 10, 307–317. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Sisti, D.; Amatori, S.; Gervasi, M.; Agostini, D.; Piccoli, G.; Bertuccioli, A.; Rocchi, M.B.L.; Stocchi, V.; Sestili, P. High-Intensity Interval Training Promotes the Shift to a Health-Supporting Dietary Pattern in Young Adults. Nutrients 2020, 12, 843. [Google Scholar] [CrossRef]
- Di Pierro, F.; Simonetti, G.; Petruzzi, A.; Bertuccioli, A.; Botta, L.; Bruzzone, M.G.; Cuccarini, V.; Fariselli, L.; Lamperti, E. A Novel Lecithin-Based Delivery Form of Boswellic Acids as Complementary Treatment of Radiochemotherapy-Induced Cerebral Edema in Patients with Glioblastoma Multiforme: A Longitudinal Pilot Experience. J. Neurosurg. Sci. 2019, 63, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Khan, A.; Bertuccioli, A.; Maffioli, P.; Derosa, G.; Khan, S.; Khan, B.A.; Nigar, R.; Ujjan, I.; Devrajani, B.R. Quercetin Phytosome® as a Potential Candidate for Managing COVID-19. Minerva Gastroenterol. 2021, 67, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bertuccioli, A.; Giuberti, R.; Saponara, M.; Ivaldi, L. Role of a Berberine-Based Nutritional Supplement in Reducing Diarrhea in Subjects with Functional Gastrointestinal Disorders. Minerva Gastroenterol. Dietol. 2020, 66, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Iablokov, S.N.; Albayrak, L.; Khanipov, K.; Uchitel, S.; Chopra, D.; Mills, P.J.; Fofanov, Y.; Rodionov, D.A.; et al. 16S RRNA Gene Profiling and Genome Reconstruction Reveal Community Metabolic Interactions and Prebiotic Potential of Medicinal Herbs Used in Neurodegenerative Disease and as Nootropics. PLoS ONE 2019, 14, e0213869. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Zonzini, G.B.; Di Pierro, F.; Moricoli, S.; Bertuccioli, A. Gut Microbiota, Metabolic Disorders and Breast Cancer: Could Berberine Turn Out to Be a Transversal Nutraceutical Tool? A Narrative Analysis. Int. J. Mol. Sci. 2022, 23, 12538. [Google Scholar] [CrossRef]
- Feng, J.; Li, Z.; Ma, H.; Yue, Y.; Hao, K.; Li, J.; Xiang, Y.; Min, Y. Quercetin Alleviates Intestinal Inflammation and Improves Intestinal Functions via Modulating Gut Microbiota Composition in LPS-Challenged Laying Hens. Poult. Sci. 2023, 102, 102433. [Google Scholar] [CrossRef]
- Lee, S.; Yu, Y.; Trimpert, J.; Benthani, F.; Mairhofer, M.; Richter-Pechanska, P.; Wyler, E.; Belenki, D.; Kaltenbrunner, S.; Pammer, M.; et al. Virus-Induced Senescence Is a Driver and Therapeutic Target in COVID-19. Nature 2021, 599, 283–289. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective Effect of Quercetin on High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice Is Mediated by Modulating Intestinal Microbiota Imbalance and Related Gut-Liver Axis Activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Marini, E.; Ivaldi, L. A Pilot Trial on Subjects with Lactose and/or Oligosaccharides Intolerance Treated with a Fixed Mixture of Pure and Enteric-Coated α- and β-Galactosidase. Clin. Exp. Gastroenterol. 2015, 8, 95–100. [Google Scholar] [CrossRef]

| Substance | Effects Described | Comments |
|---|---|---|
| Nonnutritive sweeteners (NNSs) | ||
| Aspartame | Akkermansia muciniphila ↑ Enterobacteriaceae ↑ Enterococcaceae ↓ Enterococcus ↓ Parasutterella ↓ Clostridium cluster IV ↑ Escherichia coli ↓ | The observed effect may be due to the animals stopping eating rather than aspartame intake. |
| Acesulfame-K | Escherichia coli ↓ Firmicutes ↑ Akkermansia muciniphila ↓ | The inhibition of the growth of Escherichia coli is greater at intermediate doses, decreasing to zero at the higher doses tested. In combination with sucralose. |
| Cyclamate | Bifidobacterium ↑ SCFAs ↓ Microbial count ↓ Fermentation profile ↓ | |
| Sucralose | Firmicutes ↓↑ Bacteroidetes ↓↑ Turicibacter ↑ Escherichia coli ↑ Proteobacteria ↑ Valeric acid ↑ | Opposite results are reported by several authors. |
| Saccharin | Bifidobacterium ↑ Firmicutes ↓ SCFAs ↓ | |
| Steviol glycosides | Bifidobacterium ↓ Fermentation profile ↓ Bacillus ↑ Streptococcus saliviloxodontae ↑ Staphylococcus lugdunensis ↑ | |
| Low-calorie sweeteners (LCSs) | ||
| Erythritol | SCFAs ↑ | |
| Isomalt | Bifidobacterium ↑ ß-glucosidase ↓ | |
| Lactitol | Bifidobacterium ↑ Lactobacillus ↑ Akkermansia muciniphila ↑ Bacteroidetes ↓ Clostridium ↓ Coliforms ↓ Eubacterium ↓ SCFAs ↑ | |
| Maltitol | Bifidobacterium ↑ | |
| Mannitol | SCFAs ↑ | |
| Sorbitol | Osmotic/laxative ↑ | |
| Xylitol | Firmicutes ↑ Bacteroidetes ↓ Lactobacilli ↓ Clostridium difficile ↓ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuccioli, A.; Cardinali, M.; Zonzini, G.; Neri, M.; Palazzi, C.M.; Gregoretti, A.; Cazzaniga, M.; Di Pierro, F. Clostridium butyricum Strain MIYAIRI 588 (CBM588) as a Precision Probiotic Therapy in the Ketogenic Diet: A Possible Application? Microbiol. Res. 2023, 14, 492-506. https://doi.org/10.3390/microbiolres14020036
Bertuccioli A, Cardinali M, Zonzini G, Neri M, Palazzi CM, Gregoretti A, Cazzaniga M, Di Pierro F. Clostridium butyricum Strain MIYAIRI 588 (CBM588) as a Precision Probiotic Therapy in the Ketogenic Diet: A Possible Application? Microbiology Research. 2023; 14(2):492-506. https://doi.org/10.3390/microbiolres14020036
Chicago/Turabian StyleBertuccioli, Alexander, Marco Cardinali, Giordano Zonzini, Marco Neri, Chiara Maria Palazzi, Aurora Gregoretti, Massimiliano Cazzaniga, and Francesco Di Pierro. 2023. "Clostridium butyricum Strain MIYAIRI 588 (CBM588) as a Precision Probiotic Therapy in the Ketogenic Diet: A Possible Application?" Microbiology Research 14, no. 2: 492-506. https://doi.org/10.3390/microbiolres14020036
APA StyleBertuccioli, A., Cardinali, M., Zonzini, G., Neri, M., Palazzi, C. M., Gregoretti, A., Cazzaniga, M., & Di Pierro, F. (2023). Clostridium butyricum Strain MIYAIRI 588 (CBM588) as a Precision Probiotic Therapy in the Ketogenic Diet: A Possible Application? Microbiology Research, 14(2), 492-506. https://doi.org/10.3390/microbiolres14020036








