Heterosigma akashiwo, a Fish-Killing Flagellate
Abstract
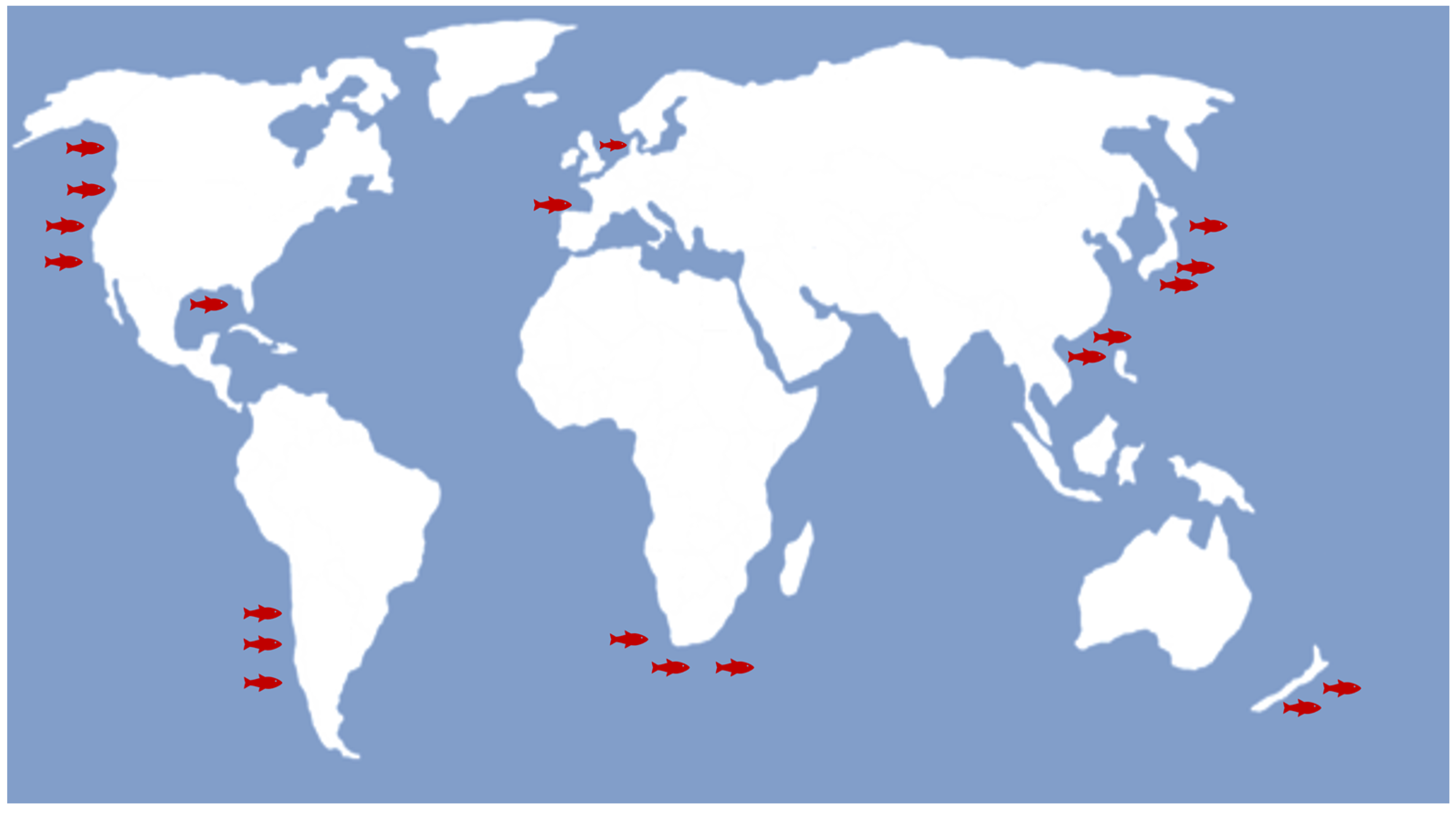
1. Introduction
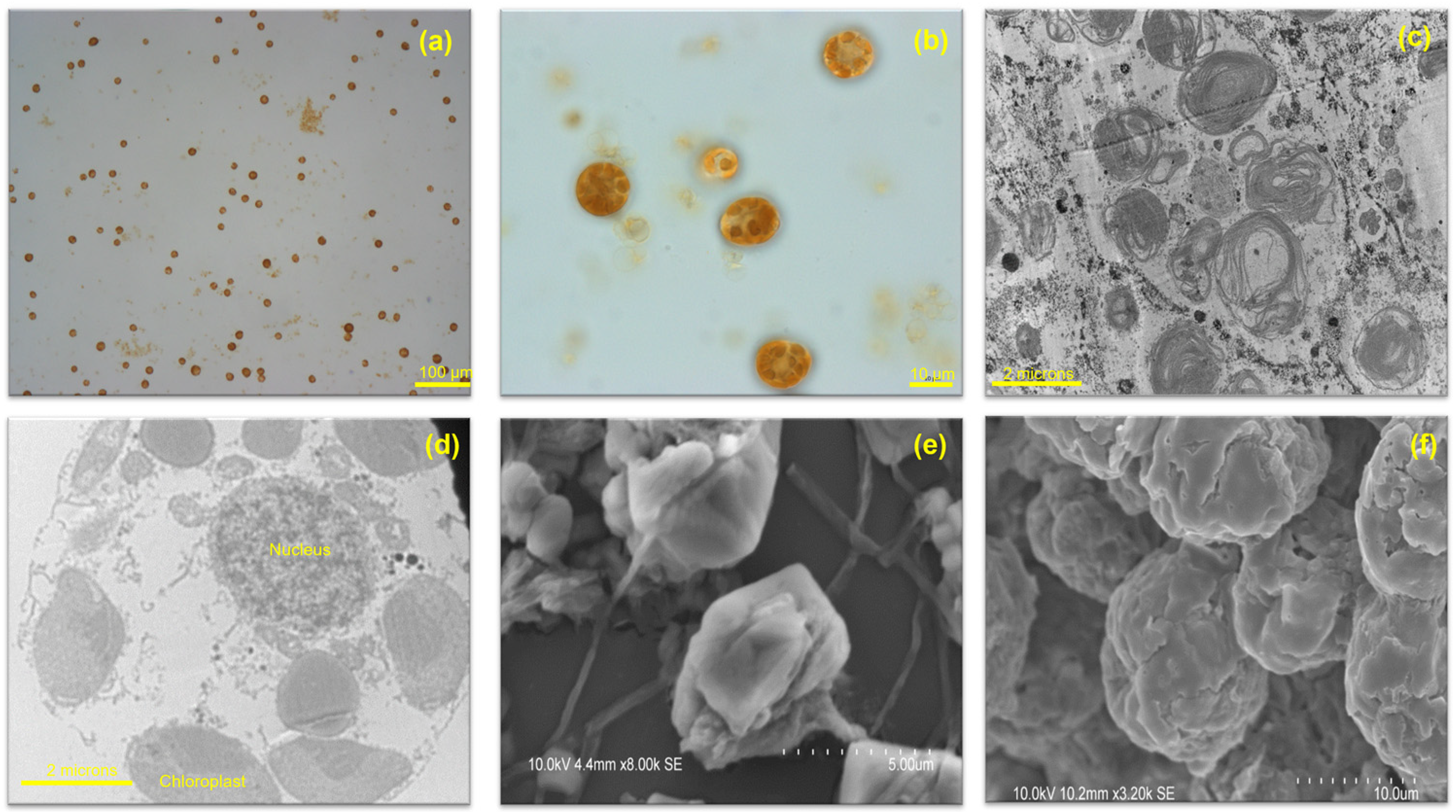
2. H. akashiwo Taxonomy and Morphology
3. The Physiological Effects of Temperature, Light, and Salinity on H. akashiwo
4. Nutrients Impact
5. Climate Change and Global Warming
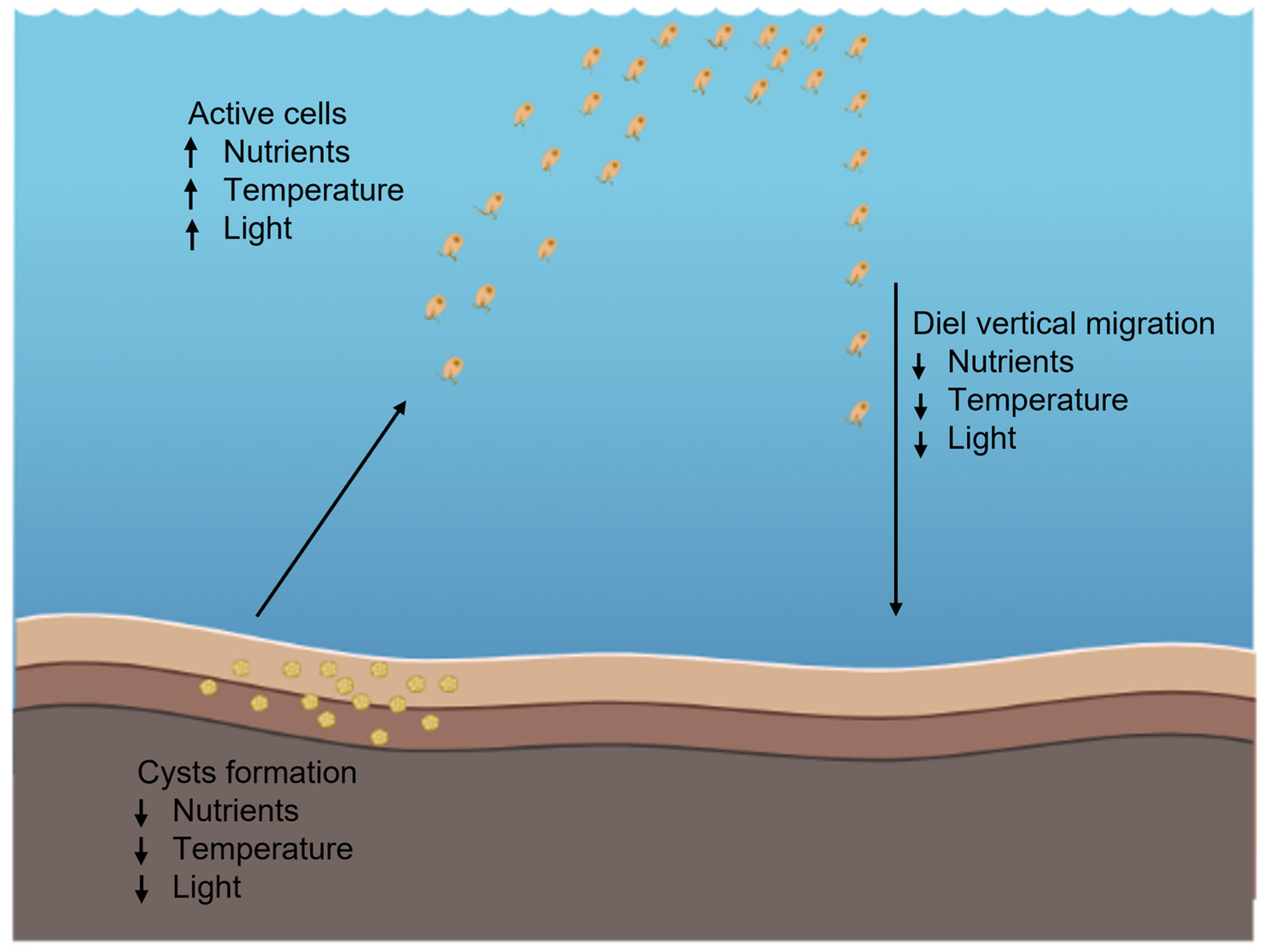
6. H. akashiwo Cyst Formation
7. H. akashiwo Fish-Killing Mechanisms
- Asphyxiation by covering fish gills and physical damage as a result of extreme mucous secretion [4].

7.1. Mucous Secretion
7.2. Reactive Oxygen Species (ROS) Production
7.3. Toxin Production
7.4. Hemolytic Activity
8. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Hara, Y.; Chihara, M. Heterosigma akashiwo morphology, ultrastructure and taxonomy of the Raphidophycean alga Heterosgima akashwio. Bot. Mag. Tokyo 1987, 100, 151–163. [Google Scholar] [CrossRef]
- Taylor, F.J.R.; Haigh, R. The Ecology of fish-killing blooms of the chloromonad flagellate Heterosigma in the Strait of Georgia and adjacent Waters. In Toxic Phytoplankton Blooms in the Sea; Elsevier: New York, NY, USA, 1993; pp. 705–710. [Google Scholar]
- Smayda, T.J. Harmful Algal Bloom Communities in Scottish Coastal Waters: Relationship to Fish Farming and Regional Comparisons—A Review Paper 2006/3. Scottish Executive, Scottish Environmental Protection Agency SEPA. Available online: https://www.webarchive.org.uk/wayback/archive/20160122225400/http://www.gov.scot/Publications/2006/02/03095327/16 (accessed on 22 January 2023).
- Chang, F.H.; Anderson, C.; Boustead, N.C. First record of a Heterosigma (Raphidophyceae) bloom with associated mortality of cage-reared salmon in Big Glory Bay, New Zealand. N. Z. J. Mar. Freshw. Res. 1990, 24, 461–469. [Google Scholar] [CrossRef]
- O’Halloran, C.; Silver, M.W.; Holman, T.R.; Scholin, C.A. Heterosigma akashiwo in central California waters. Harmful Algae 2006, 5, 124–132. [Google Scholar] [CrossRef]
- Yamoehi, S.; Abe, T. Mechanisms to initiate a Heterosigma akashiwo red tide in Osaka Bay. II. Diel vertical migration. Mar. biol. 1984, 261, 255–261. [Google Scholar] [CrossRef]
- Nagasaki, K.; Tarutani, K.; Yamaguchi, M. Growth characteristics of Heterosigma akashiwo virus and its possible use as a microbiological agent for red tide control. Appl. Environ. Microbiol. 1999, 65, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, G.; Tian, J. The Effects of the harmful alga Heterosigma akashiwo on cultures of Schmackeria Inopinus (Copepoda, Calanoida). J. Sea Res. 2010, 64, 287–294. [Google Scholar] [CrossRef]
- Hershberger, P.K.; Rensel, J.E.; Matter, A.L.; Taub, F.B. Vertical distribution of the chloromonad flagellate Heterosigma carterae in columns: Implications for bloom development. Can. J. Fish. Aquat. Sci. 1997, 2234, 2228–2234. [Google Scholar] [CrossRef]
- Rensel, J.E.; Haigh, N.; Tynan, T.J. Fraser river sockeye salmon marine survival decline and harmful blooms of Heterosigma akashiwo. Harmful Algae 2010, 10, 98–115. [Google Scholar] [CrossRef]
- Livingston, R.J. Phytoplankton bloom effects on a gulf estuary: Water quality changes and biological response. Ecol. Appl. 2007, 17, 110–128. [Google Scholar] [CrossRef]
- Ono, K.; Khan, S.; Onoue, Y. Effects of temperature and light intensity on the growth and toxicity of Heterosigma akashiwo (Raphidophyceae). Aquac. Res. 2000, 31, 427–433. [Google Scholar] [CrossRef]
- Haque, S.M.; Onoue, Y. Effects of salinity on growth and toxin production of a noxious phytoflagellate, Heterosigma akashiwo (Raphidophyceae). Bot. Mar. 2002, 45, 356–363. [Google Scholar] [CrossRef]
- Flores-Leñero, A.; Vargas-Torres, V.; Paredes-Mella, J.; Norambuena, L.; Fuenzalida, G.; Lee-Chang, K.; Mardones, J.I. Heterosigma akashiwo in Patagonian Fjords: Genetics, growth, pigment signature and role of PUFA and ROS in ichthyotoxicity. Toxins 2022, 14, 577. [Google Scholar] [CrossRef]
- Yang, A.; Bellerby, R.G.J.; Wang, Y.; Li, X. Growth and nutrient uptake characteristics of Heterosigma akashiwo (Raphidophyceae) under nitrogen and phosphorus concentrations in the East China Sea. Water 2021, 13, 3166. [Google Scholar] [CrossRef]
- Martínez, R.; Orive, E.; Laza-Martínez, A.; Seoane, S. Growth response of six strains of Heterosigma akashiwo to varying temperature, salinity and irradiance conditions. J. Plankton Res. 2010, 32, 529–538. [Google Scholar] [CrossRef]
- Engesmo, A.; Strand, D.; Gran-Stadniczeñko, S.; Edvardsen, B.; Medlin, L.K.; Eikrem, W. Development of a QPCR assay to detect and quantify ichthyotoxic flagellates along the Norwegian coast, and the first Norwegian record of Fibrocapsa japonica (Raphidophyceae). Harmful Algae 2018, 75, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Bornman, E.; Adams, J.B.; Strydom, N.A. Algal blooms of Heterosigma akashiwo and mugilidae gill alterations. Estuar. Coast. 2022, 45, 1674–1687. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.I.; Paredes-Mella, J.; Flores-Leñero, A.; Yarimizu, K.; Godoy, M.; Artal, O.; Corredor-Acosta, A.; Marcus, L.; Cascales, E.; Pablo Espinoza, J.; et al. Extreme harmful algal blooms, climate change, and potential risk of eutrophication in Patagonian Fjords: Insights from an exceptional Heterosigma akashiwo fish-killing event. Prog. Oceanogr. 2022, 210, 102921. [Google Scholar] [CrossRef]
- Guiry, G.M.; Guiry, M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: https://www.algaebase.org (accessed on 21 December 2022).
- Throndsen, J. The planktonic marine flagellates. In Identifying Marine Phytoplankton; Tomas, C.R., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 591–729. [Google Scholar]
- Verity, P.G. Expansion of Potentially Harmful Algal Taxa in a Georgia Estuary (USA). Harmful Algae 2010, 9, 144–152. [Google Scholar] [CrossRef]
- Smayda, T.J. Ecophysiology and bloom dynamics of Heterosigma akashiwo (Raphidophyceae). In Physiology Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer-Verlag: Berlin, Germany, 1998; pp. 113–132. [Google Scholar]
- Bowers, H.A.; Kempton, J.W.; Lewitus, A.J.; Oldach, D.W. Raphidophycea [chadefaud ex silva] systematics and rapid identification: Sequence analyses and real-time PCR assay. J. Phycol. 2006, 1348, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Tobin, E.D.; Grünbaum, D.; Patterson, J.; Cattolico, R.A. Behavioral and physiological changes during benthic-pelagic transition in the harmful alga, Heterosigma akashiwo: Potential for rapid bloom formation. PLoS ONE 2013, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Imai, I.; Itakura, S.; Itoh, K. Cysts of the red tide flagellate Heterosigma akashiwo, Raphidophyceae, found in bottom sediments of Northern Hiroshima Bay, Japan. Nippon Suisan Gakkaishi 1993, 59, 1669–1673. [Google Scholar] [CrossRef]
- Powers, L.; Creed, I.F.; Trick, C.G. Sinking of Heterosigma akashiwo results in increased toxicity of this harmful algal bloom species. Harmful Algae 2012, 13, 95–104. [Google Scholar] [CrossRef]
- Strom, S.L.; Harvey, E.L.; Fredrickson, K.A.; Menden-Deuer, S. Broad salinity tolerance as a refuge from predation in the harmful raphidophyte alga Heterosigma akashiwo (Raphidophyceae). J. Phycol. 2013, 49, 20–31. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al-Shehri, A.M. The Link between shrimp farm runoff and blooms of toxic Heterosigma akashiwo in Red Sea coastal waters. Oceanologia 2012, 54, 287–309. [Google Scholar] [CrossRef]
- Ikeda, C.E.; Cochlan, W.P.; Bronicheski, C.M.; Trainer, V.L.; Trick, C.G. The effects of salinity on the cellular permeability and ichthyotoxicity of Heterosigma akashiwo. J. Phycol. 2016, 53, 745–760. [Google Scholar] [CrossRef]
- Mehdizadeh Allaf, M.; Trick, C.G. Multiple-stressor design-of-experiment (DOE) and one-factor-at-a-time (OFAT) observations defining Heterosigma akashiwo growth and cell permeability. J. Appl. Phycol. 2019, 31, 3515–3526. [Google Scholar] [CrossRef]
- Haque, S.M.; Onoue, Y. Variation in toxin compositions of two harmful raphidophytes, Chattonella antiqua and Chattonella marina, at different salinities. Environ. Toxiol. 2001, 17, 113–118. [Google Scholar] [CrossRef]
- Philip, J.R. The osmotic cell, solute diffusibility, and the plant water cconomy. Plant Physiol. 1958, 33, 264–271. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Peierls, B.L.; Rossignol, K.L. Evolving paradigms and challenges in estuarine and coastal eutrophication dynamics in a culturally and climatically stressed world. Estuar. Coast. 2014, 37, 243–258. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.; Cochlan, W.P. Nitrogen utilization by the raphidophyte Heterosigma akashiwo: Growth and uptake kinetics in laboratory cultures. Harmful Algae 2007, 6, 260–270. [Google Scholar] [CrossRef]
- Ji, N.; Zhang, Z.; Huang, J.; Zhou, L.; Deng, S.; Shen, X.; Lin, S. Utilization of various forms of nitrogen and expression regulation of transporters in the harmful alga Heterosigma akashiwo (Raphidophyceae). Harmful Algae 2020, 92, 101770. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Trick, C.G. Expression and standardized measurement of hemolytic activity in Heterosigma akashiwo. Harmful Algae 2010, 9, 522–529. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Grinevald, J.; Crutzen, P.; McNeill, J. The anthropocene: Conceptual and historical. Phil. Trans. R. Soc. A 2011, 369, 842–867. [Google Scholar] [CrossRef]
- IPCC. Climate Change: The physical science basis. In Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Heal. A Glob. Access Sci. 2008, 7, 1–12. [Google Scholar] [CrossRef]
- NOAA. Trends in Atmospheric Carbon Dioxide–Mauna Loa. US Department of Commerce, National Oceanic and Atmospheric Administration. 2022. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 22 January 2023).
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean ph. Nature 2003, 425, 365. [Google Scholar] [CrossRef]
- Raven, J.; Caldeira, K.; Elderfield, H.; Hoegh-Guldberg, O.; Liss, P.; Riebesell, U.; Shepherd, J.; Turley, C.; Watson, A. Ocean acidification due to increasing. Coral Reefs 2005, 12/05, 68. [Google Scholar]
- Moore, S.K.; Mantua, N.J.; Hickey, B.M.; Trainer, V.L. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae 2013, 8, 463–477. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, B.; Wang, Y.; Ju, Q.; Yu, Q.; Tang, X. Effect of CO2 enrichment on competition between Skeletonema costatum and Heterosigma akashiwo. Chin. J. Oceanol. Limnol. 2010, 28, 933–939. [Google Scholar] [CrossRef]
- Itakura, S.; Yamaguchi, M. Morphological and physiological differences between the cysts of Alexandrium catenella and A. tamarense (Dinophyceae) in the Seto Inland Sea, Japan. Plankt. Biol. Ecol. 2005, 52, 85–91. [Google Scholar]
- Kamykowski, D.; Mccollum, S.A. The temperature acclimatized swimming speed of selected marine dinoflagellates. J. Plankton Res. 1986, 8, 275–287. [Google Scholar] [CrossRef]
- Kim, H.; Spivack, A.J.; Menden-deuer, S. pH alters the swimming behaviors of the raphidophyte Heterosigma akashiwo: Implications for bloom formation in an acidified ocean. Harmful Algae 2013, 26, 1–11. [Google Scholar] [CrossRef]
- Beardall, J.; Raven, J.A. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 2004, 43, 26–40. [Google Scholar] [CrossRef]
- Raven, J.A.; Geider, R.J. Temperature and algal growth. New Phytol. 1988, 110, 441–461. [Google Scholar] [CrossRef]
- Marinov, I.; Doney, S.C.; Lima, I.D. Response of ocean phytoplankton community structure to climate change over the 21st century: Partitioning the effects of nutrients, temperature and light. Biogeosciences 2010, 7, 3941–3959. [Google Scholar] [CrossRef]
- Fu, F.X.; Zhang, Y.; Warner, M.E.; Feng, Y.; Sun, J.; Hutchins, D.A. A comparison of future increased CO2 and temperature effects on sympatric Heterosigma akashiwo and Prorocentrum minimum. Harmful Algae 2008, 7, 76–90. [Google Scholar] [CrossRef]
- Mehdizadeh Allaf, M. The Effect of Multiple Environmental Stressors on the Growth and Toxicity of the Red Tide Alga Heterosigma Akashiwo. Ph.D. Thesis, Western University, London, ON, Canada, 2018. Available online: https://ir.lib.uwo.ca/etd/5799/ (accessed on 22 January 2023).
- Itakura, S.; Nagasaki, K.; Yamaguchi, M.; Imai, I. Cyst formation in the red tide flagellate Heterosigma akashiwo (Raphidophyceae). J. Plankton Res. 1996, 18, 1975–1979. [Google Scholar] [CrossRef]
- Camacho, F.G.; Rodríguez, J.G.; Mirón, A.S.; García, M.C.C.; Belarbi, E.H.; Chisti, Y.; Grima, E.M. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef]
- Imai, I.; Itakura, S. Importance of cysts in the population dynamics of the red tide flagellate Heterosigma akashiwo (Raphidophyceae). Mar. Biol. 1999, 133, 755–762. [Google Scholar] [CrossRef]
- Kok, J.W.K.; Leong, S.C.Y. Decline and recovery in cell population densities of Heterosigma akashiwo (Raphidophyceae) as a novel bloom driver for the species. J. Trop. Ecol. 2021, 37, 91–97. [Google Scholar] [CrossRef]
- Solovchenko, A.E. Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ. J. Plant. Physl. 2012, 59, 167–176. [Google Scholar] [CrossRef]
- Cohen, Z.; Khozin-Goldberg, I.; Adlerstein, D.; Bigogno, C. The role of triacylglycerol as a reservoir of polyunsaturated fatty acids for the rapid production of chloroplastic lipids in certain microalgae. Biochem. Soc. Trans. 2000, 28, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Yamochi, S. Mechanisms for outbreak of Heterosigma akashiwo red tide in Osaka Bay, Japan—Part 3. Release of vegetative cells from bottom mud. J. Oceanogr. Soc. Jpn. 1984, 40, 343–348. [Google Scholar] [CrossRef]
- Honjo, T. Overview of bloom dynamics and physiological ecology of Heterosigma akashiwo. In Toxic phytoplankton blooms in the sea. Proceedings of the 5th international conference on toxic marine phytoplankton, Newport, RI, USA, 28 October–1 November 1991; Elsevier: New York, NY, USA, 1993; pp. 33–41. [Google Scholar]
- Twiner, M. Azaspiracid shellfish poisoning: A review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs 2008, 6, 39–72. [Google Scholar] [CrossRef]
- Kegel, J.U.; Del Amo, Y.; Costes, L.; Medlin, L.K. Testing a microarray to detect and monitor toxic microalgae in Arcachon Bay in France. Microarrays 2013, 2, 1–23. [Google Scholar] [CrossRef]
- Yang, C.Z.; Albright, L.J.; Yousif, A.N. Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Dis. Aquat. Organ. 1995, 23, 101–108. [Google Scholar] [CrossRef]
- Oda, T.; Nakamura, A.; Shikayama, M.; Kawano, I.; Ishimatsu, A.; Muramatsu, T. Generation of reactive oxygen species by raphidophycean phytoplankton. Chem. Pharm. Bull. 1997, 61, 1658–1662. [Google Scholar]
- Twiner, M.J.; Trick, C.G. Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. Plankton Res. 2000, 22, 1961–1975. [Google Scholar] [CrossRef]
- Khan, S.; Arakawa, O.; Onoue, Y. Neurotoxins in a toxic red tide of Heterosigma akashiwo (Raphidophyceae) in Kagoshima Bay, Japan. Aquac. Res. 1997, 28, 9–14. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Holdt, S.L.; De Francisci, D.; Valverde-Pérez, B.; Plósz, B.G.; Angelidaki, I. Microplate-based method for high-throughput screening of microalgae growth potential. Bioresour. Technol. 2014, 169, 566–572. [Google Scholar] [CrossRef]
- Shimada, M.; Imahayashi, T.; Ozaki, H.S.; Murakami, T.H.; Toyoshima, T.; Okaichi, T. Effects of sea bloom, Chattonella antiqual, on gill primary lamellae of the young yellowtail, Seriola quinqueradiata. Acta Histochem. Cytochem. 1983, 16, 232–244. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Sameshima, M.; Tamura, A.; Oda, T. Histological analysis of the mechanisms of Chattonella induced hypoxemia in yellowtail. Fish. Sci. 1996, 62, 50–58. [Google Scholar] [CrossRef]
- Oda, T.; Nakamura, A.; Okamoto, T.; Ishimatsu, A.; Muramatsu, T. Lectin-induced enhancement of superoxide union production by red tide phytoplankton. Mar. Biol. 1998, 131, 383–390. [Google Scholar] [CrossRef]
- Kim, D.; Kumamoto, O.; Lee, K.S.; Kuroda, A.; Fujii, A.; Ishimatus, A.; Oda, T. Deleterious effect of Chattonella marina on short-necked clam (Ruditapes philippinarum); possible involvement of reactive oxygen species. J. Plankton Res. 2004, 26, 967–971. [Google Scholar] [CrossRef]
- Carrasquero, R. Role of associated bacteria in Heterosigma carterae toxicity to salmonids. Aquat. Toxicol. 1999, 45, 19–34. [Google Scholar] [CrossRef]
- Kim, D.; Nakamura, A.; Okamoto, T.; Komatsu, N.; Oda, T.; Iida, T.; Ishimatsu, A.; Muramatsu, T. Mechanism of superoxide anion generation in the toxic red tide phytoplankton Chattonella marina: Possible involvement of NAD(P)H oxidase. Biochim. Biophys. Acta Gen. Subj. 2000, 1524, 220–227. [Google Scholar] [CrossRef]
- Twiner, M.J.; Dixon, S.J.; Trick, C.G. Toxic effects of Heterosigma akashiwo do not appear to be mediated by hydrogen peroxide. Limnol. Oceanogr. 2001, 46, 1400–1405. [Google Scholar] [CrossRef]
- Kim, D.; Watanabe, M.; Nakayasu, Y.; Kohata, K. Changes in O2− and H2O2 production by Chattonella antiqua during diel vertical migration under nutrient stratification. Aquat. Microb. Ecol. 2005, 39, 183–191. [Google Scholar] [CrossRef]
- Liu, W.; Au, D.W.T.; Anderson, D.M.; Lam, P.K.S.; Wu, R.S.S. Effects of nutrients, salinity, pH and light:dark cycle on the production of reactive oxygen species in the alga Chattonella marina. J. Exp. Mar. Bio. Ecol. 2007, 346, 76–86. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Dorantes-Aranda, J.J.; Mardones, J.; Seger, A. Review of progress in our understanding of fish-killing microalgae: Implications for management and mitigation. In Marine and Fresh-Water Harmful Algae, Proceedings of the 17th International Conference on Harmful Algae, Florianópolis, Brazil, 9–14 October 2016; International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO 2017: Florianópolis, Brazil, 2016; pp. 148–153. [Google Scholar]
- Marshall, J.A.; Nichols, P.D.; Hallegraeff, G.M. Chemotaxonomic survey of sterols and fatty acids in six marine raphidophyte algae. J. Appl. Phycol. 2002, 14, 255–265. [Google Scholar] [CrossRef]
- Marshall, J.; de Salas, M.; Oda, T.; Hallegraeff, G.M. Superoxide production by marine microalgae. Mar. Biol. 2005, 147, 533–540. [Google Scholar] [CrossRef]
- Nakamura, A.; Okamoto, T.; Komatsu, N.; Ooka, S.; Oda, T.; Ishimatsu, A.; Muramatsu, T. Fish mucus stimurates the generation of superoxide anion by Chattonella marina and Heterosigma akashiwo. Fish. Sci. 1998, 64, 866–869. [Google Scholar] [CrossRef]
- Kawano, I.; Oda, T.; Ishimatsu, A.; Muramatsu, T. Inhibitory effect of the iron chelator desferrioxamine (desferal) on the generation of activated oxygen species by Chattonella marina. Mar. Biol. 1996, 126, 765–771. [Google Scholar] [CrossRef]
- Marshall, J.A.; Nichols, P.D.; Hamilton, B.; Lewis, R.J.; Hallegraeff, G.M. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): The synergistic role of reactive oxygen species and free fatty acids. Harmful Algae 2003, 2, 273–281. [Google Scholar] [CrossRef]
- Endo, M.; Onoue, Y.; Kuroki, A. Neurotoxin-induced cardiac disorder and its role in the death of fish exposed to Chattonella marina. Mar. Biol. 1992, 112, 371–376. [Google Scholar] [CrossRef]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.A.; Mende, T.J.; Baden, D.G. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol. 1986, 30, 129–135. [Google Scholar] [PubMed]
- Wang, D.-Z. neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef]
- Mehdizadeh Allaf, M.; Trick, C. Yeast cell as a bio-model for measuring the toxicity of fish-killing flagellates. Toxins 2021, 13, 821. [Google Scholar] [CrossRef]
- Khan, S.; Arakawa, O.; Onoue, Y. Neurotoxin production by a chloromonad Fibrocapsa japonica (Raphidophyceae). J. World Aquac. Soc. 1996, 27, 254–263. [Google Scholar] [CrossRef]
- Bourdelais, A.J.; Tomas, C.R.; Naar, J.; Kubanek, J.; Baden, D.G. New fish-killing alga in coastal delaware produces neurotoxins. Environ. Health Perspect. 2002, 110, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Sakai, T.; Kuroki, A. Histological and histochemical changes in the gills of the yellowtail Seriola quinqueradiata exposed to the raphidphycean flagellate Chattonella marina. Mar. Biol. 1985, 87, 193–197. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Waite, T.D.; Godrant, A.; Rose, A.L.; Tovar, C.D.; Woods, G.M.; Hallegraeff, G.M. Novel application of a fish gill cell line assay to assess ichthyotoxicity of harmful marine microalgae. Harmful Algae 2011, 10, 366–373. [Google Scholar] [CrossRef]
- Endo, M.; Foscarini, R.; Kuroki, A. Electrocardiogram of a marine fish, Pagrus major, exposed to red tide plankton, Chattonella marina. Mar. Biol. 1988, 97, 477–481. [Google Scholar] [CrossRef]
- De Boer, M.K.; Tyl, M.R.; Vrieling, E.G.; Van Rijssel, M. Effects of salinity and nutrient conditions on growth and haemolytic activity of Fibrocapsa japonica (Raphidophyceae). Aquat. Microb. Ecol. 2004, 37, 171–181. [Google Scholar] [CrossRef]
- Fu, M.; Koulman, A.; Van Rijssel, M.; Lützen, A.; De Boer, M.K.; Tyl, M.R.; Liebezeit, G. Chemical characterisation of three haemolytic compounds from the microalgal species Fibrocapsa japonica (Raphidophyceae). Toxicon 2004, 43, 355–363. [Google Scholar] [CrossRef]
- de Boer, M.K.; Tyl, M.R.; Fu, M.; Kulk, G.; Liebezeit, G.; Tomas, C.R.; Lenzi, A.; Naar, J.; Vrieling, E.G.; van Rijssel, M. Haemolytic activity within the species Fibrocapsa japonica (Raphidophyceae). Harmful Algae 2009, 8, 699–705. [Google Scholar] [CrossRef]
- Kuroda, A.; Nakashima, T.; Yamaguchi, K.; Oda, T. Isolation and characterization of light-dependent hemolytic cytotoxin from harmful red tide phytoplankton Chattonella marina. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, J.H.; Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms the effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]

| Empire | Eukaryota |
| Kingdom | Chromista |
| Phylum | Ochrophyta |
| Class | Raphidophyceae |
| Order | Chattonellales |
| Family | Chattonellaceae |
| Genus | Heterosigma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdizadeh Allaf, M. Heterosigma akashiwo, a Fish-Killing Flagellate. Microbiol. Res. 2023, 14, 132-147. https://doi.org/10.3390/microbiolres14010012
Mehdizadeh Allaf M. Heterosigma akashiwo, a Fish-Killing Flagellate. Microbiology Research. 2023; 14(1):132-147. https://doi.org/10.3390/microbiolres14010012
Chicago/Turabian StyleMehdizadeh Allaf, Malihe. 2023. "Heterosigma akashiwo, a Fish-Killing Flagellate" Microbiology Research 14, no. 1: 132-147. https://doi.org/10.3390/microbiolres14010012
APA StyleMehdizadeh Allaf, M. (2023). Heterosigma akashiwo, a Fish-Killing Flagellate. Microbiology Research, 14(1), 132-147. https://doi.org/10.3390/microbiolres14010012







