Abstract
The use of prenatal antibiotics should be carefully considered, owing to their potential adverse effects on neonatal outcomes. This study aimed to identify the contributing factors to early-onset neonatal infection and to determine the influence of antepartum antibiotics on women and neonates. This study included 127 pregnant women without obvious intra-amniotic infection on admission, who delivered under 34 weeks of gestation. Information on maternal and neonatal characteristics was obtained from their medical charts. Vaginal swabs were taken from all women on admission. In total, 29 (22.8%) neonates developed early-onset infection. Multivariate analysis revealed that antepartum antibiotics were the most strongly associated factor for early-onset neonatal infection (odds ratio, 11.2; 95% confidence interval, 4.08–31.02). The frequency of early-onset neonatal infection was significantly higher in women who received antibiotic therapy than in those who did not; no significant difference in prolonging their gestation or neonatal morbidities was observed. The prevalence of women who hosted vaginal microorganisms on admission was similar to that in women whose infants subsequently developed early-onset neonatal infection compared with that of women whose infants did not. Among infants of the 40 women who received antepartum antibiotic therapy, 21 developed early-onset infection. Of the women who delivered these 21 infants, 62% (13/21) showed reduced lactobacilli and 43% (9/21) had resistant bacterial strains in their vaginal microbiota at the time of delivery. The use of antepartum antibiotics is the most strongly associated factor in early-onset neonatal infection; it does not prolong gestation and would change the vaginal environment.
1. Introduction
Preterm birth (PTB), defined as birth within 37 completed weeks of gestation, affects 5–18% of pregnancies. PTB is the leading cause of perinatal mortality and morbidity. Intra-amniotic infection (IAI) or inflammation, most often resulting from ascending infection from the cervix and vagina, is a well-known etiology for PTB. IAI is associated with 20% of all PTBs and 50% of extreme PTBs (≤28 weeks of gestation) [1].
To prevent PTB and attenuate neonatal morbidity and mortality, maternal antepartum antibiotics, antenatal corticosteroids, and magnesium sulfate have been used [2]. Several studies have reported the effect of antibiotic use women with preterm premature rupture of membranes (pPROM) such as prolonged the duration of pregnancy and attenuated neonatal morbidities [3,4]. Additionally, several studies have reported the efficacy of antibiotics in women with IAI or inflammation who presented with PTL [5,6].
There are several opportunities to use antibiotics to prevent infection or to treat established infection during the antepartum, intrapartum, and postpartum periods. However, there are some concerns about the use of antibiotics in clinical medicine. It has been reported that 20–50% of all prescribed antibiotics in acute care hospitals in the United States were inappropriate [7]. An antimicrobial stewardship program was developed in 2017 by the Joint Commission to promote appropriate antibiotic use to improve infection cure rates, reduce antibiotic resistance, and decrease the spread of multidrug-resistant organisms [8]. Antimicrobial prophylaxis has been demonstrated to result in the selection of resistant endogenous microbiota, as well as the nosocomial acquisition of resistant microorganisms [9]. Awareness of the potential adverse effects of resistant bacterial infections has been increasing among perinatologists. Changes in the resistance patterns of isolated strains of Escherichia coli in newborns have been reported [10,11,12,13,14,15]. Sepsis in very-low-birth-weight neonates with ampicillin-resistant E. coli is more likely to be fatal than infection with susceptible strains [14]. In addition, isolated group B streptococcus (GBS) resistant to clindamycin led to significant changes in the intrapartum protocols designed to prevent invasive neonatal GBS disease [16,17,18,19]. Early-onset neonatal infection (EoNI), representing vertical mother-to-infant transmission of pathogens before or during delivery, increases the morbidity and mortality rates in infants [20,21]. These might be caused by an intrauterine infection or the modification of normal maternal vaginal microbiota.
We aimed to identify the antenatal factors that contribute to EoNI and determine the effect of antenatal antibiotic use on pregnancy and neonatal outcomes.
2. Materials and Methods
2.1. Study Population
This retrospective study was conducted at the Perinatal Center, University of Miyazaki Hospital, Japan. This center is the only tertiary Perinatal Center in Miyazaki Prefecture that has a population of 1,000,000 with 10,000 annual deliveries. Pregnant women were eligible for this study if they experienced PTL with intact membranes or pPROM and delivered between 22+0 and 33+6 weeks of gestation between 2016 and 2020. Women with positive results on Gram staining and/or culture of the amniotic fluid upon admission, clinical chorioamnionitis, and infants with congenital malformation were excluded from this study.
Clinical chorioamnionitis was diagnosed according to Lencki’s criteria comprising maternal temperature of ≥38.0 °C and at least one of the following four clinical signs: maternal tachycardia ≥ 100 beats/minute, uterine tenderness, maternal leukocytosis (≥15,000/mm3), or foul-smelling vaginal discharge [22]. PTL was diagnosed if there was regular, painful uterine contraction and if cervical dilation was ≤3 cm. EoNI was defined as a positive blood culture or at least two of the following laboratory findings within 72 h after birth: absolute neutrophil count < 1750/mm3 or <10% of the total white blood cell count, immature/total (I/T) neutrophil ratio ≥ 0.2, platelet count < 10,000/mm3, or C-reactive protein (CRP) level > 1 mg/dL [23].
Data on maternal and neonatal characteristics were obtained from patients’ medical charts. Fetal heart-rate-monitoring records were reviewed for nonreassuring fetal heart rate patterns, such as recurrent late decelerations with absent or decreased variability, with or without fetal tachycardia (>160 beats/minute). The women underwent transabdominal amniocentesis to determine if IAI was present upon admission if it were possible. Vaginal culture sampling with antimicrobial susceptibility was performed upon admission for all women. We observed the change of vaginal microbiota, focusing on a depletion of lactobacilli and/or bacteria with antimicrobial resistance from admission to delivery. Physical and blood examinations of all the women were performed upon admission. Neonates were tested for white blood cell counts, platelet counts, and CRP level at birth and every 8 h thereafter until 72 h to evaluate neonatal infection. Cultures were obtained from the neonates’ oral cavities and skin surfaces on admission. Umbilical cord blood was tested for blood gas analysis and culture. The identification of microorganism was conducted with bacterial culture method, not being evaluated by 16S rRNA gene sequencing. Histopathological findings of the placenta and cord were evaluated for maternal and fetal inflammatory responses according to Blanc’s classification [24] and Redline’s criteria [25].
2.2. Management of PTL with Intact Membranes or pPROM
All women in this study were hospitalized and expectantly managed, unless they had an obstetrical indication for delivery or had clinical chorioamnionitis. Pregnant women with PTL only received tocolytic therapy if indicated. Antenatal corticosteroids were administered to women up to 34 weeks of gestation when indicated. Fetal well-being was assessed for women undergoing expectant management by using daily nonstress tests and weekly biophysical profile scoring.
The antibiotic strategy for women with pPROM changed during the course of the study. From 1 January 2016 to 30 June 2018, only ampicillin was administered. From 1 July 2018 to 31 December 2020, a 7-day course of antibiotic therapy with a combination of intravenous ampicillin and erythromycin followed by oral amoxicillin and erythromycin was used to prolong gestation.
Women were treated with oral or intravenous antibiotics until the disappearance of bacteria during expectant management when they had urinary tract infection (UTI). We discontinued the antibiotics when we confirmed negative urine cultures. Prophylactic antibiotics to prevent vertical transmission of GBS during delivery or threatened preterm delivery were administered for a positive vaginal GBS culture or for those with unknown GBS culture results.
2.3. Statistical Analysis
Between-group differences were assessed using the Mann–Whitney U, Welch’s t, chi-square, or Fisher’s exact test. Results with p-values of <0.05 were considered significant. Multivariate logistic regression analysis was performed to identify any independent predictive factors of EoNI. Only predictive variables with a p-value of <0.1 on univariate analysis were entered into the logistic regression model. A stepwise forward procedure using the likelihood ratio test was employed in the multivariate logistic regression analysis. Variables with p-values of <0.05 in the final model were determined to be independent predictive factors of EoNI.
Statistical analyses were performed using SPSS version 27 for Windows (IBM SPSS Statistics, Tokyo, Japan). Data are expressed as mean ± standard deviation, n (%), or median (minimum, maximum).
3. Results
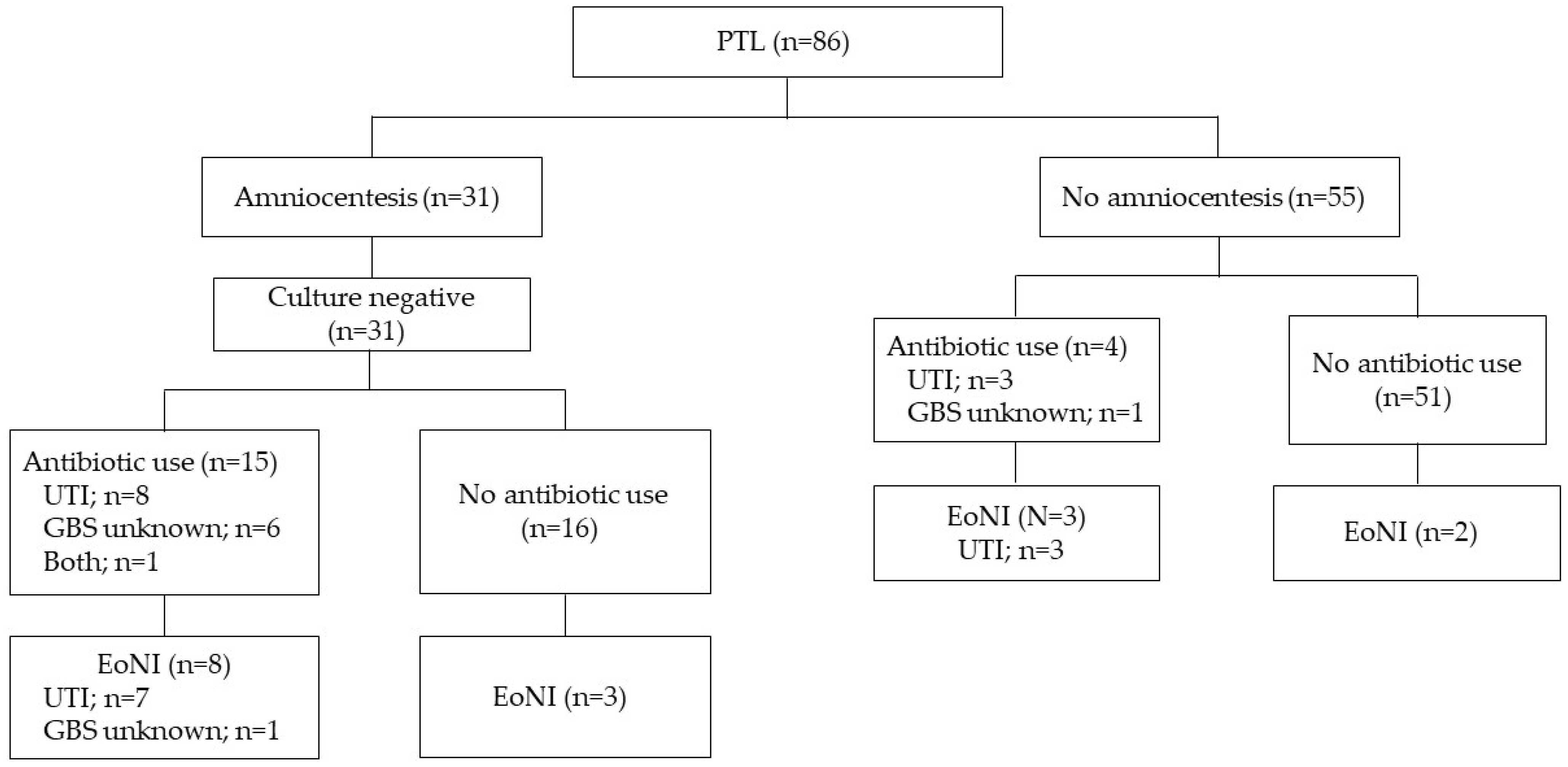
The flowchart of this study is depicted in Figure 1. A total of 246 preterm births between 22+0 and 33+6 weeks of gestation had been admitted to the hospital during the study period. Among them, 91 were excluded because of obstetrical complications, including multifetal pregnancies and placenta previa without signs of PTL, fetal growth restrictions, and hypertensive disorders of pregnancy (n = 91). Of the remaining 155 women with PTL or pPROM, exclusions were made based on obvious chorioamnionitis on admission (n = 9), congenital malformations of the neonates (n = 8), and unavailable data (n = 11). In total, 127 women with PTL or pPROM who delivered under 34 weeks of gestation were eligible for the study.
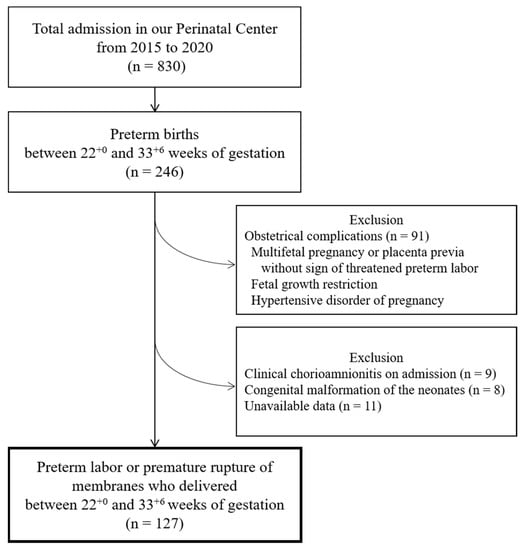
Figure 1.
A flowchart of this study.
A total of 29 (22.8%) neonates developed EoNI. The study participants were divided into EoNI and non-EoNI groups. Table 1 and Table 2 present the maternal and neonatal characteristics. The percentage of women receiving antepartum antibiotics and transabdominal amniocentesis was higher in the EoNI group than in the non-EoNI group.

Table 1.
Characteristics of the pregnant women.

Table 2.
The characteristics of neonates.
Among the infants of the 40 women exposed to antepartum antibiotics, 21 developed EoNI. Twelve neonates in EoNI group showed positive blood culture on admission. Among them, 9 mothers received antepartum antibiotics. The proportion of neontates with positive blood culture in mother used and mother not used antepartum antibiotics were 43% (9/21) and 38% (3/8), respectively. No statistical difference observed in the frequency of positive blood culture between the two groups (p = 0.66). Their mothers in EoNI group received antepartum antibiotics for 4.5 ± 2.9 days. The remaining 19 mothers without EoNI received antepartum antibiotics for 3.3 ± 1.9 days. No statistical difference was observed in the duration of antibiotic exposure between the EoNI and non-EoNI groups. The incidence of histological chorioamnionitis of the placentas diagnosed as Blanc’s classification stage III was statistically higher in women with EoNI (16/21, 76%) than in those without EoNI (8/19, 42%; p = 0.028).
In the EoNI group, 25 neonates had positive culture results from several sites, i.e., blood cultures (n = 12; 9 exposed and 3 not exposed to antepartum antibiotics), oral cavities or skin surfaces only (n = 10; 7 exposed and 3 not exposed to antepartum antibiotics), amniotic fluids (n = 3; 2 exposed and 1 not exposed to antepartum antibiotics). Among them, microorganisms of 12 neonates isolated from 7 blood culture (5 exposed and 2 not exposed to antepartum antibiotics) and 5 oral cavities or skin surfaces only (3 exposed and 2 not exposed to antepartum antibiotics) were consisted with them in maternal vaginal specimens on admission.
Table 3 lists the vaginal microorganisms isolated from all women on admission. Multiple microorganisms were isolated from women in both the EoNI and non-EoNI groups. The proportion of the number of women who carried Lactobacillus sp. and other vaginal microorganisms were similar between the two groups. However, of the 21 women in the EoNI group, a depletion of lactobacilli was observed in 13/21 (61.9%), and bacteria with antimicrobial resistance were detected in 9/21 (42.9%) at the time of delivery. There included 2 women who carried both reduced lactobacilli and bacteria with resistance.

Table 3.
The microorganisms in vaginal specimen cultures obtained from all pregnant women on admission, and the number of women carrying these bacteria.
The results of the multivariate logistic regression analysis are summarized in Table 4. Seven predictive factors, namely, first childbirth age ≥ 35-year-old, pPROM, antepartum antibiotic use, maternal temperature ≥ 38.0 °C, gestational age at delivery, birthweight, and male sex, were entered into the multivariate model. Three predictive factors, namely, antepartum antibiotic exposure, male sex, and gestational age at delivery, were found to be independent predictive factors of EoNI.

Table 4.
Results of the multivariate logistic regression analysis of the predictive factors.
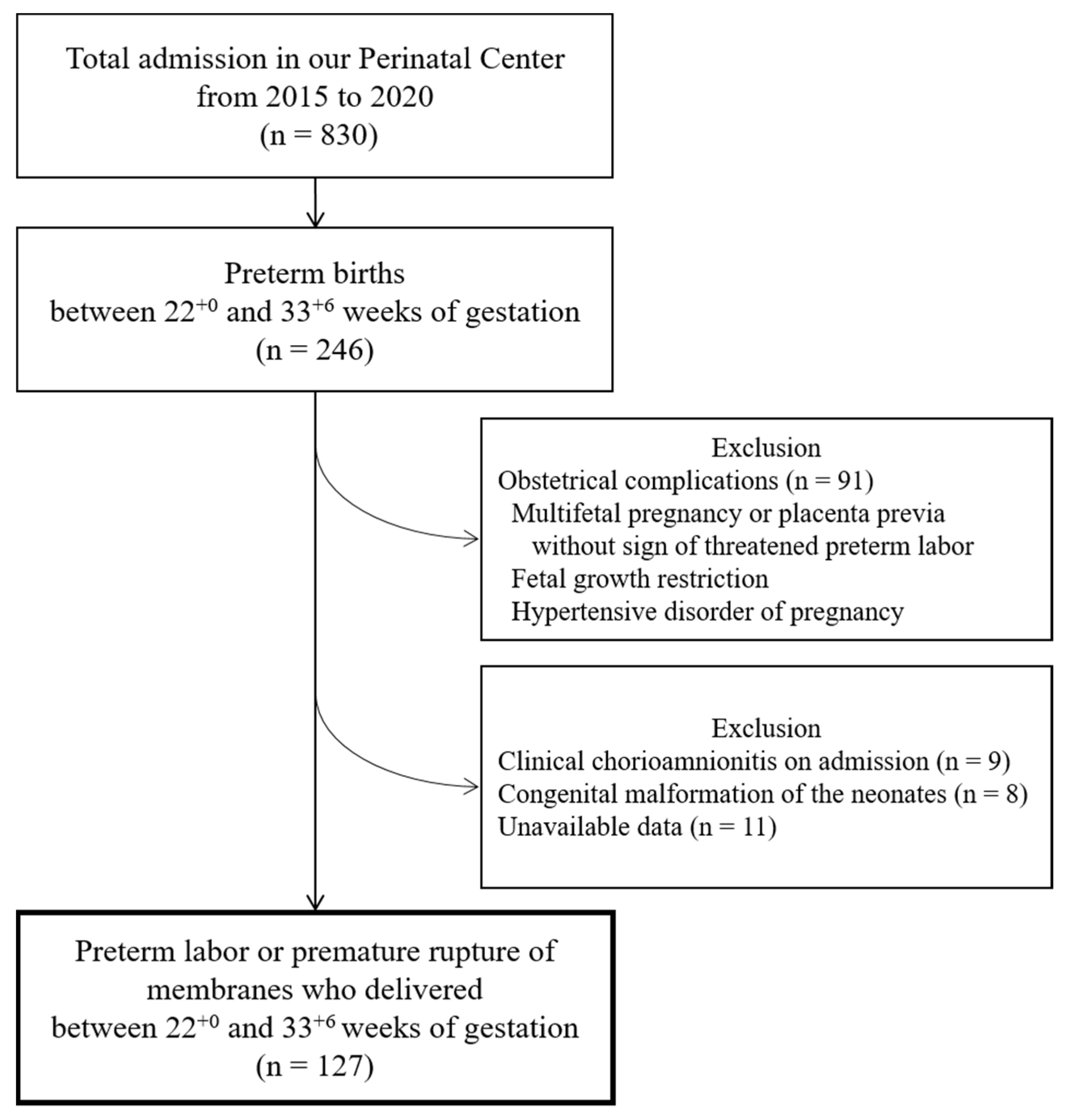
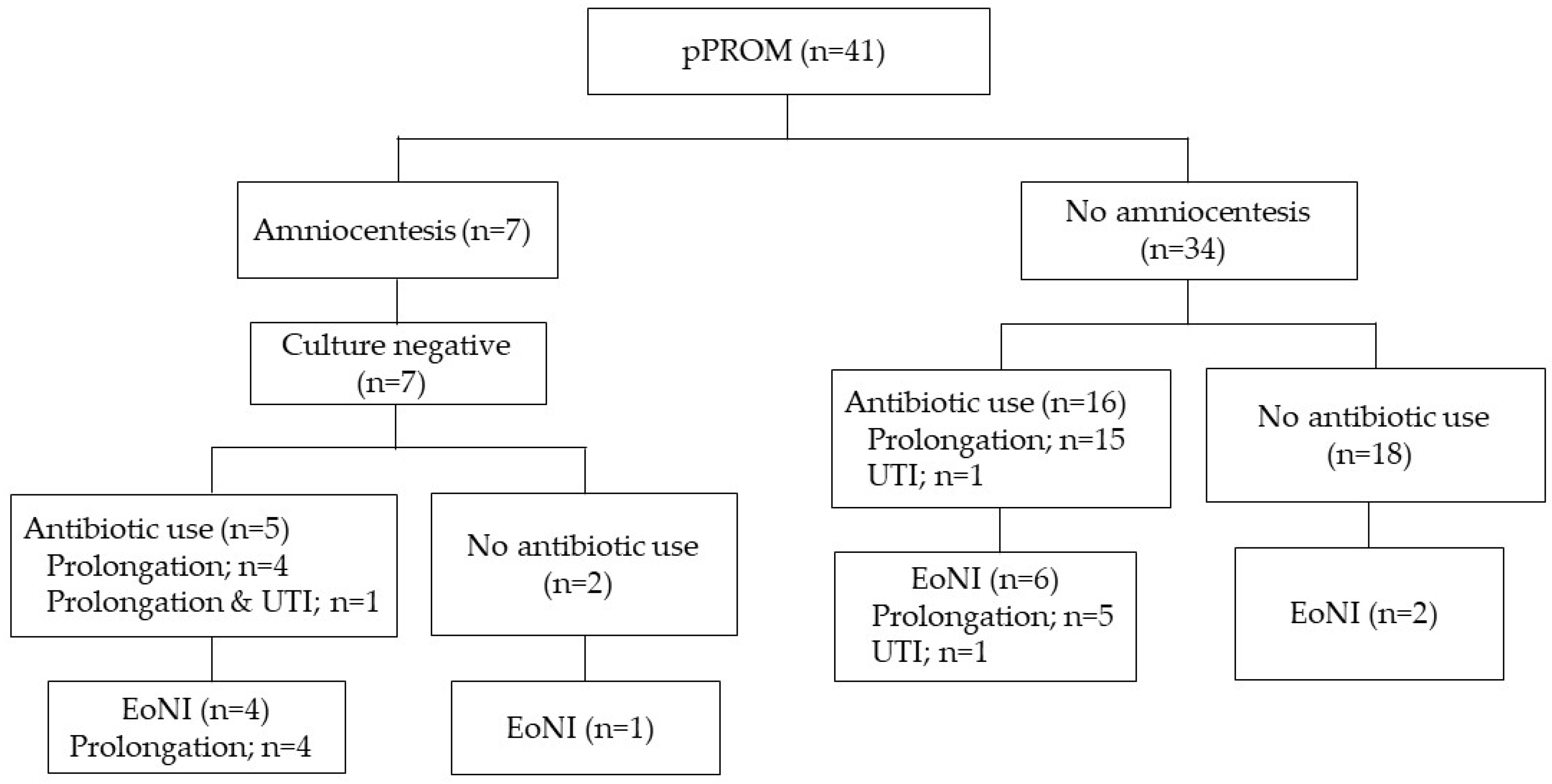
Women with pPROM and threatened PTL were assessed separately, as shown in Figure 2 and Figure 3. The proportion of EoNI in the antibiotics was statistically higher than that in the women not receiving antibiotics among both women with pPROM (p < 0.05) and those with threatened PTL (p < 0.01).
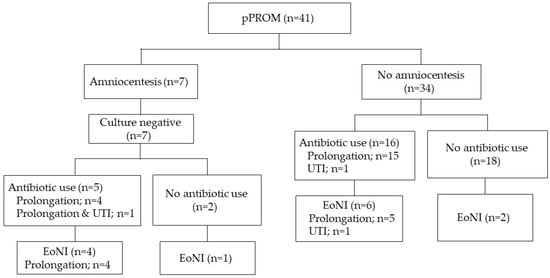
Figure 2.
Incidence of EoNI and indications for antepartum antibiotic use in pPROM. Abbreviations: EoNI, early-onset neonatal infection; UTI, urinary tract infection.
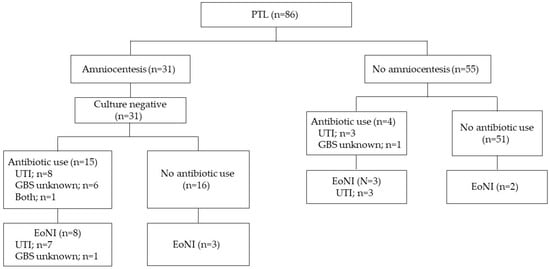
Figure 3.
Incidence of EoNI and indications for antepartum antibiotics in PTL. Abbreviations: EoNI, early-onset neonatal infection; UTI, urinary tract infection; PTL, preterm labor.
No significant differences were observed in gestation prolongation or neonatal morbidities, including RDS, NEC, grade III or IV IVH, and periventricular leukomalacia between those exposed and not exposed to antibiotics. Among women with pPROM or PTL, no statistical difference gestation prolongation was noted between women who received and those who did not receive antibiotics (Table 5).

Table 5.
The effects of antibiotics exposure on infants’ morbidities and prolongation of gestation.
4. Discussion
The results of this study indicated that antibiotic use during the antepartum period significantly associated with the risk of EoNI in women with PTL or pPROM who had no signs of clinical chorioamnionitis upon admission and delivered under 34 weeks of gestation. In addition, antibiotic use may contribute to adverse effects on vaginal microbiota, including the depletion of lactobacilli and the emergence of resistant strains of common bacteria.
We emphasize that the benefits of antibiotic therapy when indicated are clearly significant. However, its irresponsible use without any microbiological justification is dangerous and leads to adverse effects. Mercer et al. [3] reported that administration of broad-spectrum antibiotics prolonged pregnancy and reduced gestational age-dependent morbidity such as respiratory distress syndrome (RDS) and necrotizing enterocolitis (NEC). However, the frequencies of early-onset neonatal sepsis occurring ≤72 h of age, or grade 3 or 4 intraventricular hemorrhage (IVH) were similar between mothers receiving antibiotics and those receiving placebo [3]. For PTL patients, antibiotics do not show short-term neonatal benefits and may be associated with long-term harm [5,26]. A Cochrane review concluded that antibiotics intended only for pregnancy prolongation in PTL patients should not be administered [26]. Intravenous GBS prophylaxis is recommended until GBS test results are available. In this study, eight women with PTL received antibiotics due to unknown GBS results on admission, and only one infant from those women developed EoNI. However, about one-third of EoNI occurred in the infants of women with PTL who received antibiotics for UTI. Women presenting with symptoms should obviously be treated to prevent more severe sequelae from urosepsis. Yoneda et al. [5] reported that antibiotic therapy increased the risk of preterm birth in PTL patients without intra-amniotic microbes. They speculated that empirical antibiotic therapy for patients whose amniotic fluid was microbe-negative affects their intestinal bacteria and the homeostatic mechanisms required to maintain the immune system during pregnancy [5]. As PTL is frequently complicated by UTI, it may be impractical for the obstetrician to withhold treatment from those with UTI according to the findings of Yoneda et al.
The vagina, with its microbiota, has a balanced ecosystem in which the dominant bacteria are vaginal Lactobacillus sp. that play a protective role against ascending infection. The bacteria that reside in the female genital tract vary and are quite complex [27]. The degree of infectiousness of a particular vaginal pathogen depends not only on the type of microorganism and its intrinsic virulence but also on the relative amounts of the various bacteria. In this study, Lactobacillus sp. were detected in the vaginal specimens of approximately 50% of women upon admission and no differences were observed in the vaginal microbiome upon admission between women whose infants subsequently developed EoNI and those whose infants did not. Results of the present study suggest that antibiotic therapy could cause the depletion of lactobacilli and the selection of drug-resistant organisms in the vaginal microbiota of a subset of women whose infants developed EoNI. In the neonatal intensive care unit, initial empiric therapy for EoNI should consist of ampicillin and aminoglycoside. Such a regimen is sufficient for the treatment of major pathogens, including GBS and gram-negative enteric bacteria. Antenatal antibiotic exposure can result in the replacement of common pathogens with opportunistic or drug-resistant pathogens; therefore, these microbiological changes may affect the antimicrobial strategies of the neonatal care team in the near future.
Birthweight was not an independent predictive factor of EoNI in this study. This result was not consistent with that determined by Montella et al. [28]. We speculated this was due to the exclusion of FGR in our study. If fetal growth was severely affected, infants could be born small for their gestational age (SGA). It was reported that the infants born with SGA showed lower blood neutrophil counts at birth and immune response. It is difficult to separate the effect of prematurity and SGA on life threatening infections in the neonates, because both overlapping and independent factors (e.g., maternal infection/inflammation, poor placental function, reduced blood supply/oxygenation, or genetic factors) may predispose to preterm delivery and growth restriction at birth. We thought it was required to simplify the study population to observe the effect of antibiotics on early onset infection. Thus, we excluded the patients with obstetrical complications in this study.
This study has some limitations. First, the study sample was small, and it retrospectively evaluated antibiotic use. Second, the protocols of antepartum antibiotic therapy for women with pPROM changed during the study period. However, the incidence of EoNI was similar between the former and latter halves of the study period. Third, vaginal sample was not tested in all women before delivery; those least likely to be associated with clinical chorioamnionitis were not tested. Our findings thereby inferred reasonable validity. The detailed species of Lactobacillus could not be identified in this study [29,30]. Despite these limitations, our results are consistent with the hypothesis that antepartum antibiotics have an association with EoNI. The overall management policy, except for antepartum antibiotic use, was consistent throughout the study period. In addition, the perinatal mortality in the Miyazaki Prefecture throughout the study period was unchanged at <3.0 per 1000 births [31].
5. Conclusions
Antepartum antibiotic use is the most strongly associated factor for EoNI among pregnant women delivering under 34 weeks of gestation. No significant differences were observed in prolongation of gestation or neonatal morbidity between women who received and those who did not receive antibiotics. Among women receiving antepartum antibiotics, 21 delivered infants with early-onset infection. Of these women, 62% had reduced lactobacilli and 43% had resistant strains of common bacteria in their vaginal microbiota. These results highlight the potential adverse effect of antibiotic usage during the antepartum period.
Author Contributions
Conceptualization, J.M. and M.K.; Methodology, M.K.; Validation, J.M., M.K. and Y.K.; Formal Analysis, J.M. and M.K.; Investigation, J.M. and M.K.; Resources, J.M. and M.K.; Data Curation, J.M., Y.K. and K.D.; Writing—Original Draft Preparation, J.M.; Writing—Review & Editing, M.K.; Supervision, H.S.; Funding Acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly supported by a Grant-in-Aid for Clinical Research from Miyazaki University Hospital Grant Number 2021.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medicine, University of Miyazaki, Japan (number O-0825, 23 October 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions for personal information protection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG 2006, 113, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Oladapo, O.T.; Manu, A.; Gulmezoglu, A.M.; Bahl, R. New WHO recommendations to improve the outcomes of preterm birth. Lancet Glob. Health 2015, 3, e589–e590. [Google Scholar] [CrossRef] [Green Version]
- Mercer, B.M.; Miodovnik, M.; Thurnau, G.R.; Goldenberg, R.L.; Das, A.F.; Ramsey, R.D.; A Rabello, Y.; Meis, P.J.; Moawad, A.H.; Iams, J.D.; et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. JAMA 1997, 278, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, S.; Boulvain, M.; Neilson, J.P. Antibiotics for preterm rupture of membranes. Cochrane Database Syst. Rev. 2013, 12, CD001058. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Shiozaki, A.; Yoneda, N.; Ito, M.; Shima, T.; Fukuda, K.; Ueno, T.; Niimi, H.; Kitajima, I.; Kigawa, M.; et al. Antibiotic therapy increases the risk of preterm birth in preterm labor without intra-amniotic microbes, but may prolong the gestation period in preterm labor with microbes, evaluated by rapid and high-sensitive PCR system. Am. J. Reprod. Immunol. 2016, 75, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Romero, R.; Park, J.Y.; Oh, K.J.; Lee, J.; Conde-Agudelo, A.; Hong, J.S. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol 2019, 221, e1–e22. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Antibiotics Prescribing and Use in Hospital and Long-Term Care. Available online: https://www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html (accessed on 15 September 2021).
- Joint Commission on Hospital Accreditation. Approved: New antimicrobial stewardship standard. Jt. Comm. 2017, 36, 3–4. [Google Scholar]
- Archer, G.L. Alteration of cutaneous staphylococcal flora as a consequence of antimicrobial prophylaxis. Rev. Infect. Dis. 1991, 13, S805–S809. [Google Scholar] [CrossRef]
- Stiver, H.; Forward, K.R.; Tyrrell, D.L.; Krip, G.; Livingstone, R.A.; Fugere, P.; Lemay, M.; Verschelden, G.; Hunter, J.D.; Carson, G.D.; et al. Comparative cervical microflora shifts after cefoxitin or cefazolin prophylaxis against infection following cesarean section. Am. J. Obstet. Gynecol. 1984, 149, 718–721. [Google Scholar] [CrossRef]
- Towers, C.V.; Carr, M.H.; Padilla, G.; Asrat, T. Potential consequences of widespread antepartal use of ampicillin. Am. J. Obs. Gynecol. 1998, 179, 879–883. [Google Scholar] [CrossRef]
- Terrone, D.A.; Rinehart, B.K.; Einstein, M.H.; Britt, L.B.; Martin, J.N.; Perry, K.G. Neonatal sepsis and death caused by resistant Escherichia coli: Possible consequences of extended maternal ampicillin administration. Am. J. Obstet. Gynecol. 1999, 180, 1345–1348. [Google Scholar] [CrossRef]
- Friedman, S.; Shah, V.; Ohlsson, A.; Matlow, A.G. Neonatal Escherichia coli infections: Concerns regarding resistance to current therapy. Acta Paediatr. 2000, 89, 686–689. [Google Scholar] [CrossRef]
- Schuchat, A.; Zywicki, S.S.; Dinsmoor, M.J.; Mercer, B.; Romaguera, J.; O’Sullivan, M.J.; Patel, D.; Peters, M.T.; Stoll, B.; Levine, O.S.; et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: A multicenter case-control study. Pediatrics 2000, 105, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.V.; Briggs, G.G. Antepartum use of antibiotics and early-onset neonatal sepsis: The next 4 years. Am. J. Obstet. Gynecol. 2002, 187, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, M.D.; Pierson, C.L.; Faix, R.G. Frequent resistance of clinical group B streptococci isolate to clindamycin and erythromycin. Obstet. Gynecol. 1998, 92, 258–261. [Google Scholar]
- Bland, M.L.; Vermillion, S.T.; Soper, D.E.; Autin, M. Antibiotic resistance patterns of group B streptococci in late third trimester rectovaginal cultures. Am. J. Obstet. Gynecol. 2001, 184, 1125–1126. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease. MMWR 2010, 59, 1–36. [Google Scholar]
- Anonymous. Prevention of group B streptococcal early-onset disease in newborns. Committee Opinion No. 485, American College of Obstetrics and Gynecologists. Obstet. Gynecol. 2019, 134, e51–e72. [Google Scholar]
- Stoll, B.J.; Hansen, N.I.; Bell, E.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.H.; et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [Green Version]
- Stoll, B.J.; Hansen, N.I.; Bell, E.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.H.; et al. Trends in perinatal practices and neonatal outcomes of very low birth weight infants during 16-year period at NEOCOSUR centers. J. Pediatr. 2020, 225, 44–50. [Google Scholar]
- Lencki, S.G.; Maciulla, M.B.; Eglinton, G.S. Maternal and umbilical cord serum interleukin level in preterm labor with clinical chorioamnionitis. Am. J. Obstet. Gynecol. 1994, 170, 1345–1351. [Google Scholar] [CrossRef]
- Gleason, C.A.; Juul, S.E. Avery’s Diseases of the Newborn, 10th ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 557–560. [Google Scholar]
- Blanc, W.A. Pathology of the placenta, membranes, and umbilical cord in bacterial, fungal, and viral infections in man. Monogr. Pathol. 1981, 22, 67–132. [Google Scholar]
- Redline, R.W. Inflammatory responses in the placenta and umbilical cord. Semin. Fetal Neonatal Med. 2006, 11, 296–301. [Google Scholar] [CrossRef]
- Flenady, V.; Hawley, G.; Stock, O.M.; Kenyon, S.; Badawi, N. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst. Rev. 2013, 12, CD000246. [Google Scholar] [CrossRef] [Green Version]
- Larsen, B.; Monif, G.R. Understanding the bacterial flora of the female genital tract. CID 2001, 32, e69–e77. [Google Scholar] [CrossRef] [Green Version]
- Montella, E.; Ferraro, A.; Sperli, G.; Triassi, M.; Santini, S.; Improta, G. Predictive analysis of healthcare-associated blood stream infections in the neonatal intensive care unit using artificial intelligence: A single center study. Int. J. Environ. Res. Public Health 2022, 19, 2498. [Google Scholar] [CrossRef]
- Petricevic, L.; Domig, K.J.; Nierscher, F.J.; Sandhofer, M.J.; Fidesser, M.; Krondorfer, I.; Husslein, P.; Kneifel, W.; Kiss, H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2014, 4, 5136. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- e-Stat, Portal Site of Official Statistics of Japan. Available online: https://www.e-stat.go.jp/dbview?sid=0003411827 (accessed on 15 September 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).