Forces Governing the Transport of Pathogenic and Nonpathogenic Escherichia coli in Nitrogen and Magnesium Doped Biochar Amended Sand Columns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Bacterial Cultures
2.2. Biochar’s Preparation
2.3. Sand as a Model Porous Medium
2.4. Quantification of Bacterial Transport in 2% Biochar/Sand Columns
2.5. Contact Angles Measurements and Modeling
2.6. Interfacial Net Gibbs Free Energy Calculations
2.7. Derjaguin, Landau, Verwey, and Overbeek (DLVO) Theory of Colloidal Stability
2.8. Statistics
3. Results and Discussion
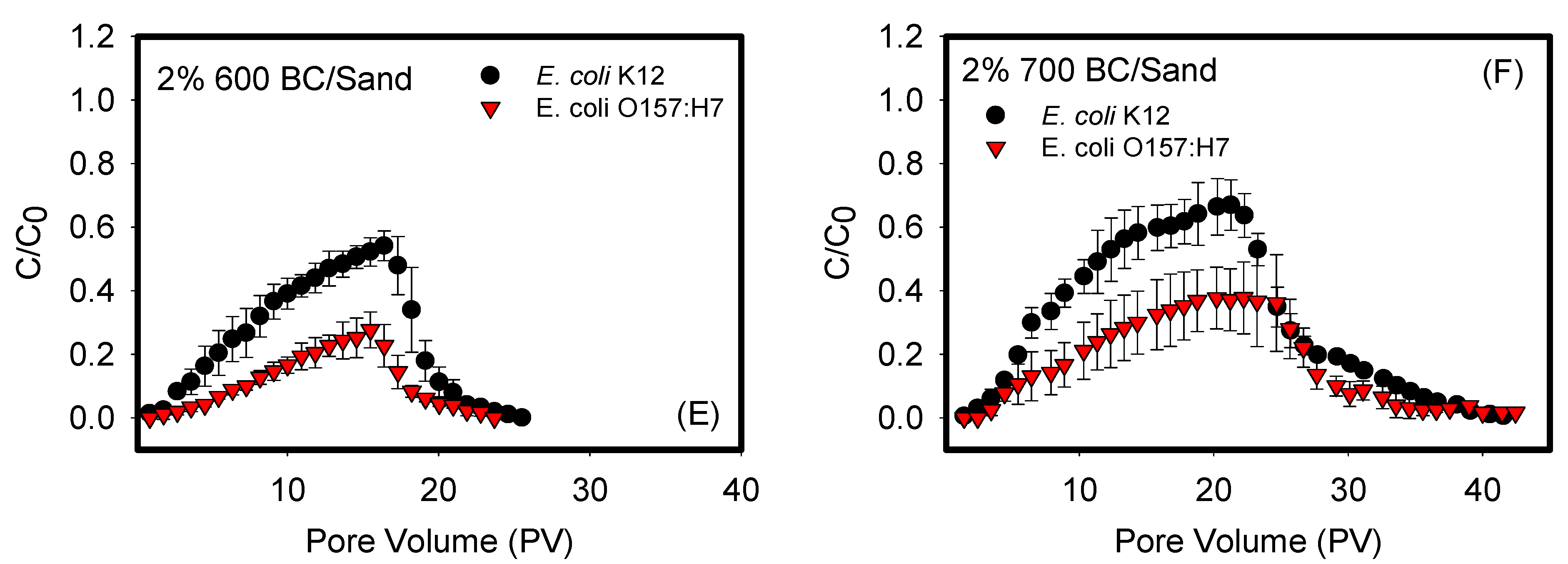
3.1. Effects of Biochar Pyrolysis Temperature on Bacterial Transport in Porous Media
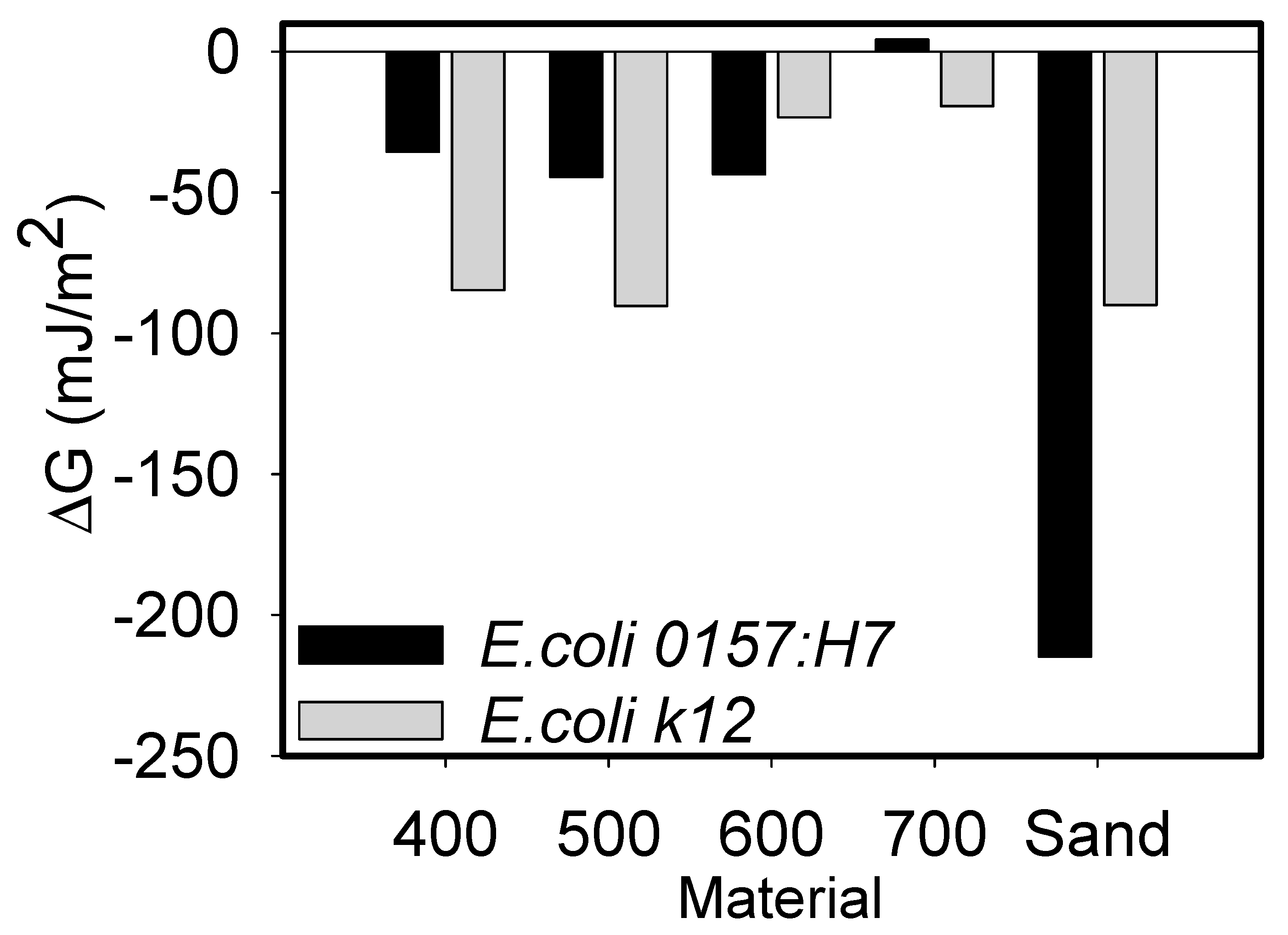
3.2. Net Gibbs Free Energy Calculations
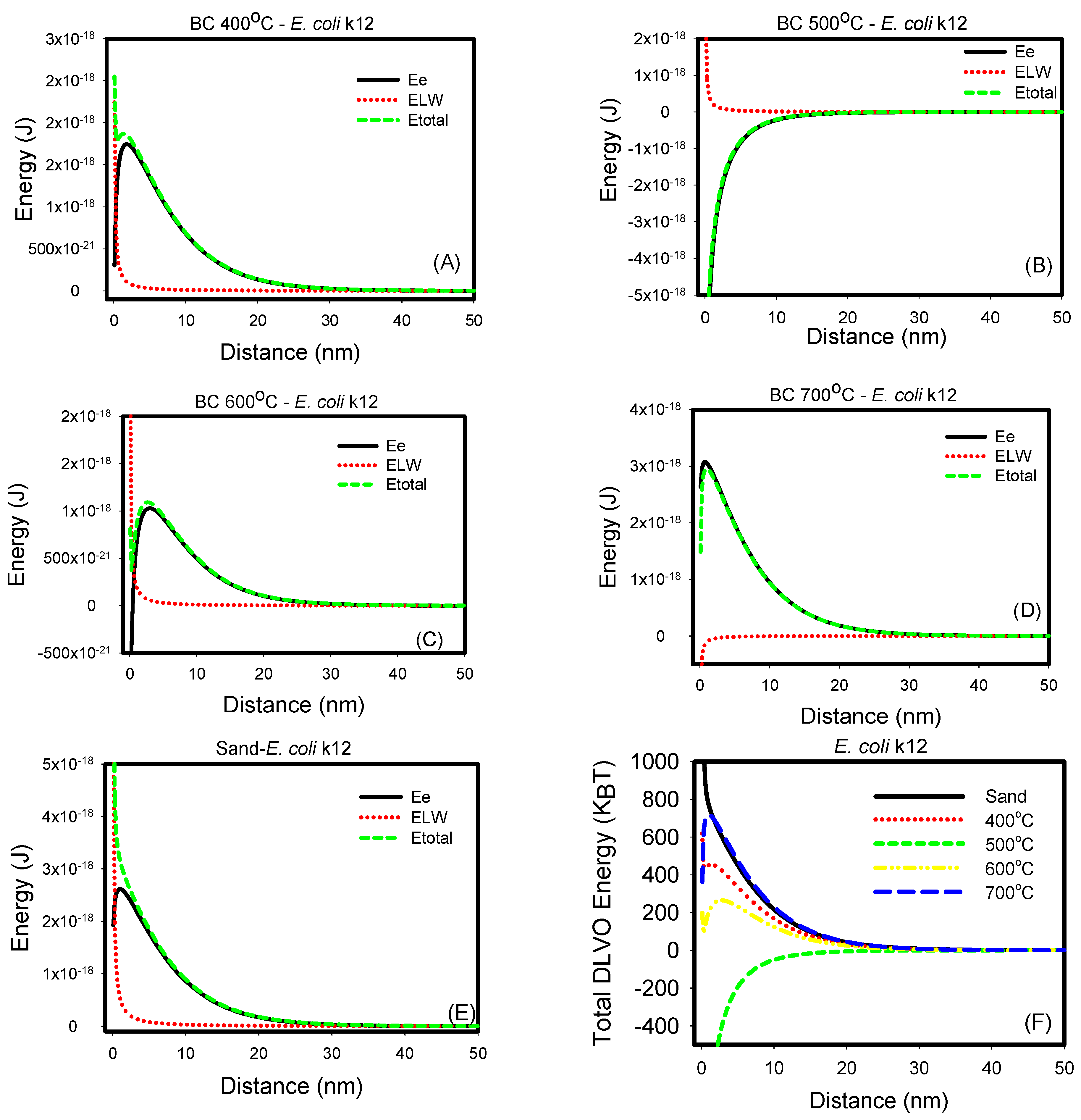
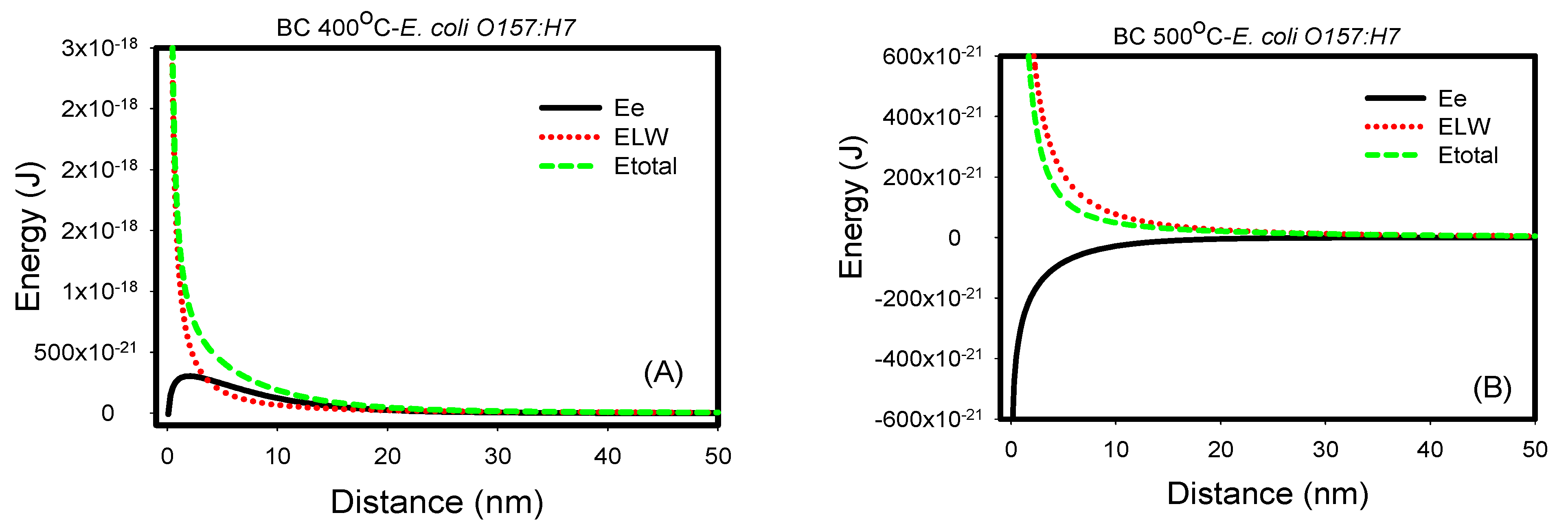
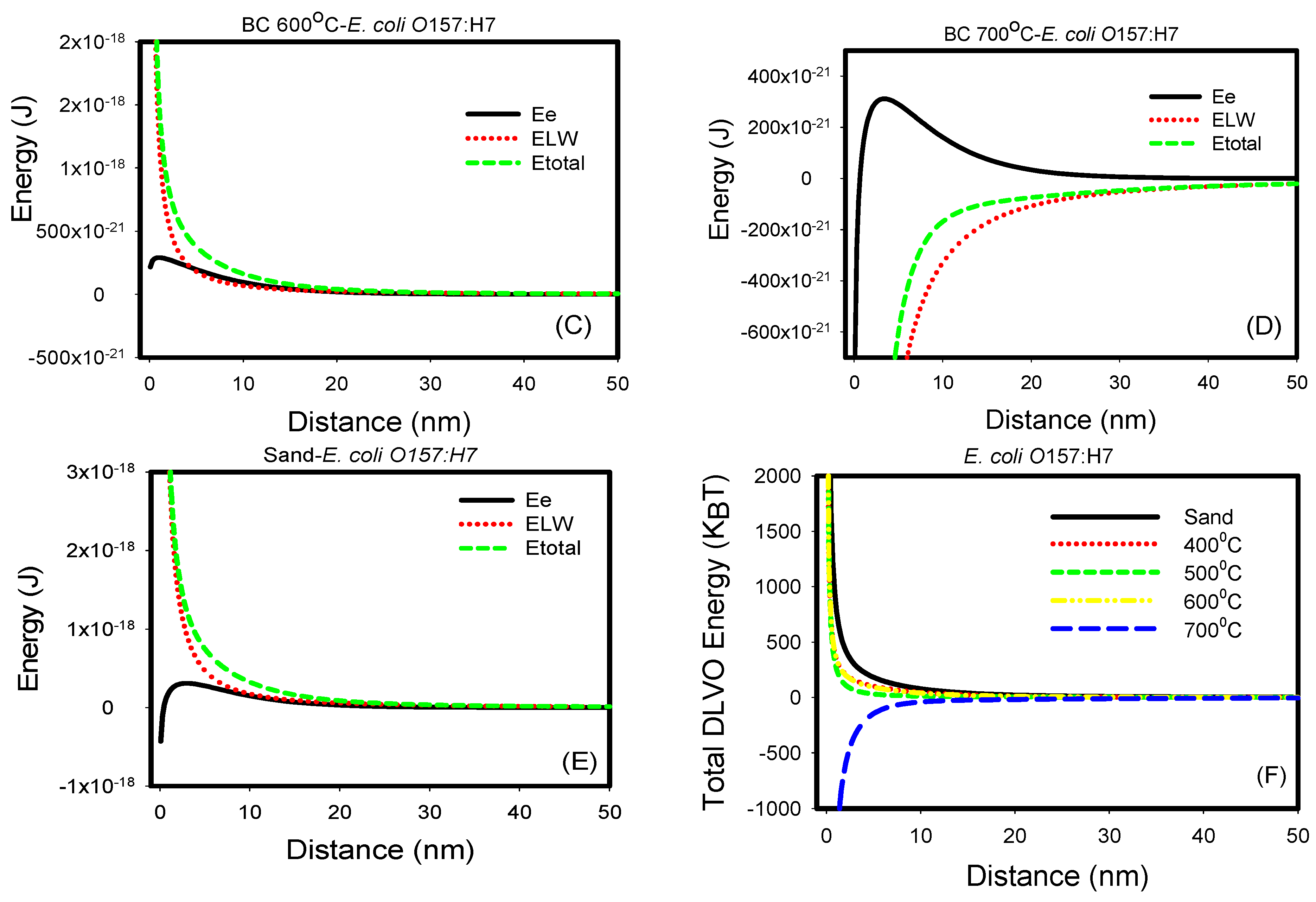
3.3. Non-Specific, Long-Range DLVO Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohanty, S.K.; Torkelson, A.A.; Dodd, H.; Nelson, K.L.; Boehm, A.B. Engineering solutions to improve the removal of fecal indicator bacteria by bioinfiltration systems during intermittent flow of stormwater. Environ. Sci. Technol. 2013, 47, 10791–10798. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.K.; Boehm, A.B. Escherichia coli removal in biochar-augmented biofilter: Effect of infiltration rate, initial bacterial concentration, biochar particle size, and presence of compost. Environ. Sci. Technol. 2014, 48, 11535–11542. [Google Scholar] [CrossRef]
- Abbasi, B.; Ta, H.X.; Muhunthan, B.; Ramezanian, S.; Abu-Lail, N.; Kwon, T.-H. Modeling of permeability reduction in bioclogged porous sediments. J. Geotech. Geoenvironmental Eng. 2018, 144, 4018016. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modeling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Wang, K.; Brown, R.C.; Homsy, S.; Martinez, L.; Sidhu, S.S. Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour. Technol. 2013, 127, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.A. The charcoal vision: A win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefining 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Abit, S.M.; Bolster, C.H.; Cai, P.; Walker, S.L. Influence of feedstock and pyrolysis temperature of biochar amendments on transport of Escherichia coli in saturated and unsaturated soil. Environ. Sci. Technol. 2012, 46, 8097–8105. [Google Scholar] [CrossRef]
- Abit, S.M.; Bolster, C.H.; Cantrell, K.B.; Flores, J.Q.; Walker, S.L. Transport of Escherichia coli, Salmonella typhimurium, and microspheres in biochar-amended soils with different textures. J. Environ. Qual. 2014, 43, 371–388. [Google Scholar] [CrossRef]
- Chung, J.W.; Foppen, J.W.; Izquierdo, M.; Lens, P.N. Removal of Escherichia coli from saturated sand columns supplemented with hydrochar produced from maize. J. Environ. Qual. 2014, 43, 2096–2103. [Google Scholar] [CrossRef]
- Sasidharan, S.; Torkzaban, S.; Bradford, S.A.; Kookana, R.; Page, D.; Cook, P. Transport and retention of bacteria and viruses in biochar-amended sand. Sci. Total Environ. 2016, 548, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lail, N.I.; Camesano, T.A. Specific and nonspecific interaction forces between Escherichia coli and silicon nitride, determined by Poisson statistical analysis. Langmuir 2006, 22, 7296–7301. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lail, N.I.; Camesano, T.A. The effect of solvent polarity on the molecular surface properties and adhesion of Escherichia coli. Colloids Surf. B Biointerfaces 2006, 51, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-J.; Abu-Lail, N.I. The role of the pH conditions of growth on the bioadhesion of individual and lawns of pathogenic Listeria monocytogenes cells. J. Colloid Interface Sci. 2011, 358, 611–620. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Fortuna, A.-M.; Garcia-Pérez, M.; Abu-Lail, N.I. Quantitative effects of biochar oxidation and pyrolysis temperature on the transport of pathogenic and nonpathogenic Escherichia coli in biochar-amended sand columns. Environ. Sci. Technol. 2017, 51, 5071–5081. [Google Scholar] [CrossRef]
- Mood, S.H.; Ayiania, M.; Jefferson-Milan, Y.; Garcia-Perez, M. Nitrogen doped char from anaerobically digested fiber for phosphate removal in aqueous solutions. Chemosphere 2020, 240, 124889. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Smith, M.W.; Helms, G.; McEwen, J.-S.; Garcia-Perez, M. Effect of pyrolysis temperature on aromatic cluster size of cellulose char by quantitative multi cross-polarization 13C NMR with long range dipolar dephasing. Carbon 2017, 116, 210–222. [Google Scholar] [CrossRef]
- Abu-Lail, N.I.; Camesano, T.A. Role of ionic strength on the relationship of biopolymer conformation, DLVO contributions, and steric interactions to bioadhesion of Pseudomonas putida KT2442. Biomacromolecules 2003, 4, 1000. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, H. Bacterial adhesion to silica sand as related to Gibbs energy variations. Colloids Surf. B Biointerfaces 2005, 44, 41–48. [Google Scholar] [CrossRef]
- Van Oss, C.J. Interfacial Forces in Aqueous Media; CRC Press: Baton Rouge, LA, USA, 2006. [Google Scholar]
- Vithanage, M.; Mayakaduwa, S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef]
- Ramezaniankeikanloo, S. Multiscale Investigations of the Effects of Chemical Stimuli on the Composition, Adhesion and Mechanics of Pseudomonas putida Cells and Biofilms. Ph.D. Thesis, Washington State University, Pullman, WA, USA, May 2018. [Google Scholar]
- Afrooz, A.R.M.N.; Pitol, A.K.; Kitt, D.; Boehm, A.B. Role of microbial cell properties on bacterial pathogen and coliphage removal in biochar-modified stormwater biofilters. Environ. Sci. Water Res. Technol. 2018, 4, 2160–2169. [Google Scholar] [CrossRef]
- Afrooz, A.N.; Boehm, A.B. Escherichia coli removal in biochar-modified biofilters: Effects of biofilm. PLoS ONE 2016, 11, e0167489. [Google Scholar] [CrossRef]
- Hermansson, M. The DLVO theory in microbial adhesion. Colloids Surf. B Biointerfaces 1999, 14, 105–119. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Li, Q.-R.; Geng, Z.-C.; Hu, F.-N.; Zhao, S.-W. Surface properties and suspension stability of low-temperature pyrolyzed biochar nanoparticles: Effects of solution chemistry and feedstock sources. Chemosphere 2020, 259, 127510. [Google Scholar] [CrossRef]
- Ramezanian, S.; Ta, H.X.; Muhunthan, B.; Abu-Lail, N. Role of ionic strength in the retention and initial attachment of Pseudomonas putida to quartz sand. Biointerphases 2018, 13, 041005. [Google Scholar] [CrossRef]
- Van Oss, C.J. The forces involved in bioadhesion to flat surfaces and particles—Their determination and relative roles. Biofouling 1991, 4, 25–35. [Google Scholar] [CrossRef]
- Dubinin, M.M. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR 1947, 55, 327–329. [Google Scholar]
- Dubinin, M.M.; Zaverina, E.D.; Radushkevich, L.V. Sorption and structure of active carbons. I. Adsorption of organic vapors. Zhurnal Fiz. Khimii 1947, 21, 151–162. [Google Scholar]

| Pyrolysis Temperature (°C) | ||||
|---|---|---|---|---|
| Physiochemical characteristic | 400 | 500 | 600 | 700 |
| Carbon content (wt. %) (dry basis) | 64.0 | 63.6 | 67.9 | 63.6 |
| Nitrogen content (wt. %) (dry basis) | 8.3 | 7.7 | 7.7 | 11.0 |
| Hydrogen content (wt. %) (dry basis) | 4.6 | 3.2 | 1.9 | 1.2 |
| Oxygen content (wt. %) (dry basis) | 11.6 | 11.5 | 7.1 | 7.7 |
| Fixed carbon content (wt. %) (dry basis) | 54.7 | 60.2 | 65.7 | 64.6 |
| Volatile matter content (wt. %) (dry basis) | 33.8 | 25.8 | 18.8 | 18.9 |
| Ash content (wt. %) (dry basis) | 11.5 | 14.0 | 15.4 | 16.5 |
| Moisture content (wt. %) ** (as received) | 9.2 | 10.0 | 6.3 | 6.0 |
| SA-CO2 (m2 g−1) * | 202.7 | 299.8 | 400.9 | 493.8 |
| Vmicro (cm3 g−1) & | 0.081 | 0.120 | 0.161 | 0.198 |
| Pcap (cm3g−1) # | 24.2 | 26.6 | 26.9 | 26.5 |
| Bulk density (g cm−3) | 1.56 | 1.551 | 1.537 | 1.561 |
| porosity (v/v) | 0.69 | 0.60 | 0.67 | 0.63 |
| pH (in H2O) | 10.1 | 10.0 | 10.0 | 10.2 |
| Mean particle diameter (µm) | 51 | 32 | 26 | 29 |
| Sphericity (%) | 0.49 | 0.49 | 0.49 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinn, K.; Mood, S.H.; Cervantes, E.; Perez, M.G.; Abu-Lail, N.I. Forces Governing the Transport of Pathogenic and Nonpathogenic Escherichia coli in Nitrogen and Magnesium Doped Biochar Amended Sand Columns. Microbiol. Res. 2023, 14, 218-228. https://doi.org/10.3390/microbiolres14010018
Quinn K, Mood SH, Cervantes E, Perez MG, Abu-Lail NI. Forces Governing the Transport of Pathogenic and Nonpathogenic Escherichia coli in Nitrogen and Magnesium Doped Biochar Amended Sand Columns. Microbiology Research. 2023; 14(1):218-228. https://doi.org/10.3390/microbiolres14010018
Chicago/Turabian StyleQuinn, Katherine, Sohrab Haghighi Mood, Elizabeth Cervantes, Manuel Garcia Perez, and Nehal I. Abu-Lail. 2023. "Forces Governing the Transport of Pathogenic and Nonpathogenic Escherichia coli in Nitrogen and Magnesium Doped Biochar Amended Sand Columns" Microbiology Research 14, no. 1: 218-228. https://doi.org/10.3390/microbiolres14010018
APA StyleQuinn, K., Mood, S. H., Cervantes, E., Perez, M. G., & Abu-Lail, N. I. (2023). Forces Governing the Transport of Pathogenic and Nonpathogenic Escherichia coli in Nitrogen and Magnesium Doped Biochar Amended Sand Columns. Microbiology Research, 14(1), 218-228. https://doi.org/10.3390/microbiolres14010018








