Abstract
The altered patterns of a microbial population colonizing an organ are increasingly recognized as a relevant item in human disease pathogenesis. The female urogenital tract is no exception, as some vaginal microbiota patterns, named community state types (CSTs), and urinary tract microbiota patterns, named urotypes (UTs), have been linked to viral, inflammatory, and gestational diseases. Treating these conditions is an issue, as antibiotic therapies alone are not always effective. Lactobacillus crispatus M247 is a strain with good intestinal and vaginal adhesion capability, combined with local antibacterial and anti-inflammatory properties; this strain also has proven nontransferable resistance to antibiotics commonly used in female genital tract infections, such as metronidazole. Lactobacillus crispatus M247 could, therefore, be considered as a potential add-on therapy to antibiotics in vaginal tract infections, with the aim to restore a favorable microbiota pattern.
1. Introduction
The vaginal microbiota constitutes a peculiar ecosystem with a significant impact on female health. Analysis identified five clearly distinguishable community status types (CSTs), of which four are characterized by the predominance of a single Lactobacilli species: CST I—Lactobacillus crispatus; CST II—Lactobacillus gasseri; CST III—Lactobacillus iners; and CST V—Lactobacillus jensenii. CST IV consists of a heterogeneous group of facultative and narrow anaerobes, including various bacteria associated with bacterial vaginosis—in this context, a poor representation of Lactobacilli (BV) [1]. CST IV is correlated in the literature with candida and chlamydial infections, the persistence of human papillomavirus (HPV), sine causal infertility, and spontaneous preterm and menopausal births with vulvovaginal atrophy, with particular impacts associated with greater biodiversity and bacterial presence [2,3,4]. As widely investigated, cervical cancer progression is related to oxidative damage caused by persistent infection by one of the oncogenic types of the HPV, with cellular damage being provoked by oxidative stress. The expression of oncoprotein by HPV causes an increase in reactive oxygen and nitrogen species, promoting tumor progression through metabolic processes [5]. Furthermore, more recently, the presence of a dysbiotic vaginal microbiota has been highlighted as a contributor to carcinogenesis, with an increase in genital inflammation and local perturbation of amino acid and nucleotide metabolisms by non-Lactobacillus-dominant communities, particularly in a setting of high-grade dysplasia [6].
Furthermore, in considering gynecological health, it is not possible to exclude an analysis of the urinary tract microbiota, which has led to the identification of eight different urotypes (UTs), where UT7, characterized by Lactobacillus crispatus, has been identified only in the female sex, while the others are found in both male and female subjects [7]. Urinary tract infections synchronous to gastroenteritis could be sustained by infectious agents, such as Enterococcus strains; it has recently been highlighted in a rat model how, as the basis of these infections, there is an important involvement of the inflammatory process, and how natural substances could provide protection [8]. In addition, it is tempting to speculate how a favorable UTs could provide a protective setting against urinary tract infections with the modulation of inflammation.
Traditionally, gynecological infections are treated with drugs such as metronidazole, antifungal drugs, and lactic acid and boric acid [9]; however, resolving vaginal microbiota dysbiosis as the basis of these infections could be proven. Therefore, beyond the standard antibiotic therapy, alternative or adjunct treatments have been considered, including probiotics or even vaginal microbiota transplants, to ensure successful treatment outcomes and reduce colonization by pathogenic microbes of the female reproductive tract [10]. Probiotics containing Lactobacillus spp. have been overlooked as a possible choice in maintaining vaginal microbiota homeostasis through an oral route or inserted vaginally; however, various strains have been evaluated with mixed results, and there is no common therapeutic behavior. Another approach that has been proposed is microbiome transplant, and some small randomized controlled trials (RCTs) are ongoing [10]. The use of probiotics during an antibiotic therapy in light of the current legislation is considered to be a meaningless medical practice as, in order to be marketed to the European Food Safety Authority (EFSA), probiotics need to show significant sensitivity to antibiotic-acting molecules [11]. The use of probiotics characterized by single, nontransferable antibiotic resistance characteristics could be considered to be a useful tool in the management of gynecological well-being [12]. Metronidazole is not included in the antibiotic resistance profiling panel of a probiotic prescribed by EFSA [10].
2. Lactobacillus crispatus M247
Lactobacillus crispatus M247 (IDA: LMG-P-23257) is a strain that was isolated in 1989 from the fecal material of a healthy child. It is greatly studied for its favorable characteristics from the point of view of aggregation, colonization, and the modulation of inflammatory phenomena, initially evaluated in the intestine [13,14,15,16]. Genetic analysis revealed a genome of 2,112,063 bp (2.1 Mbp) with 2187 coding genes, of which 236 are involved in carbohydrate metabolism, 203 in protein metabolism, 107 in DNA, 84 in RNA, 83 in biosynthesis of cell walls and capsules, and 55 in the ribosome [16]. Of particular interest is the identification of a gene previously identified in Lactobacillus crispatus ST1 that encodes Lactobacillus epithelium adesin (LEA), an element at the basis of the colonization capacity of the vaginal epithelium and of determining the competition for the biological niche with Gardnerella vaginalis [17,18] that, together with the genes encoding fibronectin (fibronectin type III domain and N-terminal fibronectin A binding protein FbpA), contributes to vaginal colonization and competition with pathogens such as Gardnerella vaginalis [18]. Widely documented is the ability of Lactobacilli to produce different types of exopolysaccharides (EPS), functional substances that support bacterial adhesion and protection from environmental factors, such as substances with an antibiotic action and drying [18]. For Lactobacillus crispatus M247, they have been characterized as having 18 genes encoding enzymes involved in the production of EPS [16]. This set of characteristics and the data published in the literature indicate that Lactobacillus crispatus M247 has suitable characteristics for the colonization of vaginal tissue following oral or local administration [19,20] if the appropriate environmental conditions occur. Furthermore, in line with what was observed with the gene analysis of other Lactobacilli, suitable characteristics for the production of bacteriocins were also found for the M247 strain, including two elveticins, a penocin, two enterolysins, and a bacteriocin of the LS2 group [21]. Although this characteristic has not yet been demonstrated at the phenotypic level, antagonistic action against certain strains of uropathogenic Staphylococcus epidermidis and Escherichia coli was documented [16]. In light of the current knowledge, the strain is considered to be safe, it does not demonstrate elements of virulence, and a plasmid presence is unlikely, showing itself to be safe from a phenotypic point of view according to the criteria established by EFSA [20,22]. The combination of these characteristics render Lactobacillus crispatus M247 a candidate strain for microbial control at the intestinal level and, above all, at the urogynecological level.
3. Functional Characteristics of Lactobacillus crispatus M247
Lactobacillus crispatus M247 shows good survival characteristics against the main physiological barriers, with survival exposed to gastric juice at pH 3 for 90 min correlated to a loss of 3 logs, to bile salts at 0.5% for 48 h with a loss of 1–2 logs (depending on the bile salts used) and 0.2% pancreatin for 3 h increasing by 1 log in experimental pancreatic juice. The adhesion properties were evaluated to be at least eight times higher on human ileostomic glycoproteins and Caco2 cells than its spontaneous nonaggregating isogenic mutant (MU5), characteristics confirmed with the evaluation of colonization in vivo, where the administration of 10 billion live and viable cells for 8 days correlated with a strong presence in all fecal samples and in the majority of biopsy samples [23]. The ability of Lactobacilli to produce hydrogen peroxide is considered by some authors to be an important mechanism in biological antagonism against unwanted microorganisms, such as pathogens, and Lactobacillus crispatus M247 is an efficient producer of hydrogen peroxide in the context of a production capacity that is rather varied [11]. Hydrogen peroxide has recently been re-evaluated, highlighting modest antimicrobial capacities compared to other substances, such as lactic acid [7,9], leading to questions regarding an effective role in vivo as an antimicrobial agent produced by the vaginal microbiota [9]. Despite this evidence, it was recently clarified how Lactobacillus crispatus M247 uses hydrogen peroxide as an inducer of PPAR-γ activation in intestinal epithelial cells, modulating the cellular response to inflammatory stimuli [13], a context where hydrogen peroxide plays the role of a signal transduction factor. This mechanism favors the production of TLR-2, with a consequent reduction in TLR-4, positively intervening in the integrity of the tight junctions and limiting the ability of Gram-characterized lipopolysaccharide (LPS) bacteria to produce TNF-α-mediated inflammatory processes [24], as described in Figure 1. This ability has been highlighted in experimental colitis models where the strain demonstrated the ability to modulate the inflammatory condition [15].
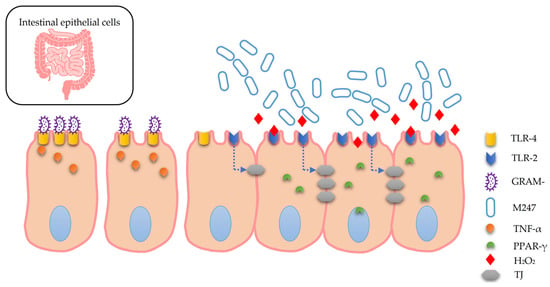
Figure 1.
GRAM- bacteria can promote inflammatory dynamics with TNF-α release. Lactobacillus crispatus M247 releases hydrogen peroxide (H2O2) that induces the upmodulation of PPAR-γ in intestinal epithelial cells. PPAR-γ causes an increase in TLR-2, and a decrease in TLR-4 and the integrity of the tight junctions (TJ). These events, on the whole, produce effects that contribute to the modulation of inflammatory processes. (see text).
3.1. Vaginal Colonization
The scientific literature has documented the ability of transferring Lactobacilli from the intestinal to the vaginal environment following continuous administration for 4–5 weeks [21]. An evaluation of the intestinal and vaginal colonization capacity of Lactobacillus crispatus M247, following the oral administration of 10 billion live cells per day for 2 weeks in healthy women, documented the presence of the M247 strain in the colon of 70% of the subjects, and in the vagina of 40% of the subjects [20]. Further evaluations describe that, following topical application for 3 days by vaginal douche, the M247 strain was recovered alive from almost 100% of the subjects, suggesting a rapid colonization capacity with this method [20].
3.2. Antibiotic Resistance Profile
The antibiotic resistance profile of Lactobacillus crispatus M247 was defined by Di Pierro et al. [22] according to European standards, such as the EFSA guidelines, comparing the MIC values obtained for the predicted antibiotics and the breakpoints defined by EFSA for the corresponding genus or species, using Lactobacillus acidophilus as a reference. In detail, the resistance profiles were assessed for a panel of 19 antibiotics, of which 10 compared with the threshold values defined by the EFSA guidelines. In addition, an evaluation of the effects exerted by boric acid, commonly used in the treatment of BV, was added. The minimal inhibitory concentrations (MICs) were determined for the 19 antibiotics and boric acid using the resistance profiles of Lactobacillus paracasei ATCC 334 as reference quality controls, as follows:
- Ampicillin, penicillin, clindamycin and linezolid (range: 0.03–16 μg/mL).
- Vancomycin and ciprofloxacin (range: 0.25–128 μg/mL).
- Neomycin, gentamicin and streptomycin (range: 0.5–256 μg/mL).
- Kanamycin (range: 2–1024 μg/mL).
- Erythromycin and quinupristin–dalfopristin (range: 0.016–8 μg/mL).
- Tetracycline, chloramphenicol, rifampicin, rifaximin and trimethoprim (range: 0.125–64 μg/mL).
- Metronidazole and sulfamethoxazole (range: 0.5–256 μg/mL).
- Boric acid (range: 16–10,000 μg/mL).
The Lactobacillus crispatus M247 strain showed significant resistance to metronidazole, sulfamethoxazole and boric acid, substances used in the clinical routine for the treatment of vaginal infections (Table 1).

Table 1.
Minimal inhibitory concentration (MIC) for L. crispatus M247 expressed in μg/mL. S: sensitive; nr: not required; /: not mentioned in the EFSA guidelines.
3.3. Adhesion Profile
The epithelial adhesion capacities of Lactobacillus crispatus M247 were investigated by Di Pierro et al. [22] regarding the intestinal and vaginal districts. Evaluations were performed in vitro on human epithelial cell lines, HT29-MTX, and the HeLa colonic epithelial model (derived from the cervix), for the vaginal epithelial model; all adhesion tests were performed in duplicate. The strain demonstrated a significant adhesion capacity, with values of 75% and 72% for the HT29-MTX and HeLa cell lines, respectively (Table 2).

Table 2.
Adhesion percentages obtained for L. crispatus M247 on HT29-MTX and HeLa cells. CFU: colony forming unit.
4. Lactobacillus crispatus LM247: Preliminary Efficacy Evaluations
A recent study on Lactobacillus crispatus strain M247 (LMGP-23257) took into consideration the obtainable effects with the administration of the version produced by Labomar SpA (Istrana, Treviso, Italy) and marketed by Pharmextracta SpA (Pontenure, Piacenza, Italy) with the trade name of (Crispact®), notified to the Italian Health Authority on 1 March 2019, with notification number 115450, using a 3-month treatment with no fewer than 20 billion colony forming units (CFUs). The declared objective of the study was to evaluate the efficacy profile of an oral treatment based on Lactobacillus crispatus M247 on a sample of 35 women affected by HPV cervical infection [24]. With regard to the inclusion criteria, the women were sexually active, aged between 18 and 65, and had a positive HPV DNA test, and/or various types of anomalies were found in the Pap test, such as ASCUS, L-SIL, or reactive cellular changes associated with tissue inflammation/atrophy. Oral treatment for 90 days with a probiotic based on Lactobacillus crispatus M247 significantly modified the composition of the vaginal microbiota of the women subjected to the treatment. In particular, 97% of the women treated (34/35) enriched their vaginal microbiota in Lactobacillus, with the latter becoming dominant (CST IV). The remaining women still showed, despite the use of the aforementioned probiotic, a very poor vaginal microbiota in Lactobacillus spp. (CST IV). In addition, the OTU value or the expression of richness was significantly decreased from 35 ± 9 to 18 ± 5 after 90 days of treatment.
As far as HPV status was concerned, oral treatment with the M247 strain allowed for its negativization in 71% of the women, compared to the HR and LR strains of HPV. The two women positive for HPV16 and for mRNA showed persistence of the infection despite the use of the probiotic strain. Of the nine women who tested positive for HPV16 at baseline, five tested negative following the treatment. Instead, the women positive for HPV18 all tested negative after 90 days. The women who were still HR-HPV-positive despite treatment had variable ages. Therefore, the oral use of Lactobacillus crispatus M247 for 90 days positively modulated the vaginal microbiota and modified the HPV status in subjects treated without distinction regardless of age, thus proving to be an effective tool in this pathological context in all ages (from 18 to 65 years old). The product, as reported by the patients, also showed good tolerability. In fact, on the one hand, adherence to the therapy was particularly high (over 95%); on the other, no significant side effects were recorded during the 90 days of treatment other than minor adverse events of transient duration, such as meteorism and flatulence, recorded in seven patients.
Furthermore, Lactobacillus M247 seems to be able to promote an increase in viral clearance. In fact, the CST deviations observed between enrollment at t = 0 and after 90 days of treatment were significant. Several authors have observed variable spontaneous increases in the viral clearance in HPV-positive subjects; recently, Petry et al. reported spontaneous viral clearance in approximately 50% of cases over 12 months [16]. In a pilot observational study, a clearance of over 70% over a period of time of less than one year was observed, suggesting that the oral use of Lactobacillus M247 could guarantee a higher increase in clearance than what would occur spontaneously. Interpretatively, we can hypothesize that the positive effects obtained from this study, although not controlled, may depend on the role played by the bacterial strain administered orally in structuring and positively modulating the vaginal microbiota, and in its ability to colonize the colon and vagina. In fact, previous evidence shows that, in the strain that we used, two specific genes have been identified (genes coding for the type III domain of fibronectin and for the N-terminal fibronectin-binding protein A; FbpA gene) that are associated with the colonization capacity of the vaginal mucosa and the competitive colonization with G. vaginalis, on which its ability to reduce the onset of bacterial vaginosis depends [18]. Furthermore, the M2427 strain is capable of producing a series of bacteriocins (i.e., two helveticins, one penoncin, two enterolysins, and one bacteriocin of the LSD group) that are capable of antagonizing some uropathogenic strains of Escherichia coli and Staphylococcus epidermidis [18]. Added to this is the ability of Lactobacilli to produce exopolysaccharides (EPS) that can facilitate adhesion to mucous membranes [19].
In addition, L. crispatus M2427 is a good producer of hydrogen peroxide. Hydrogen peroxide is considered to be an effective defense mechanism against vaginal colonization by unwanted microorganisms [20] and is able, by activating PPAR-gamma, to directly modulate the response of epithelial cells to inflammatory stimuli [13]. Furthermore, the increase in the amount of TLR-2 and the reduction in TLR-4 depend on the latter aspect, and the consequent strengthening of the tight junctions, which determines the reduction in the ability of Gram-negative bacteria equipped with LPS to induce inflammatory processes mediated by TNF-alpha [15]. The obtained results, correlated to the cited scientific evidence, can lead us to hypothesize good efficacy for oral treatment based on L Crispatus M247 against HPV infection and a possible improvement in local immunity against the virus, suggesting in this preliminary evaluation the ability to change almost all CSTs to an unfavorable type I CST in the setting of sexually active patients with persistent HPV infection, and contributing to the eradication of approximately two-thirds of the high-grade HPV present [16].
5. Discussion
As widely documented in the literature, there are several nutraceutical solutions that show good functionality as an add-on therapy in different clinical scenarios [25,26,27,28,29,30]. These, together with the appropriate lifestyle corrections [31,32], can contribute to the success of standard care. From this point of view, Lactobacillus crispatus M247 can be proposed as a precision probiotic tool. In addition to being widely documented for human use, it shows several peculiar characteristics, such as being a good intestinal and vaginal colonizer where, in addition to exerting significant anti-inflammatory effects, it contributes to the modulation of dysbiotic pictures, with the possibility of both oral and topical use. These potentials at the vaginal level could be further favored with the simultaneous use of substances that can hinder the development of other microbial species without altering the vitality of Lactobacillus crispatus M247, such as metronidazole, sulfamethoxazole, and boric acid. Metronidazole is commonly used in the treatment of BV, situations that are generally more frequent in the case of a vaginal consortium devoid of Lactobacilli (CST IV) and/or containing Lactobacilli other than L. crispatus (CST II, II and V). The simultaneous use of metronidazole and Lactobacillus crispatus M247, which shows metronidazole resistance, should rationally further favor the chances of colonization, creating a biological niche less crowded with pathogens and diners with which to compete. This is an approach that can be safely hypothesized, considering the absence of mobile genomic elements in the M247 strain, a situation that renders it impossible to transmit metronidazole resistance to other bacterial species, including pathogens [16]. A similar application can also be imagined for boric acid, commonly used in the treatment of vaginal infections, with particular reference to resistant antifungal candidiasis, where the simultaneous use of Lactobacillus crispatus M247 and boric acid could constitute an element favoring the colonization of the M247 strain. in the context of candida eradication. These potential uses become particularly interesting, especially if discussed in light of the adhesion properties of Lactobacillus crispatus M247 to vaginal cells, which is greater than 70%. Previously investigated and discussed in [22], the results confirm that they are comparable to what is already known about intestinal cells [13].
Recalling vaginal dysbiosis’s importance in sustaining human papilloma virus’s persistence, it is worth mentioning a recent work where Lactobacillus crispatus M247′s oral administration for a median period of 12 months resulted in a 15.3% HPV-DNA Pap test negativization against a 9.3% HPV-DNA negativization of patients assigned only to follow-up [33]. While this result is not statistically significative, these data could be considered to be very interesting for future developments where further investigations relating to the composition of the microbiota, both vaginal and intestinal, could clarify which factors and in which populations this application could be exploited to its previously described potential; further future and larger studies could potentially prove that Lactobacillus crispatus M247 can play an important role in cervical cancer prevention.
6. Conclusions
Lactobacillus crispatus M247 is a strain of considerable interest in the gynecological field, showing a significant colonization capacity of the vaginal epithelium, with an adhesion capacity greater than 70% and considerable versatility of use given the possibility of local and/or oral administration. Furthermore, its documented resistance to substances such as metronidazole, sulfamethoxazole, and boric acid allow for their simultaneous use, favoring a more effective eradication of bacterial and fungal vaginal pathogens, increasing the chances of colonization due to the reduced competition for the ecological niche. The combination of these characteristics favors a better management of dysbiotic condition, promoting greater possibilities of orienting the vaginal microbial consortium towards CST I, a more favorable framework for better gynecological health in the context of global intestinal and vaginal anti-inflammatory action. On the basis of these aspects, the use of Lactobacillus crispatus M247 can be hypothesized in subjects with CST IV and in subjects with CST II, CST III, or CST V and recurrent infections, a risk of preterm birth, or sine causa infertility, subjects with persistent HPV, subjects on fewer breaks with signs of vaginal atrophy, and subjects for whom other therapies for recurrent urinary infections have failed. In light of this evidence, Lactobacillus crispatus M247 can be considered to be an interesting precision probiotic tool for gynecological health, in particular but not exclusively for the simultaneous administration with metronidazole, sulfamethoxazole, and boric acid. Future clinical studies are necessary for the definition of the applicability margin, clinical impact, and possible definition of the appropriate therapeutic protocols.
Author Contributions
Conceptualization, A.B. and M.C. (Massimiliano Cazzaniga); investigation, A.B., M.C. (Massimiliano Cazzaniga) and F.D.P.; data curation, A.B., M.C. (Massimiliano Cazzaniga) and M.C. (Marco Cardinali); writing—original draft preparation, A.B., M.C. (Marco Cardinali) and G.Z.; review and editing, A.B., M.C. (Marco Cardinali) and F.D.P.; project administration, A.B. and G.Z.; figures and illustrations, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Bocking, A.; Hill, J.E.; Money, D.M.; VOGUE Research Group. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.I.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2018, 25, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Lepargneur, J.P. Lactobacillus crispatus as biomarker of the healthy vaginal tract. Ann. Biol. Clin. 2016, 74, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Preci, D.P.; Almeida, A.; Weiler, A.L.; Mukai Franciosi, M.L.; Cardoso, A.M. Oxidative damage and antioxidants in cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 265–271. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef]
- Gottschick, C.; Deng, Z.L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Filippone, A.; Peritore, A.F.; Cuzzocrea, S. Protective Effects of Xyloglucan in Association with the Polysaccharide Gelose in an Experimental Model of Gastroenteritis and Urinary Tract Infections. Int. J. Mol. Sci. 2018, 19, 1844. [Google Scholar] [CrossRef]
- Cesena, C.; Morelli, L.; Alander, M.; Siljander, T.; Tuomola, E.; Salminen, S.; Mattila-Sandholm, T.; Vilpponen-Salmela, T.; von Wright, A. Lactobacillus crispatus and its non-aggregating mutant in human colonization trials. J. Dairy Sci. 2001, 84, 1001–1010. [Google Scholar] [CrossRef]
- Martín, R.; Suárez, J.E. Biosynthesis and degradation of H2O2 by vaginal lactobacilli. Appl. Environ. Microbiol. 2010, 76, 400–405. [Google Scholar] [CrossRef]
- Strus, M.; Brzychczy-Włoch, M.; Kochan, P.; Heczko, P. Hydrogen peroxide produced by Lactobacillus species as a regulatory molecule for vaginal microflora. Med. Dosw. Mikrobiol. 2004, 56, 67–77. [Google Scholar] [PubMed]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.J.; Ser, H.L.; Kuai, Y.H.; Tan, L.T.; Arasoo, V.J.T.; Letchumanan, V.; Wang, L.; Pusparajah, P.; Goh, B.H.; Ab Mutalib, N.S.; et al. Finding a Balance in the Vaginal Microbiome: How Do We Treat and Prevent the Occurrence of Bacterial Vaginosis? Antibiotics 2021, 10, 719. [Google Scholar] [CrossRef]
- Voltan, S.; Martines, D.; Elli, M.; Brun, P.; Longo, S.; Porzionato, A.; Macchi, V.; D’Incà, R.; Scarpa, M.; Palù, G.; et al. Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-gamma in the intestinal mucosa. Gastroenterology 2008, 135, 1216–1227. [Google Scholar] [CrossRef]
- Voltan, S.; Castagliuolo, I.; Elli, M.; Longo, S.; Brun, P.; D’Incà, R.; Porzionato, A.; Macchi, V.; Palù, G.; Sturniolo, G.C.; et al. Aggregating phenotype in Lactobacillus crispatus determines intestinal colonization and TLR2 and TLR4 modulation in murine colonic mucosa. Clin. Vaccine Immunol. 2007, 14, 1138–1148. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Galeazzi, F.; Ferrari, S.; Elli, M.; Brun, P.; Cavaggioni, A.; Tormen, D.; Sturniolo, G.C.; Morelli, L.; Palù, G. Beneficial effect of auto-aggregating Lactobacillus crispatus on experimentally induced colitis in mice. FEMS Immunol. Med. Microbiol. 2005, 43, 197–204. [Google Scholar] [CrossRef][Green Version]
- AAT-Advanced Analytical Technologies. Lactobacillus crispatus LMG-P23257 in the Prevention of Recurrent Urinary Tract Infections (RUTI); Internal Document; AAT-Advanced Analytical Technologies: Fiorenzuola d’Arda, Italy, 2017. [Google Scholar]
- Edelman, S.M.; Lehti, T.A.; Kainulainen, V.; Antikainen, J.; Kylväjä, R.; Baumann, M.; Westerlund-Wikström, B.; Korhonen, T.K. Identification of a high-molecular-mass Lactobacillus epithelium adhesin (LEA) of Lactobacillus crispatus ST1 that binds to stratified squamous epithelium. Microbiology 2012, 158 (Pt 7) Pt 7, 1713–1722. [Google Scholar] [CrossRef]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bertuccioli, A.; Cattivelli, D.; Soldi, S.; Elli, M. Lactobacillus crispatus M247: A possible tool to counteract CST IV. Nutrafoods 2018, 17, 169–172. [Google Scholar]
- Ojala, T.; Kankainen, M.; Castro, J.; Cerca, N.; Edelman, S.; Westerlund-Wikström, B.; Paulin, L.; Holm, L.; Auvinen, P. Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis. BMC Genom. 2014, 15, 1070. [Google Scholar] [CrossRef]
- Strus, M.; Chmielarczyk, A.; Kochan, P.; Adamski, P.; Chełmicki, Z.; Chełmicki, A.; Pałucha, A.; Heczko, P.B. Studies on the effects of probiotic Lactobacillus mixture given orally on vaginal and rectal colonization and on parameters of vaginal health in women with intermediate vaginal flora. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 163, 210–215. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Sagheddu, V.; Cattivelli, D.; Soldi, S.; Elli, M. Antibiotic resistance profile and adhesion properties of Lactobacillus crispatus M247. Nutrafoods 2019, 2, 89–94. [Google Scholar] [CrossRef]
- Tachedjian, G.; O’Hanlon, D.E.; Ravel, J. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 2018, 6, 29. [Google Scholar] [CrossRef]
- Petry, K.U.; Horn, J.; Luyten, A.; Mikolajczyk, R.T. Punch biopsies shorten time to clearance of high-risk human papillomavirus infections of the uterine cervix. BMC Cancer 2018, 18, 318. [Google Scholar] [CrossRef]
- Di Pierro, F.; Zacconi, P.; Bertuccioli, A.; Togni, S.; Eggenhoffner, R.; Giacomelli, L.; Scaltrini, S. A naturally-inspired, curcumin-based lecithin formulation (Meriva® formulated as the finished product Algocur®) alleviates the osteo-muscular pain conditions in rugby players. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4935–4940. [Google Scholar] [PubMed]
- Dipierro, F.; Simonetti, G.; Petruzzi, A.; Bertuccioli, A.; Botta, L.; Bruzzone, M.G.; Cuccarini, V.; Fariselli, L.; Lamperti, E. Anovel lecithin-based delivery form of Boswellic acids as complementary treatment of radiochemotherapy-induced cerebral edema in patients with glioblastoma multiforme: A longitudinal pilot experience. J. Neurosurg. Sci. 2019, 63, 286–291. [Google Scholar] [CrossRef]
- Dipierro, F.; Khan, A.; Bertuccioli, A.; Maffioli, P.; Derosa, G.; Khan, S.; Khan, B.A.; Nigar, R.; Ujjan, I.; Devrajani, B.R. Quercetin Phytosome® as a potential candidate for managing COVID-19. Minerva Gastroenterol. 2021, 67, 190–195. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Giuberti, R.; Saponara, M.; Ivaldi, L. Role of a berberine-based nutritional supplement in reducing diarrhea in subjects with functional gastrointestinal disorders. Minerva Gastroenterol. Dietol. 2020, 66, 29–34. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Marini, E.; Ivaldi, L. A pilot trial on subjects with lactose and/or oligosaccharides intolerance treated with a fixed mixture of pure and enteric-coated α- and β galactosidase. Clin. Exp. Gastroenterol. 2015, 8, 95–100. [Google Scholar] [CrossRef][Green Version]
- Bertuccioli, A.; Ninfali, P. The Mediterranean Diet in the era of globalization: The need to support knowledge of healthy dietary factors in the new socio-economical framework. Mediterr. J. Nutr. Metab. 2014, 7, 75–86. [Google Scholar] [CrossRef]
- Zeppa, S.D.; Sisti, D.; Amatori, S.; Gervasi, M.; Agostini, D.; Piccoli, G.; Bertuccioli, A.; Rocchi, M.B.L.; Stocchi, V.; Sestili, P. High-intensity interval training promotes the shift to a health-supporting dietary pattern in young adults. Nutrients 2020, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus crispatus M247 oral administration: Is it really an effective strategy in the management of papillomavirus-infected women? Infect. Agent Cancer 2022, 17, 53. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).