Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández, P.; Dalle Zotte, A. Influence of Diet on Rabbit Meat Quality. In Nutrition of the Rabbit; De Blas, C., Wiseman, J., Eds.; CAB International: Wallingford, UK, 2010; pp. 163–178. [Google Scholar]
- Dalle Zotte, A.; Szendrő, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Para, P.A.; Ganguly, S.; Wakchaure, R.; Sharma, R.; Mahajan, T.; Praveen, P.K. Rabbit meat has the potential of being a possible alternative to other meats as a protein source: A brief review. Int. J. Phar. Biomedi. Rese. 2015, 2, 17–19. [Google Scholar]
- Dalle Zotte, A.; Cossu, M.E. Dietary inclusion of tannin extract from red quebracho trees (Schinopsis spp.) in the rabbit meat production. Ital. J. Anim. Sci. 2009, 8, 784–786. [Google Scholar] [CrossRef]
- Kowalska, D.; Bielanski, P. Meat quality of rabbit fed a diet supplemented with fish oil and antioxidant. Anim. Sci. Pap. Rep. 2009, 2, 139–148. [Google Scholar]
- De Blas, J.C. Nutritional impact on health and performance in intensively reared rabbits. Animal 2013, 7, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Gidenne, T. Dietary fibers in the nutrition of the growing rabbit and recommendations to preserve digestive health: A review. Animal 2015, 9, 227–242. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Bio-processing of agro-byproducts to animal feed. Crit. Rev. Biotechnol. 2012, 32, 382–400. [Google Scholar] [CrossRef]
- Celia, C.; Cullere, M.; Gerencsér, Z.; Matics, Z.; Giaccone, V.; Kovács, M.; Bónai, A.; Szendrő, Z.; Dalle Zotte, A. Dietary supplementation of Digestarom® herbal formulation: Effect on apparent digestibility, faecal and caecal microbial counts and live performance of growing rabbits. World Rabbit Sci. 2016, 24, 95–105. [Google Scholar] [CrossRef]
- del Toro, M.I.; Aguilar, Y.M.; Navarro, M.V.; Chipres, D.S.; Cortés, M.R. Comportamiento productivo y características de la canal de conejos alimentados con harina de Agave tequilana. Rev. Electron. Vet. 2016, 17, 1–12. [Google Scholar]
- Dalle Zotte, A.; Cullere, M.; Sartori, A.; Szendrő, Z.; Kovàcs, M.; Giaccone, V.; Dal Bosco, A. Dietary Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) supplementation to growing rabbits: Effects on raw and cooked meat quality, nutrient true retention and oxidative stability. Meat Sci. 2014, 98, 94–103. [Google Scholar] [CrossRef]
- Phuoc, T.L.; Jamikorn, U. Effects of probiotic supplement (Bacillus subtilis and Lactobacillus acidophilus) on feed efficiency, growth performance, and microbial population of weaning rabbits. Asian-Australas. J. Anim. Sci. 2017, 30, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, C.A.; Maldonado Herrera, J.A.; Méndez-Zamora, G.; Hernández-Luna, C.E.; Gutiérrez-Soto, G. Enzymatic extract of Trametes maxima CU1 on productive parameters and carcass yield of rabbits. Can. J. Anim. Sci. 2017, 97, 683–688. [Google Scholar] [CrossRef][Green Version]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Akinfemi, A.; Adu, O.A.; Doherty, F. Conversion of sorghum stover into animal feed with white-rot fungi: Pleurotus ostreatus and Pleurotus pulmonarius. Afr. J. Biotechnol. 2010, 9, 1706–1712. [Google Scholar] [CrossRef]
- Medina-González, G.E.; Bernal-Barragán, H.; Hernández-Luna, C.E.; Hernández-Martínez, C.A.; Gutiérrez-Soto, G. Uso de basidiomicetos nativos en la biotransformación del pasto buffel (Cenchrus ciliaris) para mejorar la calidad nutricional. Rev. Mex. Micol. 2016, 43, 31–35. [Google Scholar]
- Shrivastava, B.; Thakur, S.; Khasa, Y.P.; Gupte, A.; Puniya, A.K.; Kuhad, R.C. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation 2011, 22, 823–831. [Google Scholar] [CrossRef] [PubMed]
- van Kuijk, S.J.A.; Sonnenberg, A.S.; Baars, J.J.; Hendriks, W.H.; Cone, J.W. Fungal treated lignocellulosic biomass as ruminant feed ingredient: A review. Biotechnol. Adv. 2015, 33, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Hatakka, A.; Maijala, P. Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes? Int. Biodeterior. Biodegrad. 2007, 59, 32–39. [Google Scholar] [CrossRef]
- Kuhar, F.; Castiglia, V.; Levin, L. Enhancement of laccase production and malachite green decolorization by co-culturing Ganoderma lucidum and Trametes versicolor in solid-state fermentation. Int. Biodeterior. Biodegrad. 2015, 104, 238–243. [Google Scholar] [CrossRef]
- Gutiérrez-Soto, G.; Medina-Gonzalez, G.E.; Treviño-Ramírez, J.E.; Hernández-Luna, C.E. Native macrofungi that produce lignin-modifying enzymes, cellulases, and xylanases with potential biotechnological applications. Bioresources 2015, 10, 6676–6689. [Google Scholar] [CrossRef]
- Gutiérrez-Soto, G.; Medina-González, G.E.; García-Zambrano, E.A.; Treviño-Ramírez, J.E.; Hernández-Luna, C.E. Selection and characterization of a native Pycnoporus sanguineus strain as a lignocellulolytic extract producer from submerged cultures of various agroindustrial wastes. Bioresources 2015, 10, 3564–3576. [Google Scholar] [CrossRef]
- Hernández-Martínez, C.A.; Treviño-Cabrera, G.F.; Hernández-Luna, C.E.; Silva-Vázquez, R.; Hume, M.E.; Gutiérrez-Soto, G.; Méndez-Zamora, G. The effects of hydrolysed sorghum on growth performance and meat quality of rabbits. World Rabbit Sci. 2018, 26, 155–163. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana. Especificaciones Técnicas para la Producción, Cuidado y Uso de Animales de Laboratorio. NOM-062-ZOO. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 29 November 2022).
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Van Soest, J.P.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3594. [Google Scholar] [CrossRef] [PubMed]
- Safwat, A.M.; Sarmiento-Franco, L.; Santos-Ricalde, R.H.; Nieves, D.; Sandoval-Castro, C.A. Estimating apparent nutrient digestibility of diets containing Leucaena leucocephala or Moringa oleifera leaf meals for growing rabbits by two methods. Asian Australas. J. Anim. Sci. 2015, 2, 1155–1162. [Google Scholar] [CrossRef][Green Version]
- Attia, K.A.; Saleh, S.Y.; El-Hamid Safaa, S.A.; ZakiAmal, A.; El-Sawy, M.A. Effects of exogenous multi-enzyme feed additive (kemzyme) on the activities of certain digestive enzymes and intestinal morphology in growing rabbits. J. Agric. Sci. 2012, 4, 35–44. [Google Scholar]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi–Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Sonnenberg, A.S.; Baars, J.J.; Hendriks, W.H.; Cone, J.W. Fungal treatment of lignocellulosic biomass: Importance of fungal species, colonization and time on chemical composition and in vitro rumen degradability. Anim. Feed Sci. Technol. 2015, 28, 40–50. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Del Rio, J.C.; Rencoret, J.; Gutiérrez, A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; Cone, J.W. Selective ligninolysis of wheat straw and wood chips by the white-rot fungus Lentinula edodes and its influence on in vitro rumen degradability. J. Anim. Sci. Biotechnol. 2016, 7, 55–74NRC. [Google Scholar] [CrossRef]
- Oduguwa, O.O.; Edema, M.O.; Ayeni, A.O. Physico-chemical and microbiological analyses of fermented corn cob, rice bran and cowpea husk for use in composite rabbit feed. Bioresour. Technol. 2008, 99, 1816–1820. [Google Scholar] [CrossRef]
- Ribeiro, L.; Pinheiro, V.; Outor-Monteiro, D.; Mourão, J.; Bezerra, R.M.F.; Dias, A.A.; Bennett, R.N.; Marques, G.; Rodrigues, M.A.M. Effects of the dietary incorporation of untreated and white-rot fungi (Ganoderma resinaceum Boud) pre-treated olive leaves on growing rabbits. Anim. Feed Sci. Technol. 2012, 173, 244–251. [Google Scholar] [CrossRef]
- Andrade, E.; Pinheiro, V.; Gonçalves, A.; Cone, J.W.; Marques, G.; Silva, V.; Ferreira, L.; Rodrigues, M. Potential use of cowpea (Vigna unguiculata (L.) Walp.) stover treated with white-rot fungi as rabbit feed. J. Sci. Food Agric. 2017, 97, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Thomas, P.A.; Sheu, J.R.; Geraldine, P. In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Int. Food Res. J. 2011, 44, 851–861. [Google Scholar] [CrossRef]
- El-Badawi, A.Y.; El-Wardany, I.; El-Moez, S.A.; Helal, F.I.S.; Ali, N.G.; Shourrap, M.I.; Aboelazab, O.M. Impact of dietary Moringa oleifera leaves on intestinal pathogenic load and histological structure of growing rabbits raised under heat-stress conditions. Anim. Prod. Sci. 2017, 58, 1901–1907. [Google Scholar] [CrossRef]
- Gidenne, T. Dietary fibers: Their analysis in animal feeding, and their role in rabbit nutrition and health. JITV 2013, 23, 195–213. [Google Scholar]
- El-Maaty, H.M.A.; Sherif, S.K.; Foda, L.S. Dietary sugar beet tops and prebiotic effect on nutrient digestibility, caecal activity and organ histology of weaning rabbits. J. Agric. Sci. 2018, 10, 162–177. [Google Scholar] [CrossRef][Green Version]
| Ingredient | g kg−1 |
|---|---|
| Alfalfa hay | 496.7 |
| Ground sorghum * | 300.0 |
| Soya meal | 146.0 |
| Soya oil | 12.5 |
| Methionine | 1.3 |
| Molasses | 30.0 |
| Phosphate | 4.5 |
| Vitamin and mineral premix | 2.5 |
| Component (%) | Sorghum | SSF-Sorghum | Diet T1 | Diet T2 |
|---|---|---|---|---|
| Dry Matter | 94.25 | 94.42 | 93.93 | 94.99 |
| NDF DM | 17.89 | 15.90 | 75.51 | 43.17 |
| ADF DM | 6.46 | 3.81 | 49.37 | 31.21 |
| Lignin DM | 2.55 | 0.51 | 16.28 | 5.57 |
| Hemicellulose DM | 12.09 | 11.43 | 26.14 | 11.96 |
| Cellulose DM | 5.95 | 1.26 | 33.09 | 25.64 |
| Ash | 9.16 | 8.580 | 1.36 | 1.62 |
| Nitrogen | 1.42 | 1.42 | 2.68 | 2.36 |
| Crude protein | 8.85 | 8.89 | 16.73 | 14.75 |
| PC DM | 9.39 | 9.41 | 17.81 | 15.52 |
| Day | Tx | DM | ASH | OM | NDF DM | ADF DM | LIGNIN | HEMICEL | CEL |
|---|---|---|---|---|---|---|---|---|---|
| (%) | |||||||||
| 7 | T1 | 92.45 a | 7.02 a | 85.42 b | 72.93 a | 52.86 a | 11.03 b | 20.07 a | 41.83 a |
| T2 | 92.62 a | 4.99 b | 87.63 a | 71.10 a | 51.56 a | 21.17 a | 19.55 a | 30.38 b | |
| 21 | T1 | 92.97 a | 7.25 a | 85.72 b | 73.28 a | 52.39 a | 13.70 b | 20.89 a | 38.70 a |
| T2 | 93.85 a | 6.26 b | 87.59 a | 68.27 b | 48.09 b | 19.07 a | 20.18 a | 29.02 b | |
| 35 | T1 | 93.79 a | 7.19 b | 86.60 a | 62.6 a | 42.37 a | 19.70 a | 20.23 a | 22.67 b |
| T2 | 92.78 a | 7.86 a | 84.92 b | 58.17 b | 40.27 a | 15.39 b | 17.91 a | 24.88 a | |
| 49 | T1 | 93.41 a | 6.71 b | 86.70 a | 66.28 a | 47.17 a | 25.72 a | 19.11 a | 21.45 a |
| T2 | 93.25 a | 7.85 a | 85.39 b | 62.91 a | 44.7 a | 19.63 b | 18.21 a | 25.08 a | |
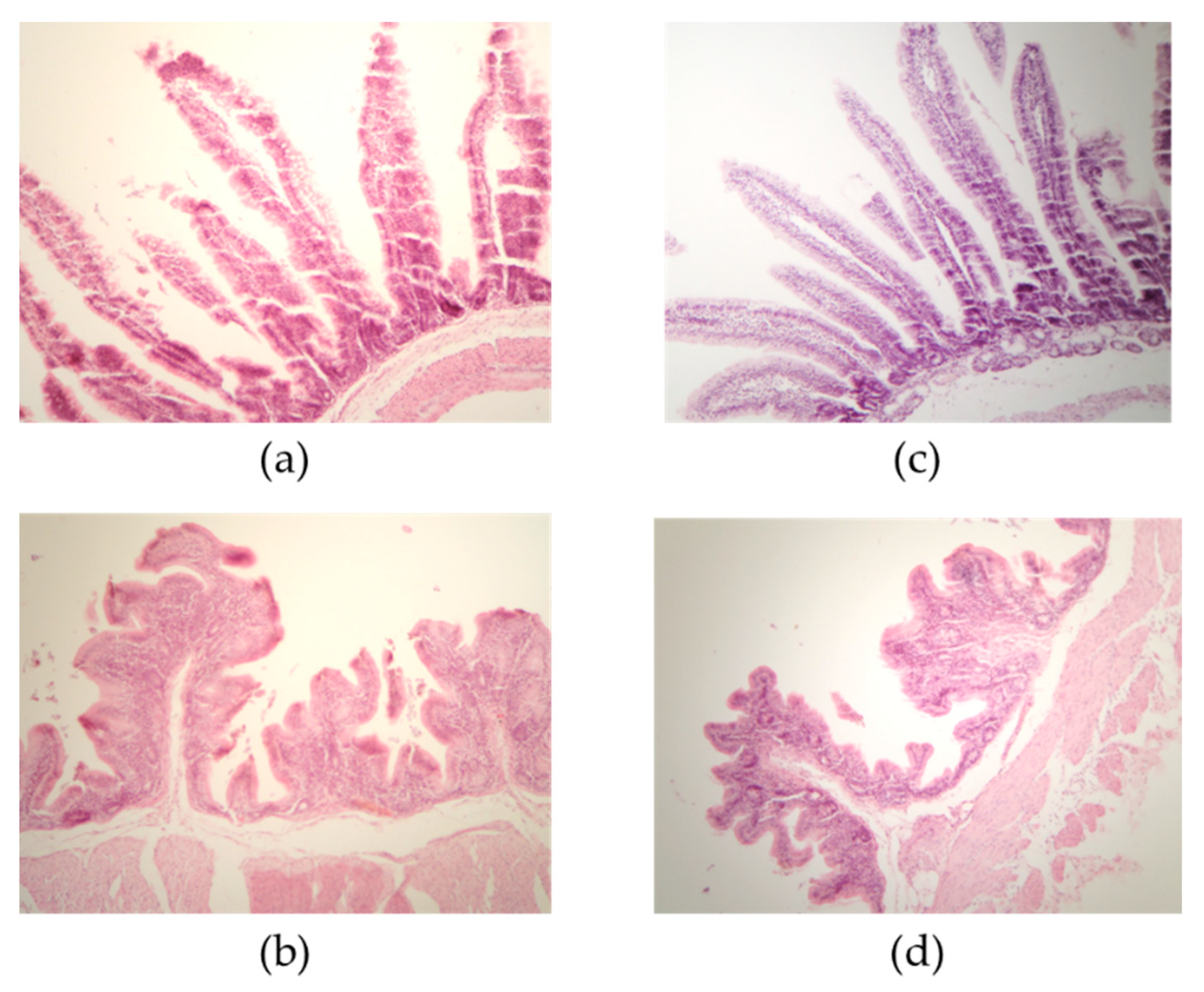
| Segment | T1 * | T2 * | p-Value |
|---|---|---|---|
| Villus Length in Small Intestine and Cecum | |||
| Duodenum | 363.86 ± 93.25 a | 350.85 ± 49.23 a | 0.5019 |
| Jejunum | 424.83 ± 36.43 b | 513.69 ± 70.35 a | <0.0001 |
| Ileum | 872.73 ± 25.47 a | 675.46 ± 88.96 b | <0.0001 |
| Cecum | 157.02 ± 18.44 b | 241.04 ± 53.90 a | <0.0001 |
| Crypt depth in small intestine and cecum | |||
| Duodenum | 58.77 ± 6.59 a | 59.60 ± 6.42 b | 0.6218 |
| Jejunum | 97.43 ± 13.17 a | 84.03 ± 19.95 b | 0.0032 |
| Ileum | 112.53 ± 18.41 a | 93.03 ± 12.83 b | <0.0001 |
| Cecum | 256.87 ± 8.21 b | 356.85 ± 13.37 a | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Martínez, C.A.; Levin, L.; Treviño-Cabrera, G.; Hernández-Luna, C.E.; Bernal-Barragán, H.; Castillo-Velázquez, U.; Rodríguez-Tovar, L.E.; Dávila-Martínez, C.; Trejo-Chávez, A.; Méndez-Zamora, G.; et al. Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition. Microbiol. Res. 2022, 13, 1018-1026. https://doi.org/10.3390/microbiolres13040074
Hernández-Martínez CA, Levin L, Treviño-Cabrera G, Hernández-Luna CE, Bernal-Barragán H, Castillo-Velázquez U, Rodríguez-Tovar LE, Dávila-Martínez C, Trejo-Chávez A, Méndez-Zamora G, et al. Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition. Microbiology Research. 2022; 13(4):1018-1026. https://doi.org/10.3390/microbiolres13040074
Chicago/Turabian StyleHernández-Martínez, Carlos A., Laura Levin, Griselda Treviño-Cabrera, Carlos E. Hernández-Luna, Hugo Bernal-Barragán, Uziel Castillo-Velázquez, Luis Edgar Rodríguez-Tovar, Cesar Dávila-Martínez, Armando Trejo-Chávez, Gerardo Méndez-Zamora, and et al. 2022. "Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition" Microbiology Research 13, no. 4: 1018-1026. https://doi.org/10.3390/microbiolres13040074
APA StyleHernández-Martínez, C. A., Levin, L., Treviño-Cabrera, G., Hernández-Luna, C. E., Bernal-Barragán, H., Castillo-Velázquez, U., Rodríguez-Tovar, L. E., Dávila-Martínez, C., Trejo-Chávez, A., Méndez-Zamora, G., & Gutiérrez-Soto, G. (2022). Effect of a Diet Based on Biotransformed Sorghum on Rabbit Intestinal Morphology and Fecal Fiber Composition. Microbiology Research, 13(4), 1018-1026. https://doi.org/10.3390/microbiolres13040074







