Abstract
Bioflocculation has become the method of choice in wastewater treatment because of its effectiveness, environmental friendliness and innocuousness to humans. In this study, the bioflocculant-producing bacterium was isolated and its bioflocculant was used in wastewater treatment. The isolate was identified by 16S rRNA gene sequencing analysis. Its culture conditions (inoculum size, carbon and nitrogen sources, pH, temperature and time) were optimised using the one-factor-at-a-time assay. The cytotoxicity of the bioflocculant was assessed on human colorectal adenocarcinoma cells (Caco2) by tetrazolium-based colorimetric method. The ability of the bioflocculant to reduce biochemical oxygen demand (BOD) and chemical oxygen demand (COD) in wastewater was evaluated using Jar test. The bacterium was identified as Bacillus subtilis CSM5 and the maximum flocculating activity of 92% was observed when fructose and urea were used as nutrients and the culture conditions were adjusted to 30 °C, pH 9, 160 rpm and 72 h of incubation. Caco2 exhibited 90% viability when the highest bioflocculant concentration of 200 µg/µL was used. The reduction of BOD and COD was achieved at 59 ± 3.1 and 75 ± 0.4%, respectively. In conclusion, B. subtilis CSM5 is a good candidate for bioflocculant production and its bioflocculant has good potential for use in wastewater treatment.
1. Introduction
A fast growing population, especially in cities, and the constant growth in industrialization have increased the demand for potable water [1]. However, water pollution has become a challenging issue that worsen the unavailability of clean water [2]. The major source of water pollution is the discharge of effluents from industries such as coal mines [3]. Mine wastewater degrades the quality of water in different water bodies, it imposes a health risk to humans and animals and has a negative impact on the environment [4]. Waterborne diseases kill more than 1.8 million people and cause about 4 billion cases of illness yearly due to consumption of contaminated water [5]. Moreover, mine wastewater tends to be pernicious to aquatic life [6]. Thus, it is imperative to implement sustainable and effective practices in wastewater treatment.
Flocculation refers to the physicochemical process that is widely used in the treatment of industrial wastewater due to its efficiency and simplicity [7]. Colloidal particles, microbial cells and suspended materials are separated and removed from solution using flocculants. Flocculants are chemical materials that are employed in the flocculation process. They are generally grouped into (1) inorganic flocculants, (2) organic synthetic flocculants and (3) naturally occurring flocculants [8]. Inorganic flocculants comprise, among others, aluminium sulphate, alum, aluminium chloride, polyaluminium chloride, ferrous sulphates and ferric chloride whereas organic synthetic flocculants include polyacrylamide and polyethylene amine [9,10]. Inorganic and organic synthetic flocculants are predominately used due to their accessibility, high efficiencies and cost-effectiveness. However, they have been regarded as posing a threat to the environmental and human health in relation to their toxicity and non-degradable nature [11,12].
The naturally occurring flocculants such as microbial flocculants can be used as alternatives that can mitigate the health risks imposed by inorganic and organic synthetic flocculants on the environment [13]. Microbial flocculants are secreted by microorganisms during their metabolic processes and interactions with the environment [14]. They are mainly composed of carbohydrates, proteins, lipids and functional groups such as amines, hydroxyl and carboxyl groups [15,16]. Although the mechanisms of action of bioflocculants are poorly understood, they predominately utilize the bridging and charge neutralization mechanisms [17]. Bioflocculants are highly biodegradable; thus, they are environmentally friendly and are innocuous to humans and animals due to their negligible toxicity [12]. However, their application is still limited due to low yields and flocculating efficiencies in comparison to the inorganic and organic synthetic flocculants [18]. Consequently, it is important to screen and identify novel bioflocculant-producing microorganisms which are characterized by high bioflocculant yields and flocculating efficiencies [12,19].
Extensive research has been done on bioflocculant-producers from ecological niches such as sludge and soil [4,20]. However, the constant pressure to discover novel bioflocculants has recently necessitated the search for bioflocculant-producers from unique niches such as marine environments [21]. Marine environments exhibit extreme environmental variations that include high salinity, low temperatures, high hydrostatic pressure, low pH and limited nutrient supply when compared to terrestrial environments [22]. Therefore, marine microorganisms biosynthesize extracellular polymeric substances such as bioflocculants, which enable them to form protective biofilms against stress caused by biotic and abiotic conditions in the sea [23]. The abilities of marine microorganisms to adapt to the extreme environments are perceived to be the signpost for production of bioflocculants of high yields and efficiencies [24].
The intent in this study was to isolate a marine bioflocculant-producing bacterium from the beach of Sodwana Bay in KwaZulu Natal, South Africa; characterize and apply its bioflocculant in the treatment of coal mine wastewater.
2. Materials and Methods
2.1. Chemicals and Culture Media
All chemicals, reagents and media used were procured from Sigma-Aldrich (St. Louis, MO, USA). The chemicals included 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), bacteriological agar, actinomycin D and 1% penicillin-streptomycin. The standard production medium was composed of glucose (20.0 g), KH2PO4 (2.0 g), K2HPO4 (5.0 g), (NH4)2SO4 (0.2 g), NaCl (0.1 g), urea (0.5 g), MgSO4 (0.2 g) and yeast extract (0.5 g) in a litre of filtered marine water. The medium was autoclaved at 121 °C for 15 min. The other media were minimum essential medium (MEM) and 10% foetal calf serum.
2.2. Sample Collection
Marine water was sampled from the beach of Sodwana Bay in Kwa-Zulu Natal, South Africa (27.5398° S, 32.6783° E). The autoclaved Schott bottles were used to sample water on three different sites at the beach. The physico-chemical parameters which included; (1) temperature, (2) pH, (3) dissolved oxygen, (4) total dissolved solid, (5) salinity, (6) pressure and (7) specific conductivity were measured in-situ using HI 98194 PH/E/DO multiparameter. Thereafter, the samples were placed into ice box and transported to the laboratory at the Department of Water and Sanitation at the University of Limpopo, Limpopo, South Africa.
2.3. Isolation of Target Bacteria
Water samples (1 mL) were poured into different test tubes containing 9 mL of sterile saline solution (0.85%) and the 10 fold serial dilutions were done, separately. About 100 µL of each serial dilution was spread on the selective production medium supplemented with 20 g of bacteriological agar (see Section 2.1). The agar plates were incubated at 30 °C for 5 days. Colonies with different colour, shape and sizes were selected. Thereafter, the isolates were screened for bioflocculant production by evaluating the flocculating activity of each isolate [25].
2.4. Determination of the Flocculating Activity
The broth production medium (see Section 2.1) was inoculated with the pure bacterial isolates and incubated at 30 °C at the shaking speed of 160 rpm for 72 h. After incubation, the broth cultures (2 mL) were centrifuged at 12,000 rpm for 16 min at 4 °C. The cell-free supernatants were collected and used to evaluate the flocculating activity. The flocculating activity was assessed in accordance to the method by More et al. [26]. Briefly, 2 mL of the supernatants and 3 mL of 1% CaCl2 were poured into 95 mL of kaolin solution (3 g/L). The mixtures were shaken for a minute, poured into the measuring cylinders and left to stand for 5 min. The autoclaved distilled water (2 mL) served as the control. The top layer of the supernatants (2 mL) were collected and their optical density (OD) were read at 550 nm using a spectrophotometer (Spectro-quant, Pharo 300 Merck, Boston, MA, USA). Thereafter, the percentage flocculating activity (% FA) was calculated following the equation (%FA) = (A1 − A2/A1) × 100, whereby A1 represents the OD at 550 nm of the control and A2, the OD at 550 nm of the test samples. The isolate which demonstrated the highest flocculating activity was selected, preserved on glycerol and stored at −80 °C for further experiments.
2.5. Molecular Identification of the Bioflocculant-Producing Bacterium
The most promising bioflocculant producing strain was identified by molecular technique based on the 16S rRNA gene amplification by polymerase chain reaction (PCR), followed by sequencing of the amplified gene at Inqaba Biotechnical Industries (Pty) Ltd., Pretoria, South Africa. Briefly, the genomic DNA of the bacterium was extracted using a ZR Fungal/Bacterial Kit™ in accordance to the manufacturer’s protocol. The 16S rRNA gene of the bioflocculant-producer was amplified with 16S-1492R primer (5′-CGGTTACCTTGTTACGACTT-3′) and 16S-27F primer (5′-AGAGTTTGATCMTGGCTCAG-3′) in the presence of the DreamTaq™ DNA polymerase. PCR products were sequenced in the forward and reverse directions on the ABI PRISM™ 3500 xl Genetic Analyser and cleaned with ExoSAP-it™. The sequences were analyzed using CLC Bio Main Workbench v7.6, followed by the Basic Local Alignment Search Tool (BLAST) program using the National Centre for Biotechnology Information (NCBI) database to find the closest bacterial species [27].
2.6. Optimization of the Medium Composition and Culture Conditions
The composition of the original medium (see Section 2.1) and the growth conditions of the isolate were optimized using the one-factor-at-a-time method in order to enhance bacterial growth and maximize bioflocculant production. The influence of inoculum size on the production of the bioflocculant was assessed by varying the inoculum size from 0.5 to 2.0 mL (v/v), representing 1 to 5% (v/v). The effect of carbon sources on the bioflocculant production of the isolate was determined by substituting the glucose in the original medium with the equal amount of starch, maltose, sucrose, lactose or fructose, and other factors remained the same. The multiple nitrogen sources in the medium were replaced with the equivalent amount (1.2 g/L) of the nitrogen sources such as casein, yeast extract, urea, peptone and ammonium sulphate ((NH4)2SO4). The effect of pH of the production medium on the bioflocculant production was assessed at the acidic, basic and neutral states. pH of the medium was adjusted in a range of 3 to 12 using NaOH and 1 N HCl. The influence of temperature on bioflocculant production was also determined by varying the temperatures within the range of 20 to 40 °C. Lastly, the effect of time on bioflocculant production was determined. Briefly, the bacterium was grown under the obtained optimal culture conditions in this study. Thereafter, 2 mL of the culture broth was drawn every 12 h up to 96 h. The samples were then centrifuged at 12,000 rpm for 16 min. The supernatant was then used to evaluate the flocculating activity as previously described (see Section 2.2) [28].
2.7. Extraction and Purification of the Bioflocculant
The bacterium was first inoculated into a 1 L of the modified medium and cultured using the obtained optimum culture conditions. Thereafter, the broth culture was centrifuged (5000 rpm, 30 min); the supernatant was poured into the sterile distilled water (1 volume) and centrifuged to remove the insoluble materials. Absolute ethanol (2 volumes) was added to the supernatant, shaken and left to precipitate at 4 °C for 12 h. The obtained precipitate was vacuum-dried and the crude bioflocculant was dissolved in the sterile distilled water (100 mL). Then, 100 mL of the mixture of chloroform and butanol (5:2 v/v) was added, agitated and left to stand at room temperature for 12 h. Lastly, the mixture was centrifuged (5000 rpm, 30 min) to remove the impurities and the purified bioflocculant was vacuum-dried [29].
2.8. Effect of Dosage Size and Cations on Flocculating Activity
The effect of dosage size on flocculating activity was evaluated. Briefly, different concentrations of the bioflocculant were prepared in a range of 0.2 to 1.0 mg/mL (w/v) and added into the mixture of kaolin solution (95 mL of 4 g/L) and CaCl (3 mL of 1%). Thereafter, the flocculating activity was assessed [30]. The synergistic effect of the monovalent (KCl, NaCl and LiCl), divalent (CaCl2, MnCl2 and BaCl2) and trivalent (FeCl3) metal ions (3 mL of 1% (v/v)) on the flocculating activity of the bioflocculant was determined in kaolin solution (4 g/L), which was previously adjusted to pH 7. The control was the mixture of kaolin solution without the metal ion [31].
2.9. Characterization of the Bioflocculant
The analysis of the elemental composition of the bioflocculant was done by scanning electron microscope-energy dispersive X-ray detector (SEM-EDX) (Oxford Instruments-X-Max N). The FT-IR spectroscopy (Perkin Elmer System 2000, Cambridge, UK) was employed to evaluate the functional groups within the obtained bioflocculant. The pyrolysis profile of the bioflocculant was evaluated by thermogravimetric analyser (Perkin Elmen Pyris 6 TGA, Germany).
2.10. Cytotoxic Effect of the Bioflocculant
The cytotoxicity of the obtained bioflocculant was evaluated against the human colorectal adenocarcinoma cells (Caco2) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The cells were grown to confluency of 80–90% in 25 cm3 flasks using the complete culture medium (CCM: minimum essential medium (MEM), 10% foetal calf serum, 1% penicillin-streptomycin) which was trypsinized and incubated overnight at 37 °C in 5% CO2 using a 96 well plate. Thereafter, the CCM was removed and treated with different concentrations (50–200 µg/µL) of the bioflocculant. The cells that were treated with 0.2% dimethyl sulfoxide (DMSO) served as the negative control and Actinomycin D (40 μg/μL) as positive control and incubated at 37 °C in 5% CO2. After 24 h of incubation, the old medium was supplemented with 100 µL of the fresh CCM. Thereafter, 10 µL of MTT reagent (5 mg/mL in phosphate buffered saline) was pipetted into the wells and incubated at 37 °C in 5% CO2 for 4 h. The MTT solution was aspirated from the microwells and the formazan crystals were solubilized in 100 µL of DMSO. Reduction of MTT was determined by reading the optical density (OD) of the samples at 595 nm using the spectrophotometer (BioTek µQuant microplate reader, Winooski, VT, USA). The percentage cell viability (%CV) was calculated using the formula %CV = (OD of untreated cells − OD of treated cells/OD of untreated cells) × 100 [32].
2.11. Flocculation Mechanism of the Bioflocculant
The flocculation mechanism was evaluated by measuring the zeta potentials of different samples using Zetasizer Nano (Malvern, UK). The samples included the bioflocculant solution, kaolin clay suspension, the mixture of the kaolin particles and BaCl2 and the kaolin particles flocculated by the bioflocculant in the presence of BaCl2. About 30 zeta runs were performed for each sample; the temperature was set at 25 °C with the count rate of 7.5 kcps [33].
2.12. Removal Efficiencies of the Bioflocculants on Wastewater
The biological oxygen demand (BOD) and chemical oxygen demand (COD) of the wastewater from one of the local coal mine wastewater in South Africa were measured using appropriate test kits according to the manufacturers’ protocols, prior to the treatment. The Jar test method was used to assess the removal efficiency of the bioflocculant. Briefly, a mixture of 3 mL of 1% (w/v) BaCl2 and 2 mL of the bioflocculant solution (0.6 mg/mL) were poured into 100 mL of the coal mine wastewater and adjusted to pH 7. The mixture was first agitated at 200 rpm for 3 min. The speed was then reduced to 40 rpm for 5 min and then poured into 100 mL measuring cylinder. Thereafter, the top supernatants (2 mL) were collected after been left to stand for 5 min. Aluminium sulphate and ferric chloride served as positive controls. The percentage removal efficiency (%RE) of each flocculant was calculated using the formula: (%RE) = (A1 − A2/A1) × 100, where A1 and A2 represent the values obtained before and after treatment, respectively [30].
2.13. Software and Statistical Analysis
The experiments were all performed in triplicates and expressed as mean standard deviation. The data was subjected to one-way analysis of variance using Graph Pad Prism TM 6.1. The p values ≤ 0.05 were regarded as significant.
3. Results
3.1. Physicochemical Parameters of Water
The physicochemical parameters of the marine water were determined in-situ. The temperature was 23.1 ± 0.5 °C, pH 7.1 ± 0.05, dissolved oxygen was 41.4 ± 5.5, total dissolved solids was 24.0 ± 5.0, salinity was 31.0 ± 7.14, pressure was 760.0 ± 0.5 mmHg and specific conductivity was 47.1 ± 4.1 mS/cm.
3.2. Selection of the Bacterial Strain for Bioflocculant Production
Bacterial strains were isolated and screened for bioflocculant production. Nine out of 20 bacterial strains demonstrated the flocculating activities higher than 60% and are tabulated in Table 1. The highest flocculating activity of 80.3 ± 0.8% was observed with isolate SDN9. The least activity was shown by isolate SDM4 with the flocculating activity of 60.4 ± 0.1%. Therefore, the isolate with the highest promising flocculating activity (isolate SDN9) was selected and identified by 16S rRNA sequence analysis. SDN9 showed 99% similarity to Bacillus subtilis with accession number of CSM5 when compared to the NCBI data base using the BLAST program (Table 1).

Table 1.
Flocculating activity of the screened bacterial strains.
3.3. Optimization of the Culture Conditions of B. subtilis CSM5
3.3.1. Effect of Inoculum Size
Table 2 illustrates the effect of the inoculum size of B. subtilis CSM5 on the bioflocculant production. The most preferred inoculum size was 1% (0.5 mL v/v), which demonstrated the flocculating activity of 75.3 ± 1%. The increase in the inoculum size led to a slight insignificant decrease (p < 0.05) in flocculating activity. Therefore, the inoculum size of 1% was used in other assays that followed.

Table 2.
Effect of inoculum size, carbon and nitrogen sources, pH, temperature and time on flocculation.
3.3.2. Effect of Carbon and Nitrogen Sources on Bioflocculant Production
Table 2 shows the effect of the carbon sources on bioflocculant synthesis. Among the carbon sources (glucose, starch, sucrose, lactose, maltose and fructose) used, fructose was the most preferred, revealing 96.1 ± 1% of the flocculating activity. Glucose gave the least flocculating activity of 73.8 ± 4%. Table 2 also displays the effect of nitrogen sources on the production of the bioflocculant. B. subtilis CSM5 grew optimally when urea was utilized, giving the maximum flocculating activity of 91.1 ± 5%. Casein was poorly utilized and gave the least flocculating activity (31.7 ± 1%). All subsequent experiments were performed using fructose as the carbon source and urea as the nitrogen source.
3.3.3. Effect of pH on Bioflocculant Production
The effect of pH of the culture medium on the biosynthesis of the bioflocculant was evaluated and the results are presented in Table 2. The optimal pH for the bioflocculant production by B. subtilis CSM5 was in the range of 9–10, with the highest flocculating activity of 92 ± 1% observed at pH 10. The lowest flocculating activity (57 ± 3%) was obtained at the acidic pH of 3.
3.3.4. Effect of Temperature on Bioflocculant Production
The effect of temperature on bioflocculant production was assessed (Table 2). The flocculating activity increased constantly when set within the temperature range of 20–30 °C. The maximum flocculating activity of 83% was observed when 30 °C was used. Thereafter, the flocculating activity decreased with the increase in temperature from 30 °C to 40 °C. Therefore, 30 °C was used as the optimum temperature for bioflocculant production in the subsequent experiments.
3.3.5. Effect of Time on Bioflocculant Production
The effect of time on the production of the bioflocculant by B. subtilis CSM5 was assessed (Table 2). The flocculating activity constantly increased with an increase in time from 12 to 72 h. The flocculating activity reached its peak at 72 h, with the flocculating activity of 92%. Thereafter, there was a slight, insignificant decrease observed with the increase in time from 72 to 96 h.
3.4. Bioflocculant Yield and the Effect of Dosage Size on Flocculation
The bioflocculant was extracted from the broth culture after 72 h and purified. The mass of the resulting purified bioflocculant was 1.5 g. The effect of the dosage size of the partially purified bioflocculant from B. subtilis CSM5 is displayed in Table 3. The highest flocculating activity of 85.8 ± 1% was obtained at a concentration of 0.6 mg/mL. The lowest activity (80.1 ± 0.5%) was observed when 0.2 mg/mL of the bioflocculant was utilized.

Table 3.
Effect of dosage size and cations on flocculating activity.
3.5. Effect of Cations on Flocculating Activity of the Bioflocculant
The synergistic effect of metal ions on the flocculating activity of the purified bioflocculant from B. subtilis CSM5 is depicted in Table 3. The metal ions did significantly (p < 0.05) improve the flocculating activity of the purified bioflocculant except for Fe3+. The most active metal ion was BaCl2, with the maximum flocculating activity of 85.8 ± 1%, followed by MnCl2 with 83.7 ± 1.4%.
3.6. Elemental Composition of the Bioflocculant
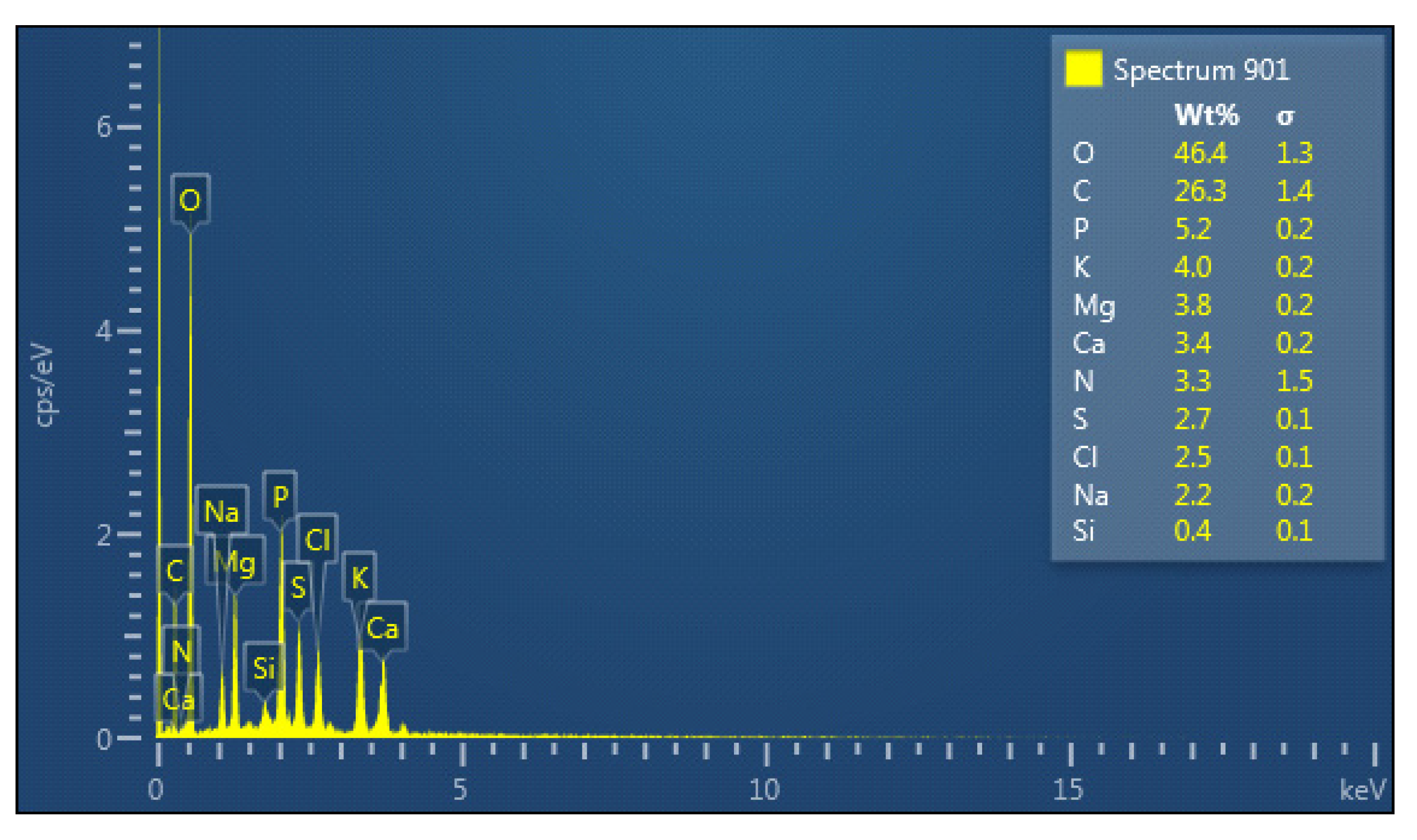
The elemental composition of the bioflocculant is indicated in Figure 1. The main constituents was O (46.4%), followed by C (26.3%) and N (5.2%). Si was the least component with 0.4%.
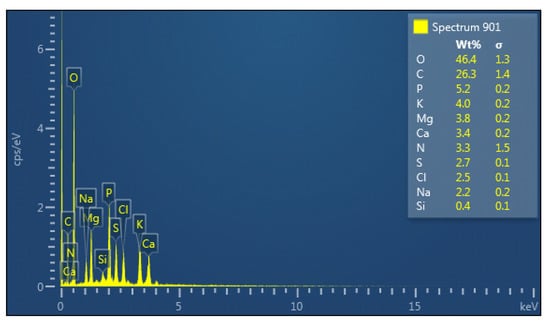
Figure 1.
Elemental composition of the bioflocculant from B. subtilis CSM5.
3.7. Functional Groups of the Bioflocculant from B. subtilis CSM5
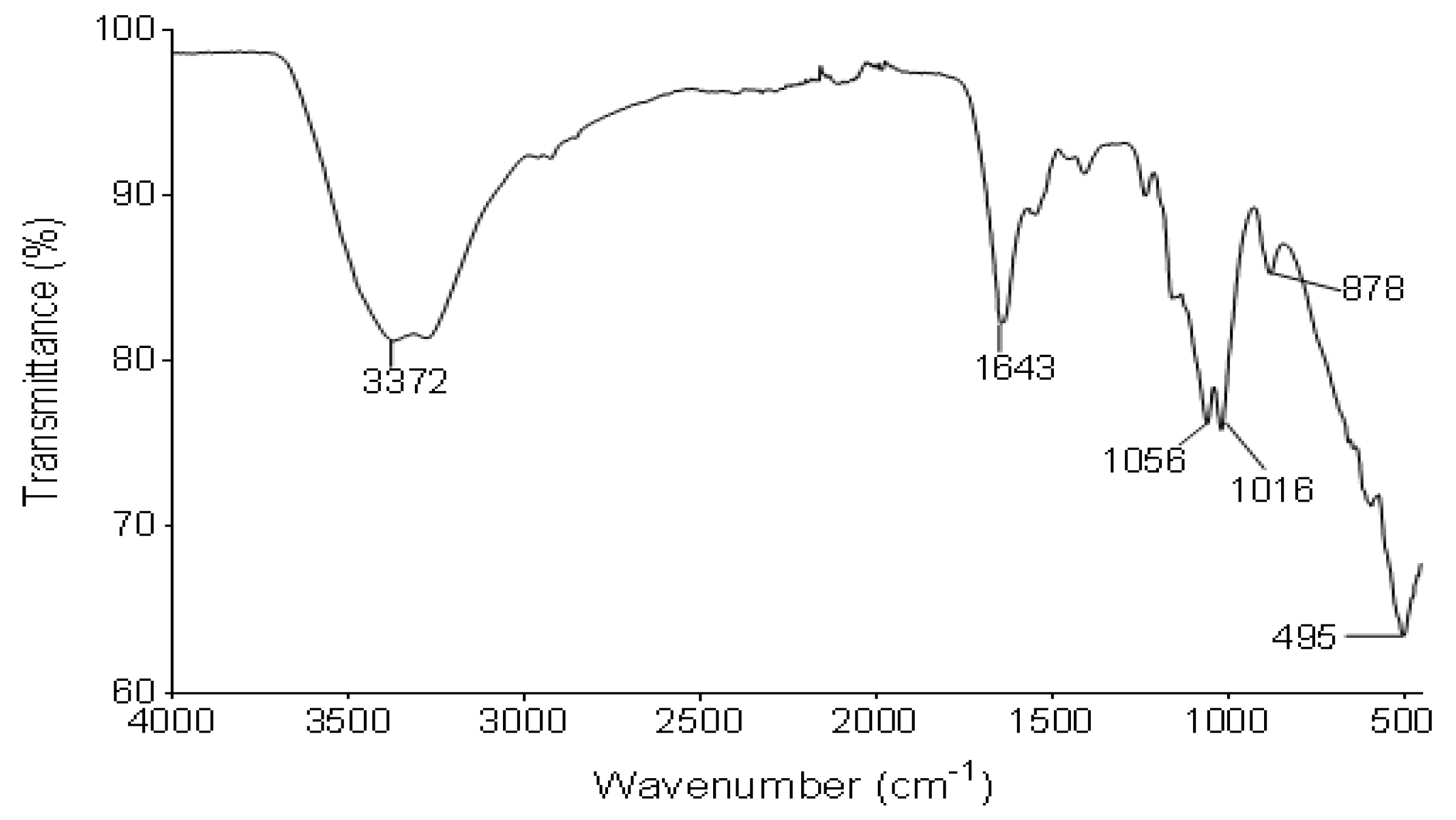
Figure 2 illustrates the IR spectrum of the functional groups of the bioflocculant from B. subtilis CSM5. The IR spectrum displayed the presence of hydroxyl or amine (3372 cm−1), amide (1643 cm−1) and carboxyl groups (1056 and 1056 cm−1) as the dominant functional groups.
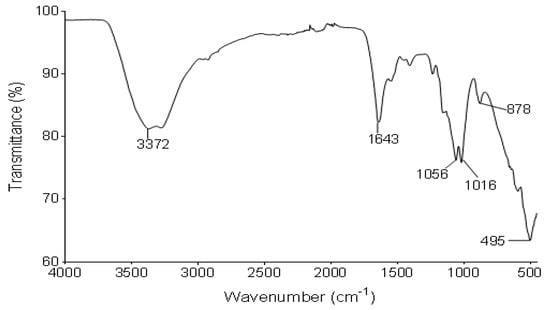
Figure 2.
IR spectrum of the bioflocculant from B. subtilis CSM5.
3.8. Pyrolysis Profile of the Bioflocculant
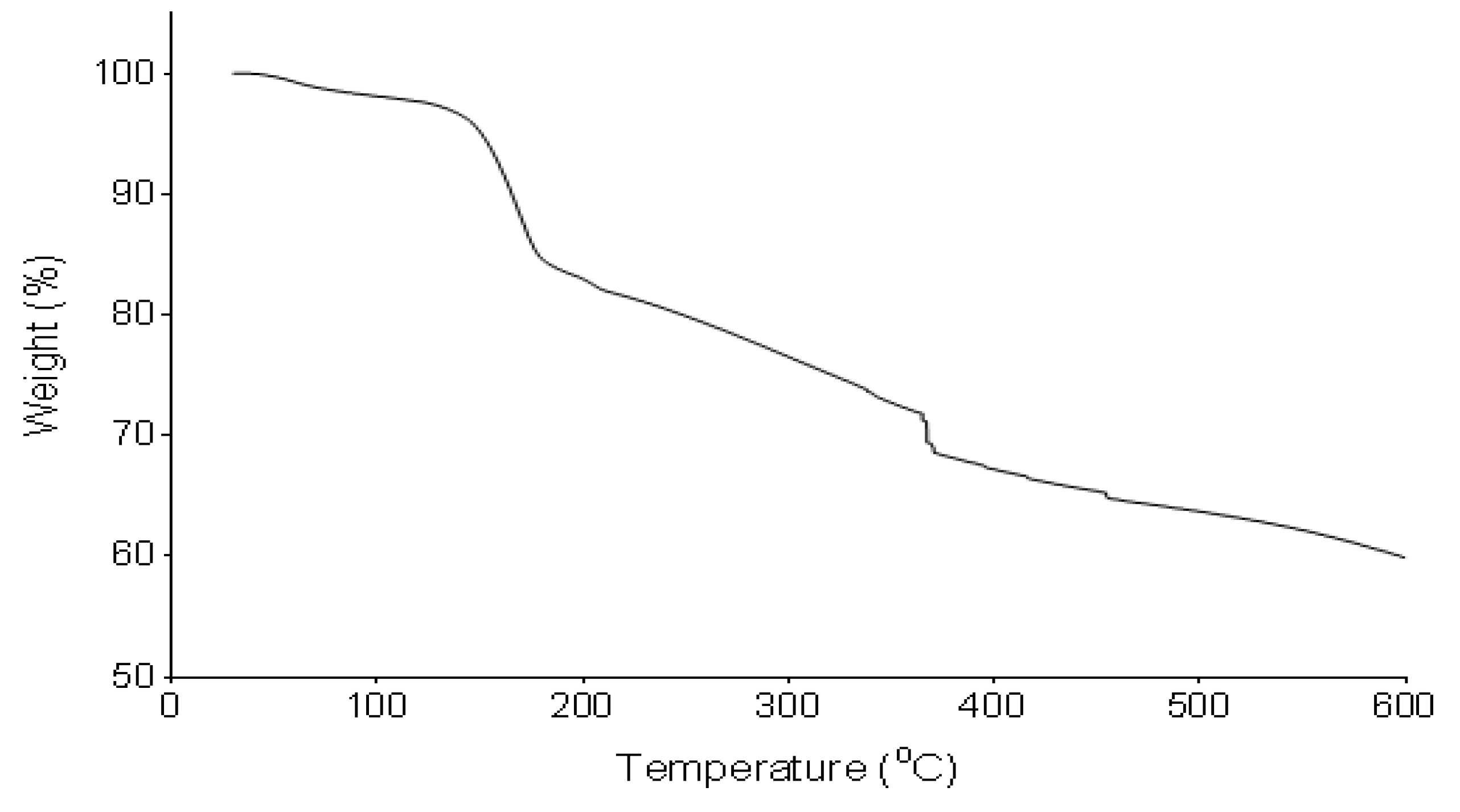
The pyrolysis profile of the bioflocculant was determined by TGA (Figure 3). The bioflocculant revealed an initial weight loss of 3% between 30 and 130 °C. It further showed degradation at 150 °C and an increase in temperature to 375 and 460 °C further resulted in degradation and weight loss.
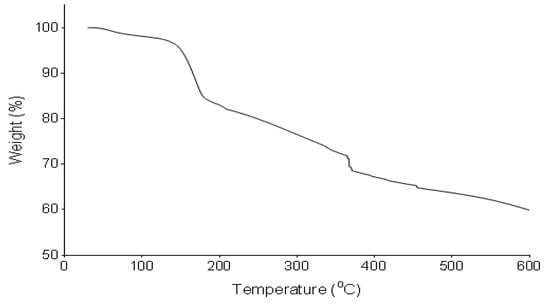
Figure 3.
Pyrolysis profile of the bioflocculant from B. subtilis CSM5.
3.9. Cytotoxic Effect of the Bioflocculant
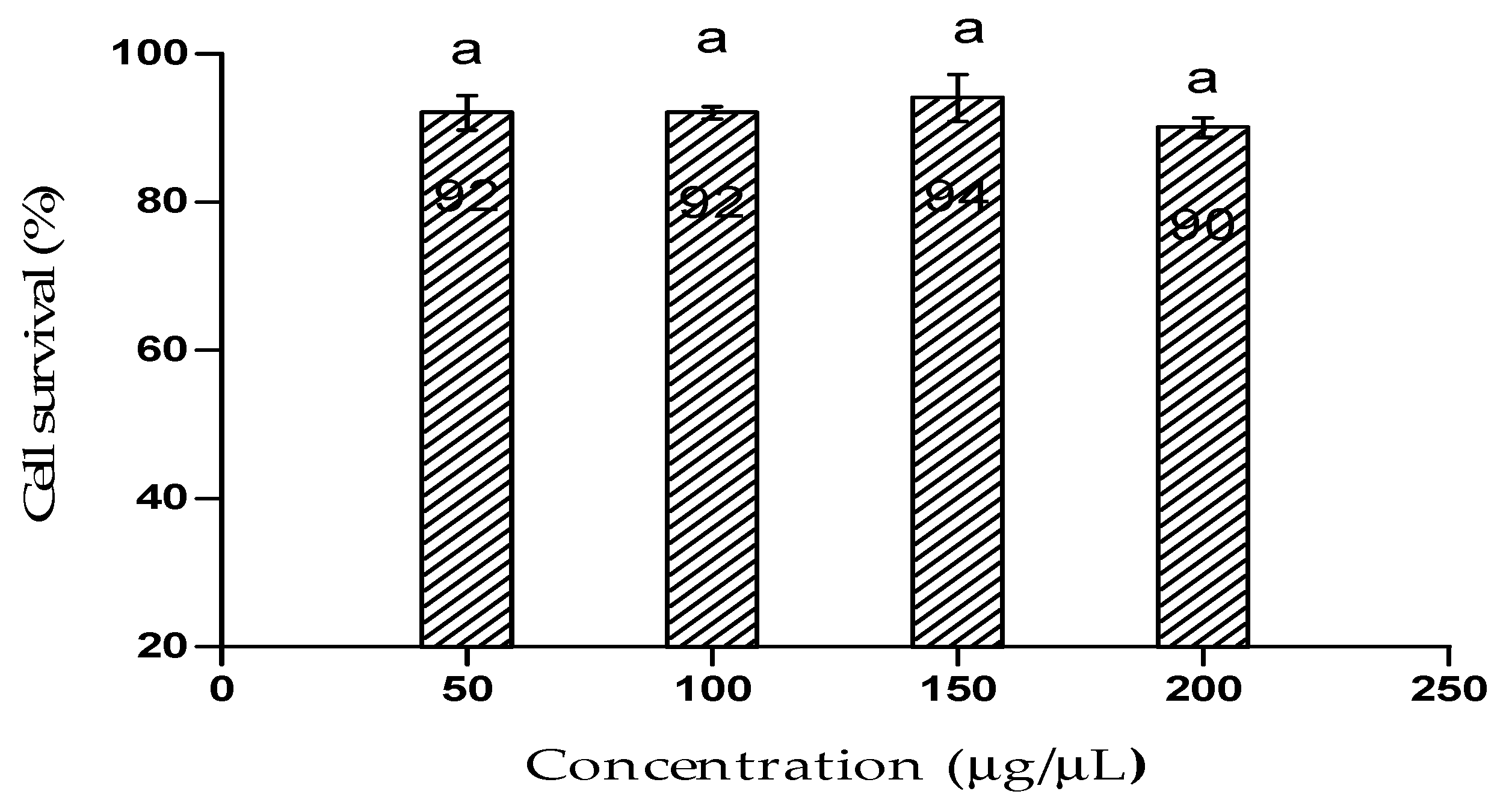
Figure 4 represents the cytotoxic effect of the bioflocculant from B. subtilis CSM5 on Caco2 cell line. Caco2 exhibited 90% cell viability after it has been treated with the highest concentration of the bioflocculant (200 µg/µL). Moreover, it is worth to state that there was no statistical (p < 0.05) differences of the effect of the bioflocculant observed within the used concentrations.
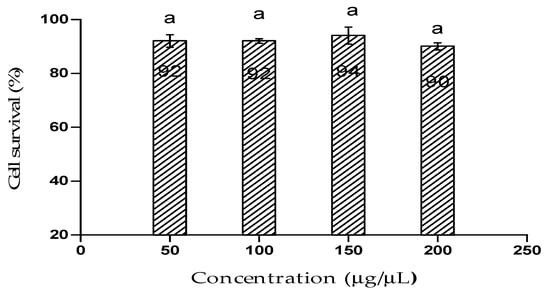
Figure 4.
Cytotoxic effect of the bioflocculant on Caco2 cells. The letter (a) denotes statistical significance at p < 0.05.
3.10. Proposed Flocculation Mechanism of the Bioflocculant
Table 4 shows the zeta potentials of the samples. The bioflocculant had the lowest zeta potential of −16.5 ± 1.07 mV whereas the mixture of the kaolin flocculated by the bioflocculant in the presence of BaCl2 had the highest zeta potential of −5.5 ± 2.1 mV.

Table 4.
Zeta potential of sample used to evaluate flocculation mechanisms of the bioflocculant.
3.11. Wastewater Treatment by the Bioflocculant in Comparison to the Conventional Flocculants
Table 5 displays the reduction efficiency of the bioflocculant from B. subtilis CSM5 on BOD and COD of the wastewater from one of the local mine wastewater plant in South Africa. The bioflocculant demonstrated lower reduction efficiency of 59 ± 3.1% on BOD in comparison to aluminium sulphate and FeCl3, which had 65 ± 0.5% and 60 ± 2.8%, respectively. However, the bioflocculant showed better reduction of COD (75 ± 0.4%) than the aluminium sulphate and FeCl3 (Table 5).

Table 5.
Mine wastewater treatment by the flocculants.
4. Discussion
Geographic location and environmental parameters are vital factors that affect the distribution and bioactivity of marine bacteria [22]. Thus, measurement of the physicochemical parameters is an important aspect when isolating microorganisms. The physicochemical conditions of marine water from Sodwana Bay were vital for the growth of the isolates and their ability to produce the bioflocculants. The highest flocculating activity was revealed by isolate SDN9, which was identified as B. subtilis CSM5. The genus Bacillus are Gram positive rod-shaped bacteria that are known to produce bioflocculants [34]. B. subtilis have been previously reported to be the active bioflocculant-producer [35]. Thus, this study also confirmed B. subtilis as the profound bioflocculant-producer.
The effect of the inoculum size of B. subtilis CSM5 was assessed and 1% inoculum size was the most preferred. This inoculum size enabled sufficient bacterium growth and production of bioflocculant. The inoculum size greater than 1% led to the decrease in flocculating activity. The decrease might have been due to the extreme niche overlap of B. subtilis CSM5, which might have resulted in the inhibition of the bioflocculant production [28]. Generally, the inoculum sizes that fall within the range of 1 to 5% are of economic preferences [36]. Thus, the B. subtilis CSM5 can be regarded as economic.
The effect of carbon source on bioflocculant production was determined and fructose was the most potent stimulant. This implied that B. subtilis CSM5 was able to effectively assimilate fructose as a carbon source for its growth, energy and bioflocculant production. The results contradicted those of Ogunlaja et al. [20], where fructose slightly suppressed the bioflocculant production by Bacillus cereus and Bacillus tropicus. However, in the same study, fructose highly promoted the bioflocculant production by Bacillus thuringiensis. There was maximum flocculating activity observed when urea was utilized as the nitrogen source, implying that urea effectively stimulated the growth of B. subtilis CSM5 and bioflocculant production. Urea was found to promote bioflocculant production of other Bacillus species such as Bacillus pumilus LBPMA-BLD07 and Bacillus toyonensis LBPMA-ACOPR1.Isox [37].
pH of the culture medium determines the electrification of the bacterial cells and oxidation–reduction potential [38]. The optimal initial pH of the culture medium of B. subtilis CSM5 was 10. The alteration in pH from the optimum resulted in the decrease in flocculating activity, implying that the change might have affected the absorption of nutrients and metabolic reactions, consequently leading to poor bioflocculant production. Similar results were observed by Ugbenyen et al. [39], whereby Bacillus sp. Gilbert had the highest flocculating activity at pH 9 and 10.
The bioflocculant from B. subtilis CSM5 demonstrated maximum flocculating activity when the bacterium was incubated at 30 °C. This might be due to the fact that the metabolic reactions of B. subtilis CSM5 were enhanced for its growth and bioflocculant production [40]. A rise in temperature above the optimum (>30 °C) might have tempered with the metabolic functions of B. subtilis CSM5, as denaturation might have set-in, consequently resulting in poor growth and bioflocculant production. This phenomenon is evidenced by the significant drop in the flocculating activity observed at higher temperatures. At lower temperatures (<30 °C), the bacterial growth rate might have been very slow, consequently leading to poor bioflocculant production. According to Salehizadeh and Shojaosadati [41] and Zhang et al. [42], the enzymes for bioflocculant production are generally active within a temperature range of 25 to 37 °C. Thus, this study affirmed their hypothesis.
The relationship between bioflocculant production and culturing time was determined. The sufficient bioflocculant was produced at 72 h, giving the peak flocculating activity of 92%. The short production time (≤72 h) intervals are the most preferred in industries as they are cost effective and less time consuming. Therefore, the bioflocculant production by B. subtilis CSM5 was produced at early stationary stage, indicative of the economic friendliness. Arafa et al. [43], obtained similar results whereby the flocculating activity was maximum when B. cereus was grown for 72 h.
Low production yields of the purified bioflocculants is the major challenge and the limiting factor for their use in industries. Thus, the screening of novel bioflocculant-producers and method of extraction are important. About 1.5 g of the purified bioflocculant was obtained from the liter of the culture broth of B. subtilis CSM5. The obtained yield was higher than the yields mostly obtained from single bacterial strains [44].
The effective flocculation was observed at the optimum concentrations of 0.6 mg/mL. At this concentration, the bioflocculant was able to effectively bind to the kaolin particles and flocculate them. Low dosage sizes (<0.6 mg/mL) caused inadequate functional groups of the bioflocculant to adsorb the kaolin particles in solution, consequently leading to low flocculating activity. Moreover, higher doses (>0.6 mg/mL), also resulted in low flocculating activity, indicating that high bioflocculant doses inhibited floc formation due to the strong repulsion force between them [45]. The profound flocculating activity of this bioflocculant at low dosage size signifies its economic friendliness. The results were comparative to those of Cosa and Okoh [46].
Metal ions stimulate flocculation through neutralization and stabilization of the negative charges of the functional groups of the colloidal particles and the bioflocculant [47]. The bioflocculant showed an outstanding flocculating activity when Ba2+ was used. Ba2+ was able to neutralize the negative charges of the functional groups of the bioflocculant and kaolin particles, thereby shortening the distance between them, consequently resulting with the high flocculating activity. However, Fe3+ resulted in the lowest flocculating efficiency. Fe3+ might have lowered the adjusted pH (pH 7) of the kaolin solution, consequently affecting the flocculating activity of the bioflocculant [48]. Moreover, the results affirmed the bioflocculant from B. subtilis CSM5 as cation independent.
The elemental analysis of the bioflocculant showed the presence of different elements. This elements play an important role for the flexibility and stability of the bioflocculant structure and flocculating activity [49]. The presence of carbon, oxygen and nitrogen elements imply that the bioflocculant is possibly a glycoprotein biopoymer [50]. The results were in alignment with those obtained Okaiyeto et al. [51], whereby the bioflocculant MBF-UFH revealed different elements.
The functional groups of the bioflocculants provide adsorption sites for different colloids in suspension [52]. The absorption peak at 3372 cm−1, which is a characteristic of a hydroxyl group, is due to the vibration of -OH or -NH in the sugar ring of carbohydrates. The band at 1643 cm−1 is linked to proteins, and is due to N-H bending and C-N stretching vibrations in CO-NH of the proteins. The peaks at 1056 and 1016 cm−1 revealed C-O, which is distinctive absorption peak for carbohydrates. In conclusion, the IR spectrum mainly demonstrated the absorption peaks in favor of carbohydrates and proteins and showed the presence of hydroxyl, amine, amide and carboxyl. The revealed functional groups tend to mostly serve as binding sites for metal ions and colloids during flocculation as they have been found in other bioflocculants [53,54].
TGA spectrum revealed the weight loss of the bioflocculant between 35 and 100 °C. The weight loss was attributed to the loss of moisture content, which was from the carboxyl and hydroxyl groups. Further decline in the weight of the bioflocculant at higher temperatures were attributed to the degradation of the bioflocculant. The pyrolysis property of the bioflocculant translated its thermal stability [55].
MTT assay was used to assess the cell viability of Caco2 after treated with the bioflocculant. The bioflocculant showed a margin of biosafety as there was no significant cytotoxic effect on Caco2. Thus, the results affirmed the probable safe use of the bioflocculant in wastewater treatment. Sharma et al. [56] reported similar findings, whereby the biopolymer from Acinetobacter haemolyticus demonstrated insignificant cytotoxicity on sheep blood cells.
The zeta potential analyses were carried out in order to ascertain the flocculation mechanism of the purified bioflocculant. The two main flocculation mechanisms are: (1) charge neutralization and (2) bridging mechanisms [57,58]. Charge neutralization occur when the bioflocculant is oppositely charged, as compared to the colloids in suspension whereas bridging dominates as the results of the extension of the functional groups beyond the surface of the kaolin particles, consequently binding the kaolin adsorption sites. The zeta potentials of the bioflocculant and the flocculated kaolin clay were negative. If charge neutralization was the main mechanism, flocculation could have occurred when the zeta potential of the particles was sufficiently low to eliminate repulsion between them. However, the zeta potential of the mixture of the kaolin particles flocculated by the bioflocculant in the presence of BaCl2 and retained large negative value (Table 4), suggesting bridging mechanism as the predominant flocculation mechanism. The observations were comparable to those reported by Guo et al. [11], whereby the bioflocculant utilized bridging mechanism to flocculate kaolin particles in the presence of CaCl2.
High levels of COD and BOD often lead to anaerobic conditions, bad odors and stagnant waters that do not support aquatic life [59]. When compared to the conventional chemical flocculants, the bioflocculant showed comparable removal efficiencies on the tested parameters. Therefore, the removal the efficiency of the bioflocculant implies its potential industrial applicability. The results were in agreement with those obtained by Agunbiade et al. [60] and Pathak et al. [61], whereby the bioflocculants were efficient in removing pollutants in wastewater.
5. Conclusions
B. subtilis CSM5 demonstrated maximum flocculating activity of 92% and about 1.5 g/L yield when the optimum culture conditions were used. The purified bioflocculant revealed diverse functional groups (hydroxyl, carboxyl and amine) which were responsible for the profound flocculating activity at low dosage size (0.6 mg/mL). The zeta potential analysis revealed the bridging mechanism as predominant during flocculation process. Moreover, the bioflocculant was found to be nontoxic and effective in the reduction of pollutants in coal mine wastewater. The revealed properties suggested its potential applicability in the industrial fields. For further studies, the bioflocculant will be applied in treatment of other wastewater effluents and in the removal of dyes from solutions.
Author Contributions
Conceptualization, T.N.S. and P.M.; validation, T.N.S.; formal analysis, T.N.S.; investigation, T.S.M.; writing—original draft preparation, T.S.M.; writing—review and editing, K.M.; supervision, T.N.S.; project administration, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the staff and postgraduate students in the Department of Biochemistry, Microbiology and Biotechnology and the Department of Water and Sanitation at the University of Limpopo for their outstanding support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Emaminejad, S.A.; Avval, S.S.; Bonakdarpour, B. Gaining deeper insights into the bioflocculation process occurring in a high loaded membrane bioreactor used for the treatment of synthetic greywater. Chemosphere 2019, 230, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Bisht, V.; Lal, B. Exploration of performance kinetics and mechanism of action of a potential novel bioflocculant BF-VB2 on clay and dye wastewater flocculation. Front. Microbiol. 2019, 10, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, O.; Lu, C.; Liu, A.; Zhu, L.; Wang, P.-M.; Qian, C.-D.; Jiang, X.-H.; Wu, X.-C. Optimization and characterization of polysaccharide-based bioflocculant produced by Paenibacillus elgii B69 and its application in wastewater treatment. Bioresour. Technol. 2013, 134, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Alias, J.; Hasan, H.A.; Abdullah, S.R.S.; Othman, A.R. Properties of bioflocculant-producing bacteria for high flocculating activity efficiency. Environ. Technol. Innov. 2022, 27, 102529. [Google Scholar] [CrossRef]
- Manetu, W.M.; Karanja, A.M. Waterborne disease risk factors and intervention practices: A review. Open Access Libr. J. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Rizal, A.; Apriliani, I.M. Analysis of several water environment parameters affecting the accumulation of heavy metal lead in the body of blood shells in the coastal waters of Muara Gembong sub-district. World Sci. News 2021, 153, 65–79. [Google Scholar]
- Al-Anzi, B.S.; Naik, M.-u.-d.; Ahmad, M. The imperative need of metal salt for the treatment of industrial wastewater via the synergic coagulation-flocculation method. Polymers 2022, 14, 165. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Mnif, W.; M Siddeeg, S. Microbial flocculants as an alternative to synthetic polymers for wastewater treatment: A review. Symmetry 2018, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Sun, D.; Liu, J.; Zhu, J.; Liu, W. Recent advances and perspectives in efforts to reduce the production and application cost of microbial flocculants. Bioresour. Bioprocess. 2021, 8, 51. [Google Scholar] [CrossRef]
- Maliehe, T.S.; Basson, A.K.; Dlamini, N.G. Removal of pollutants in mine wastewater by a non-cytotoxic polymeric bioflocculant from Alcaligenes faecalis HCB2. Int. J. Environ. Res. Public Health 2019, 16, 4001. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Yu, J.; Xin, X.; Zou, C.; Cheng, Q.; Yang, H.; Nengzi, L. Characterization and flocculation mechanism of a bioflocculant from hydrolyzate of rice stover. Bioresour. Technol. 2015, 177, 393–397. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Yan, N. Recent advances in extracellular biopolymer flocculants. Biotechnol. Adv. 2014, 32, 1506–1522. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Seki, H. Evaluation of flocculation performance of polysaccharide-protamine complex flocculant by flocculation model. Biochem. Eng. J. 2022, 180, 108356. [Google Scholar] [CrossRef]
- Ding, G.; Li, X.; Lin, W.; Kimochi, Y.; Sudo, R. Enhanced flocculation of two bioflocculation-producing bacteria by secretion of Philodina erythrophthalma. Water Res. 2017, 112, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, G.; Alam, M.A.; Mofijur, M.; Jahirul, M.; Lv, Y.; Xiong, W.; Ong, H.C.; Xu, J. Modern developmental aspects in the field of economical harvesting and biodiesel production from microalgae biomass. Renew. Sustain. Energy Rev. 2021, 135, 110209. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Menkiti, M.C. Bio-coagulation-flocculation (BCF) of municipal solid waste leachate using Picralima nitida extract: RSM and ANN modelling. Curr. Res. Green Sustain. Chem. 2021, 4, 100078. [Google Scholar] [CrossRef]
- Lai, H.; Fang, H.; Huang, L.; He, G.; Reible, D. A review on sediment bioflocculation: Dynamics, influencing factors and modeling. Sci. Total Environ. 2018, 642, 1184–1200. [Google Scholar] [CrossRef]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Characterization of a bioflocculant produced by a consortium of Halomonas sp. Okoh and Micrococcus sp. Leo. Int. J. Environ. Res. Public Health 2013, 10, 5097–5110. [Google Scholar] [CrossRef] [Green Version]
- Ogunlaja, A.; Ibidunni, B.; Oyende, K.; Ogunlaja, O. Optimization of bioflocculant production by bacteria isolated from oil-polluted soil and fermented maize effluent. Ife J. Sci. 2020, 22, 201–210. [Google Scholar] [CrossRef]
- Awolusi, O.O.; Ademakinwa, A.N.; Ojo, A.; Erasmus, M.; Bux, F.; Agunbiade, M.O. Marine actinobacteria bioflocculant: A storehouse of unique biotechnological resources for wastewater treatment and other applications. Appl. Sci. 2020, 10, 7671. [Google Scholar] [CrossRef]
- Poli, A.; Finore, I.; Romano, I.; Gioiello, A.; Lama, L.; Nicolaus, B. Microbial diversity in extreme marine habitats and their biomolecules. Microorganisms 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Vaseashta, A. The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World J. Microbiol. Biotechnol. 2022, 38, 117. [Google Scholar] [CrossRef] [PubMed]
- Finore, I.; Di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation technologies for the optimization of marine microbial exopolysaccharide production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V.S.R. Isolation, identification and characterization of bioflocculant-producing bacteria from activated sludge of Vulindlela Wastewater Treatment Plant. Appl. Microbiol. 2021, 1, 586–606. [Google Scholar] [CrossRef]
- More, T.T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Biopolymers production by mixed culture and their applications in water and wastewater treatment. Water Environ. Res. 2015, 87, 533–546. [Google Scholar] [CrossRef]
- Pillay, S.; Amoako, D.G.; Abia, A.L.K.; Somboro, A.M.; Shobo, C.O.; Perrett, K.; Bester, L.A.; Essack, S.Y. Characterisation of Campylobacter spp. isolated from poultry in KwaZulu-Natal, South Africa. Antibiotics 2020, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Tsilo, P.H.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V. Isolation and optimization of culture conditions of a bioflocculant-producing fungi from Kombucha tea SCOBY. Microbiol. Res. 2021, 12, 950–966. [Google Scholar] [CrossRef]
- Ngema, S.; Basson, A.; Maliehe, T. Synthesis, characterization and application of polyacrylamide grafted bioflocculant. Phys. Chem. Earth Parts A/B/C 2020, 115, 102821. [Google Scholar] [CrossRef]
- Maliehe, T.; Simonis, J.; Basson, A.; Reve, M.; Ngema, S.; Xaba, P. Production, characterisation and flocculation mechanism of bioflocculant TMT-1 from marine Bacillus pumilus JX860616. Afr. J. Biotechnol. 2016, 15, 2352–2367. [Google Scholar]
- Makapela, B.; Okaiyeto, K.; Ntozonke, N.; Nwodo, U.U.; Green, E.; Mabinya, L.V.; Okoh, A.I. Assessment of Bacillus pumilus isolated from fresh water milieu for bioflocculant production. Appl. Sci. 2016, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Aljuboori, A.H.R.; Idris, A.; Al-Joubory, H.H.R.; Uemura, Y.; Abubakar, B.I. Flocculation behavior and mechanism of bioflocculant produced by Aspergillus flavus. J. Environ. Manag. 2015, 150, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Ugbenyen, A.M.; Simonis, J.J.; Basson, A.K. Screening for bioflocculant-producing bacteria from the marine environment of Sodwana Bay, South Africa. Ann. Sci. Technol. 2018, 3, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Ntozonke, N.; Okaiyeto, K.; Okoli, A.S.; Olaniran, A.O.; Nwodo, U.U.; Okoh, A.I. A marine bacterium, Bacillus sp. isolated from the sediment samples of Algoa Bay in South Africa Produces a Polysaccharide-Bioflocculant. Int. J. Environ. Res. Public Health 2017, 14, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugbenyen, A.; Cosa, S.; Mabinya, L.; Babalola, O.O.; Aghdasi, F.; Okoh, A. Thermostable bacterial bioflocculant produced by Cobetia spp. isolated from Algoa Bay (South Africa). Int. J. Environ. Res. Public Health 2012, 9, 2108–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, J.G.; Silva, A.L.d.S.; dos Santos, E.C.; Martins, E.S.; López, A.M. Optimization of bioflocculant production by Bacillus spp. from sugarcane crop soil or from sludge of the agroindustrial effluent. Braz. J. Chem. Eng. 2019, 36, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Vimala, R. Production and flocculating performance of bioflocculant by bacterial strain and its application for municipal wastewater treatment. J. Pure Appl. Microbiol. 2019, 13, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Ugbenyen, A.; Cosa, S.; Mabinya, L.; Okoh, A. Bioflocculant production by Bacillus sp. Gilbert isolated from a marine environment in South Africa. Appl. Biochem. Microbiol. 2014, 50, 49–54. [Google Scholar] [CrossRef]
- Diao, H.; Li, L.M.; Liang, J.; Ding, X. Screening of High-performance Flocculant-producing Bacteria and Optimization of the Conditions for Flocculation of Wheat Distillery Wastewater. BioResources 2018, 13, 7738–7757. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Shojaosadati, S. Extracellular biopolymeric flocculants: Recent trends and biotechnological importance. Biotechnol. Adv. 2001, 19, 371–385. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Bo, L.; Xia, S.-Q.; Wang, X.-J.; YANG, A.-M. Production and application of a novel bioflocculant by multiple-microorganism consortia using brewery wastewater as carbon source. J. Environ. Sci. 2007, 19, 667–673. [Google Scholar] [CrossRef]
- Arafa, R.A.; El-Rouby, M.N.; Abass, H.A.; El-Khier, Z. Bioflocculants produced by bacterial isolates from Egyptian soil 1-characterization and application of extracellular bioflocculants and nanoparticles for treatment of river Nile water. J. Pharm. Biol. Sci. 2014, 9, 103–114. [Google Scholar] [CrossRef]
- Lin, J.; Harichund, C. Isolation and characterization of heavy metal removing bacterial bioflocculants. Afr. J. Microbiol. Res. 2011, 5, 599–607. [Google Scholar]
- Guo, J.; Yu, J. Sorption characteristics and mechanisms of Pb (II) from aqueous solution by using bioflocculant MBFR10543. Appl. Microbiol. Biotechnol. 2014, 98, 6431–6441. [Google Scholar] [CrossRef] [PubMed]
- Cosa, S.; Okoh, A. Bioflocculant production by a consortium of two bacterial species and its potential application in industrial wastewater and river water treatment. Pol. J. Environ. Stud. 2014, 23, 689–696. [Google Scholar]
- He, J.; Zou, J.; Shao, Z.; Zhang, J.; Liu, Z.; Yu, Z. Characteristics and flocculating mechanism of a novel bioflocculant HBF-3 produced by deep-sea bacterium mutant Halomonas sp. V3a’. World J. Microbiol. Biotechnol. 2010, 26, 1135–1141. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, Z.; He, J.; Li, Q. Novel strategy for controlling colloidal instability during the flocculation pretreatment of landfill leachate. Chemosphere 2022, 287, 132051. [Google Scholar] [CrossRef]
- Gong, W.-X.; Wang, S.-G.; Sun, X.-F.; Liu, X.-W.; Yue, Q.-Y.; Gao, B.-Y. Bioflocculant production by culture of Serratia ficaria and its application in wastewater treatment. Bioresour. Technol. 2008, 99, 4668–4674. [Google Scholar] [CrossRef]
- Sekelwa, C.; Anthony, U.M.; Vuyani, M.L.; Anthony, O.I. Characterization of a thermostable polysaccharide bioflocculant produced by Virgibacillus species isolated from Algoa bay. Afr. J. Microbiol. Res. 2013, 7, 2925–2938. [Google Scholar]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoli, A.S.; Okoh, A.I. Characterization of a bioflocculant (MBF-UFH) produced by Bacillus sp. AEMREG7. Int. J. Mol. Sci. 2015, 16, 12986–13003. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, Y.; Yu, Y.; Li, Q.; Wang, H.; Chen, R.; He, N. Production and characterization of a novel bioflocculant from Bacillus licheniformis. Appl. Environ. Microbiol. 2010, 76, 2778–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-El-Haleem, D.A.; Al-Thani, R.F.; Al-Mokemy, T.; Al-Marii, S.; Hassan, F. Isolation and characterization of extracellular bioflocculants produced by bacteria isolated from Qatari ecosystems. Pol. J. Microbiol. 2008, 57, 231–239. [Google Scholar] [PubMed]
- Tang, W.; Song, L.; Li, D.; Qiao, J.; Zhao, T.; Zhao, H. Production, characterization, and flocculation mechanism of cation independent, pH tolerant, and thermally stable bioflocculant from Enterobacter sp. ETH-2. PLoS ONE 2014, 9, e114591. [Google Scholar] [CrossRef] [Green Version]
- Agunbiade, M.O.; Van Heerden, E.; Pohl, C.H.; Ashafa, A.T. Flocculating performance of a bioflocculant produced by Arthrobacter humicola in sewage waste water treatment. BMC Biotechnol. 2017, 17, 51. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Kaur, T.; Bridle, H.; Ghosh, M. Antimicrobial efficacy and safety of mucoadhesive exopolymer produced by Acinetobacter haemolyticus. Int. J. Biol. Macromol. 2017, 94, 187–193. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Du, C.; Zhong, Y.; Yang, C. Preparation, performances, and mechanisms of microbial flocculants for wastewater treatment. Int. J. Environ. Res. Public Health 2020, 17, 1360. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.; Pimentel, M.; Russo, A.; Cabral, W. Charge neutralization mechanism efficiency in water with high color turbidity ratio using aluminium sulfate and flocculation index. Water 2020, 12, 572. [Google Scholar] [CrossRef] [Green Version]
- Kamaruddin, M.A.; Yusoff, M.S.; Aziz, H.A.; Basri, N.K. Removal of COD, ammoniacal nitrogen and colour from stabilized landfill leachate by anaerobic organism. Appl. Water Sci. 2013, 3, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Agunbiade, M.; Oladipo, B.; Ademakinwa, A.N.; Awolusi, O.; Adesiyan, I.M.; Oyekola, O.; Ololade, O.; Ojo, A. Bioflocculant produced by Bacillus velezensis and its potential application in brewery wastewater treatment. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Pathak, M.; Devi, A.; Bhattacharyya, K.; Sarma, H.; Subudhi, S.; Lal, B. Production of a non-cytotoxic bioflocculant by a bacterium utilizing a petroleum hydrocarbon source and its application in heavy metal removal. RSC Adv. 2015, 5, 66037–66046. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).