Abstract
Clinically significant portal hypertension is associated with most complications of advanced chronic liver disease (ACLD), including variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatic encephalopathy. Gut dysbiosis is a hallmark of ACLD with portal hypertension and consists of the overgrowth of potentially pathogenic bacteria and a decrease in autochthonous bacteria; additionally, congestion makes the intestinal barrier more permeable to bacteria and their products, which contributes to the development of complications through inflammatory mechanisms. This review summarizes current knowledge on the role of the gut–liver axis in the pathogenesis of portal hypertension, with a focus on therapies targeting portal hypertension and the gut microbiota. The modulation of the gut microbiota on several levels represents a major challenge in the upcoming years; in-depth characterization of the molecular and microbiological mechanisms linking the gut–liver axis to portal hypertension in a bidirectional relationship could pave the way to the identification of new therapeutic targets for innovative therapies in the management of ACLD.
Keywords:
gut microbiome; portal hypertension; cirrhosis; hepatic encephalopathy; HVPG; CSPH; ACLD; inflammation; FMT; NSBBs 1. Introduction
Humans live in cooperation with their gut microbiome, as an integrated superorganism [1,2]. More than 100 trillion microorganisms, including over 1000 species of bacteria, archaea, viruses, fungi, and protozoa, are hosted in our gastrointestinal tract and are now recognized as the variable part of our genome [3,4]. The composition of the gut microbiome is the final result of the interplay between a complex network of factors, including the genetic landscape and environmental agents, immune response, and dietary habits [5]. Beyond its critical role in many metabolic pathways [6], the gut microbiome is involved in the maintenance of the intestinal barrier’s integrity, the protection of the host against pathogens, and the regulation of both innate and adaptive host immunity [7]. A perturbation of this balance results in dysbiosis, a condition that can contribute to the pathogenesis and the further evolution of different disorders, including liver diseases [8].
The gut–liver axis is an entity that stems from the close anatomical and functional relationship between the gastrointestinal tract and the liver [9]. Under physiological conditions, this system allows only a small amount of bacteria and their products to reach the liver through portal circulation, where they are readily eliminated. In this way, the hepatic firewall prevents the dissemination of potential inflammatory triggers into the systemic bloodstream, maintaining a balanced immune response [10]. With the development and progression of liver disease, the gut–liver axis undergoes a gradual and profound change, characterized by a breakdown of the intestinal barrier, dysbiosis, bacterial overgrowth, and excessive bacterial translocation. This causes a dysfunctional immune response perpetuating hepatic and systemic inflammation, which worsens liver damage into a vicious cycle [11].
It is, therefore, not surprising that pathological changes in the gut microbiome have been associated with advanced chronic liver disease (ACLD) and its complications, such as hepatic encephalopathy, spontaneous bacterial peritonitis, and hepatocellular carcinoma [12]. In recent years, increasing attention has been paid to the role that portal hypertension plays in shaping the gut–liver axis [13,14]. Since portal hypertension represents the primary driver of hepatic decompensation, which, in turn, is associated with increased mortality and morbidity in cirrhotic patients, a proper understanding of its link with the gut microbiome is of paramount importance for diagnostic, prognostic, and therapeutic approaches [15]. Indeed, the last Baveno VII consensus in portal hypertension underlined the importance of the gut microbiome as one of the fields that needs to be explored in the future in order to improve the management of portal hypertension in patients with ACLD [16].
This review aims to summarize current knowledge on the effects of portal hypertension on gut–liver axis remodeling. In addition, we provide a reinterpretation of currently available therapeutic approaches, emphasizing their impact on the gut–liver axis.
2. Pathogenesis of Portal Hypertension in Liver Disease
Portal hypertension represents one of the major consequences of ACLD; it is defined as an increase in the hepatic venous pressure gradient (HVPG) of >5 mmHg [16]. Clinically significant portal hypertension (CSPH) develops in the case of HVPG > 10 mmHg and is related to all of the complications of ACLD, such as gastroesophageal variceal bleeding, hepatic encephalopathy, and ascites. These complications represent the first cause of death and the main indication for liver transplantation in these patients [17].
The development of portal hypertension in ACLD results from both an increased inflow and an obstructed outflow in the hepatic venous system. Indeed, structural modifications of hepatic sinusoids due to fibrosis and regenerative nodules, together with vasoconstriction in the intrahepatic circulation due to decreased production of vasodilators from sinusoidal endothelial cells, are responsible for the rise in intrahepatic vascular resistance [18,19]. On the other hand, splanchnic vasodilation, as a consequence of the huge amount of nitric oxide (NO) released by hyperactive vascular endothelial cells, causes an increase in portal venous inflow [20].
The pathophysiology of portal hypertension has also been linked to intrahepatic microvascular thrombosis. While, in the past, cirrhosis was considered a pro-hemorrhagic condition, it is now accepted that cirrhosis is rather characterized by a delicate hemostatic balance [21]. Parenchymal extinction, which results from the death of the hepatocytes and their replacement with fibrotic tissue following the thrombotic occlusion of intrahepatic veins and sinusoids, is involved in the progression of cirrhosis and in worsening portal hypertension [22,23,24]. Several studies have demonstrated that anticoagulation therapy can reduce hepatic fibrosis and portal hypertension, and delay hepatic decompensation [25,26].
3. Gut–Liver Axis Composition and Function
Everything that connects the intestine to the liver contributes to the realization of the gut–liver axis.
The intestinal barrier is the most exposed part to the external environment; it is composed of the mucus layer, produced by intestinal goblet cells [27], the enterocytes connected by intercellular tight junctional complexes [28], Paneth cells, the gut-associated lymphoid tissue (GALT) [29,30,31], and the gut vascular barrier [32]. The gut microbiome resides on top of the intestinal barrier, in the intestinal lumen and the outer mucus layer, along with many substances that serve as host defense and regulate the gut ecosystem, such as antimicrobial peptides (AMPs), IgA, and bile acids [33,34,35,36,37].
Under normal conditions, a limited amount of gut microbiome-associated molecular patterns (MAMPs) and pathogen-associated molecular patterns (PAMPs), which include lipopolysaccharide (LPS) and other components of the bacterial cell membrane, flagellin, and bacterial DNA [38], can cross the epithelial barrier. These molecules activate pattern-recognition receptors (PRRs) on antigen-presenting cells and on B and T cells located in the GALT and the mesenteric lymph nodes (MLNs) [29,30,31,39], which is crucial for modeling the immune system and avoiding a systemic immune response [40].
On the inner side of the gut–liver axis, the hepatic sinusoids act as the final filter of the substances collected by the splanchnic vessels. This functional unit is composed of fenestrated sinusoidal endothelial cells (SECs), resident macrophages named Kupffer cells, and hepatic stellate cells (HSCs); the latter are located in the space of Disse, between the endothelium and the hepatocytes, and are involved in tissue repair and fibrogenesis [41,42].
In summary, the gut–liver axis is an extremely dynamic system, regulated by several host cytokines, vasoactive mediators, and microbial metabolites, in a constant balance between pro-inflammatory and tolerance factors [43,44,45]. The disruption of its homeostasis participates in the development of portal hypertension, which leads to the dysfunction of the gut–liver axis at several points, not only causing liver damage and systemic inflammation, but also worsening liver hemodynamics in a vicious cycle (Figure 1).
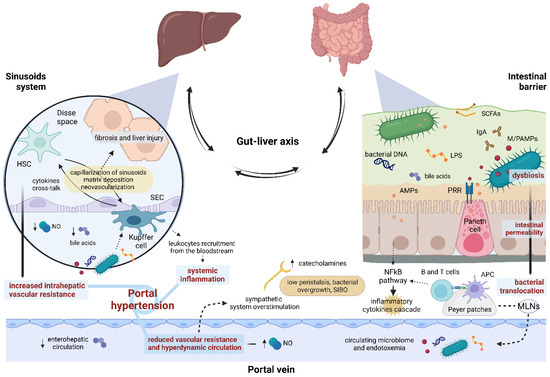
Figure 1.
Gut–liver axis and portal hypertension. The intestinal barrier and the hepatic sinusoid system represent the two hinges of the firewall involved in containing bacterial translocation within the gut–liver axis. Impairment of continuous, bi-univocal communication between all these elements at several points gives rise to a vicious cycle leading to portal hypertension. AMPs, antimicrobial peptides; APC, antigen-presenting cell; HSC, hepatic stellate cell; LPS, lipopolysaccharide; M/PAMPs, microbiome/pathogen-associated molecular patterns; MLNs, mesenteric lymph nodes; NO, nitric oxide; PRR, pattern-recognition receptor; SCFAs, Short-chain fatty acids; SEC, sinusoidal endothelial cell; SIBO, small intestinal bacterial overgrowth.
4. Gut–Liver Axis Impairment and Portal Hypertension: A Two-Way Street
The gut microbiome shows qualitative and quantitative alterations in cirrhotic patients, and portal hypertension plays a central role in this process through intestinal mucosal congestion and atrophy, which reduce gastric acid production and peristalsis, impairing bacterial clearance [10,40,46]. This mechanism results in a reduced ratio between autochthonous and potentially pathogenic taxa [47], with a decrease in Lactobacillus, Bifidobacterium, Ruminococcaceae, Lachnospiraceae, Clostridium cluster IV, and Bacteroides, and an increase in Streptococcus, Veillonella, Fusobacterium, Enterococcaceae, and Proteobacteria (in particular Enterobacteriaceae) [13,48,49]. Small intestinal bacterial overgrowth (SIBO) is also a frequent finding in patients with cirrhosis, which can be explained by the impaired intestinal motility associated with the high sympathetic tone in portal hypertension [50,51].
Bacterial translocation has been recognized as a key pathological mechanism triggering the onset and the progression of portal hypertension. In cirrhotic patients, the abnormal bacterial translocation overcomes MLNs’ defense capacity, consequently engaging the sinusoid system [19,52,53,54,55,56]. Kupffer cells are overstimulated in producing pro-inflammatory mediators, such as interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-alpha), and chemokines, through a series of pathways, including toll-like receptor 4 (TLR-4), Myeloid differentiation primary response 88 (MyD88), and nuclear factor kappa B (NFκB), in a cross-talk with SECs and HSCs, which acquire an activated, fibrinogenic phenotype [10,42,45,57,58,59,60]. All of this results in a series of maladaptive consequences: capillarization of liver sinusoids, extracellular matrix deposition and fibrosis, liver damage, and neovascularization [61,62]. Nevertheless, the inflammatory response extends beyond the liver; inflammatory mediators overflow into the systemic circulation, causing the recruitment of leukocytes from the bloodstream [63,64] and the release of vasoactive mediators. Among others [65,66], NO, produced by endothelial and inducible NO synthases (eNOS and iNOS), plays a key role in steering the hemodynamic changes in liver disease. While NO is reduced in the intrahepatic microcirculation, causing vascular hypertonus and increasing microvascular resistance, in the splanchnic and systemic bed, both iNOS and eNOS are upregulated, resulting in arterial vasodilation, reduced vascular resistance, and hyperdynamic circulation [40,67,68,69].
Therefore, gut–liver axis disruption plays a crucial role in the development and progression of portal hypertension [70]. Although it is difficult to determine whether the chicken or the egg comes first and, in particular, at what point of liver disease, growing attention has been paid to identifying the mechanisms through which the gut microbiome can modulate portal hypertension.
5. Influence of the Gut Microbiome on Portal Hypertension
Recently, various studies have suggested a strong interplay between the gut microbiota and the development and progression of portal hypertension (Table 1) [13,14].

Table 1.
Features of the gut microbiome associated with portal hypertension in animal models and human studies.
A recent study comparing conventional and germ-free mice showed that the presence of the gut microbiota stimulates the proliferation of vessels and lymphatic collectors in the intestinal wall, which depends, at least in part, on the production by Paneth cells of Angiogenin-4 (Ang-4), a ribonuclease with angiogenic and antimicrobial properties [77,78]. This was paralleled by the increase in portal pressure, outlining the hypothesis that gut microbiota may per se drive portal hypertension, irrespective of bacterial translocation, systemic inflammation, and the development of ACLD [79].
Microbial metabolites represent an additional pathophysiological link between portal hypertension and the gut–liver axis. Hydrogen sulfite (H2S) [14] is produced by sulfur-reducing gut bacteria (i.e., Bilophila and Desulfovibrio genera, both belonging to the Proteobacteria phylum) and by the host via H2S-catalyzing enzymes variably expressed in many organs [80]. H2S induces vasodilation when interacting with endothelial and smooth muscle cells, and suppresses the contraction of HSCs in experimental cirrhosis [81,82]. Furthermore, it reduces gastrointestinal motility, favoring bacterial overgrowth and the development of SIBO [83].
Short-chain fatty acids (SCFAs) (acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid) result from the gut microbiome fermentation of non-absorbable carbohydrates; they regulate the function of the intestinal barrier both directly, providing energy to enterocytes, and indirectly, exerting anti-inflammatory effects on the innate and adaptive immune system [84,85,86]. SCFAs have been found in the portal and peripheral blood, participating in several processes, including the modulation of liver hemodynamics [87]. A study enrolling 62 patients with cirrhosis showed how the level of circulating SCFAs was inversely associated with the severity of liver disease and the model for end-stage liver disease (MELD) score; above them, butyric acid was inversely correlated with the HVPG values, inflammatory markers, such as TNF-alpha and IL-6, and NO in hepatic, peripheral, and portal blood [88]. Nevertheless, SCFAs were related to hemodynamic changes, not only at a portal level, but also at a systemic level, being directly correlated with systemic vascular resistance and inversely correlated with the cardiac index.
Bile acids are key protagonists of intestinal functions and act as signaling molecules, regulating several metabolic and physiological processes. Bile acids have anti-microbial and immune-modulating properties in the gut [89,90] while participating in the regulation of intrahepatic vascular resistance by interacting with the sinusoid system via farnesoid x receptor (FXR) signaling [91,92]. In advanced cirrhosis, both primary and particularly secondary bile acid production is reduced [93], thus contributing to dysbiosis, SIBO, and bacterial translocation [13], as well as altered sinusoidal vasoregulation, consequently affecting the progression and the severity of portal hypertension.
Antimicrobial peptides (AMPs) are a wide and diverse group of small proteins implied in the host–microbiome interplay [94,95,96]. Defensins, cathelicidins, and lectins are the most common AMPs in the gut, mainly derived from Paneth cells and enterocytes; they operate in a complex and synergistic dynamic in the regulation of the gut microbiome, both directly by damaging microbes and indirectly by interacting with the host intracellular signaling pathways and stimulating the immune response [95]. Many intestinal bacterial strains also produce specific AMPs, i.e., the bacteriocins, involved in the mechanisms of bacterial competition and communication, as well as biodiversity and environmental niche formation [97,98,99]. There is some evidence of the relevance of the AMPs network in liver disease. In mice models of ethanol-induced liver injury, a deficiency of the regenerating islet-derived 3 beta and gamma (REG3B and REG3G), two gut lectins with bactericidal properties against Gram-negative bacteria, was associated with an increase in mucosa-associated bacteria and in bacterial translocation, together with worsening disease progression [100]. Of interest, in experimental cirrhosis, increased bacterial translocation was associated with a depletion of Paneth cells and a reduced expression of AMPs [101]; however, in the same study, this association was not observed in mice with acute portal hypertension without cirrhosis. While a considerable amount of data is already available on the gut microbiota composition associated with liver cirrhosis, the detailed analysis of which is beyond the scope of this paper, evidence on microbiota signatures associated with portal hypertension and its severity is still lacking. Some attempts have been made to demonstrate the value of the gut microbiota profile as a noninvasive diagnostic marker of portal hypertension. In particular, the circulating microbiome has been identified as a possible target in this context, reasonably mirroring bacterial translocation from the gut [49,102,103]. A recent study aimed to find microbial signatures of portal hypertension in blood compartments of patients with cirrhosis, particularly in peripheral circulation and hepatic veins [71]. While there were significant differences in the circulating microbial composition compared with controls and in association with MELD or biomarkers of inflammation, no predictive value regarding portal hypertension severity could be demonstrated. It has also recently been shown that specific components of the microbiome in the peripheral blood flow at baseline can predict the reduction in HVPG after direct-acting antiviral (DAA) therapy in HCV-related cirrhosis [72]. However, the study enrolled only 32 patients, including people with HIV and HCV coinfection, making it difficult to draw strong conclusions. Another study analyzed the gut microbiome of 12 inpatients with esophageal and gastric varices compared with 24 healthy controls, showing a higher relative abundance of Lactobacillales and a reduction in Clostridium cluster IV and cluster IX [73]; in this setting, no distinction was made concerning the severity of portal hypertension or in comparison with cirrhotic patients without CSPH.
6. Effects of Gut-Microbiota Modulation on Portal Hypertension
6.1. Rifaximin
Rifaximin is a derivative of rifamycin, an oral broad-spectrum antibiotic; it shows negligible absorption, with a low risk of inducing bacterial resistance, and has known eubiotic properties [104,105]. Rifaximin administration is recommended for the prophylaxis and treatment of hepatic encephalopathy; notwithstanding, there is growing evidence for a broader benefit of rifaximin in patients with liver disease.
A case–control study evaluated the cumulative incidence and frequency of complications in 200 patients with decompensated cirrhosis, randomized at a ratio of 1:1 to receive or not receive rifaximin 400 mg twice daily for 6 months [106]. In addition to the significantly lower overall complication rate in the treatment group, a reduction in the incidence of esophageal and gastric variceal bleeding was observed. Another study including 30 patients with alcoholic cirrhosis confirmed that receiving rifaximin at 1200 mg/day can reduce HVPG and endotoxemia after 29 days of treatment [107]. A total of 23 out of the 30 patients included in this study who responded with improved HVPG were enrolled to continue rifaximin treatment. After a 5-year follow-up, the treatment arm showed a significantly lower risk of variceal bleeding (35% vs 59.5%, P=0.011) compared with the controls. In addition, rifaximin was found to be independently associated with a lower rate of hepatic encephalopathy, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome [108]. Furthermore, the combination therapy with propranolol plus rifaximin seemed to be more effective than propranolol alone, not only in lowering HVPG, but also in reducing markers of bacterial translocation (e.g., LPS, LPS-binding protein, IL-6, and TNF-alpha) [109].
The exact mechanisms leading to the effects of rifaximin on the gut–liver axis and liver hemodynamics are being widely investigated. Most of the studies have been focused on hepatic encephalopathy. Rifaximin administration results in the functional modulation of the gut microbiome, rather than its composition [110]. Bajaj et al. evaluated the impact of rifaximin on patients with mild hepatic encephalopathy; notably, the metabolomic analysis revealed an increase in serum saturated and unsaturated fatty acids, as well as a positive modulation of the bacterial metabolic network, with only a slight change in the microbiota composition itself [111]. Based on recent findings, rifaximin may affect certain components of the gut microbiome that metabolically contribute to hepatic encephalopathy, and the effectiveness or failure of therapy may depend on this [112]. Instead, the TLR4/LPS pathway could be the key to understanding the effect of rifaximin on portal hypertension. In bile duct-ligated TLR4-mutant mice, rifaximin did not reduce portal hypertension, angiogenesis, and liver fibrosis as it did in wild-type mice, suggesting that the attenuation of portal hypertension induced by rifaximin is mediated by TLR4, and that acting on this pathway can influence the fibrogenic activity of HSCs and endothelial cells [113]. Moreover, rifaximin upregulates pregnane X receptor (PXR) target genes, which contribute to the integrity of the intestinal barrier and further prevent the activation of NFκB, along with the following pro-inflammatory cascade in the gut [114,115,116].
Rifaximin is undoubtedly the best-studied molecule in the modulation of portal hypertension. Little evidence is available regarding other antibiotics; in the early 2000s, norfloxacin’s effects were evaluated in small cohorts, with absent or weak results in ameliorating portal hypertension [117,118].
6.2. Probiotics
Probiotics administration, providing the gut ecosystem with beneficial bacteria, is extensively used in intestinal and extra-intestinal diseases, with often unclear evidence for the wide variety of products on the market and the heterogeneous experimental settings.
In animal models of cirrhosis, the administration of Bifidobacterium pseudocatenulatum CECT 7765, which upregulates anti-inflammatory markers and molecules associated with intestinal barrier integrity in experimental cirrhosis, was associated with a reduction in portal flow and the portal vein area and an increase in serum NO [74,119]. This was paralleled by the improvement in the gut microbiota profile, with a reduction in the relative abundance of Proteobacteria and an increase in that of Bacteroidetes, and the amelioration of liver function. The action of B. pseudocatenulatum CECT 7765 seems to be dose-dependent, as the increase in the dose is inversely related to the endotoxin serum levels and markers of intestinal permeability [120].
There is limited evidence on the effect of probiotics on portal hypertension from human studies; the most relevant data on this topic concern VSL3, a widely known probiotic mixture associated with inflammatory bowel disease and other gastrointestinal conditions. Jayakumar et al. sought to demonstrate the impact of a high dose of VSL3 (4 sachets/day for two months) on portal hypertension compared with a placebo in 17 patients with decompensated cirrhosis. They observed a decrease in HVPG not reaching statistical significance, either with respect to the gut microbiota composition or to endotoxins and inflammatory cytokines, probably due to the small sample size [121]. Similarly, another study analyzed the administration of VSL3 in preventing portal hypertension-related complications in 94 cirrhotic patients with large esophageal varices, showing that the combination of propranolol plus VSL3 was able to achieve a reduction in HVPG of up to 58% compared with 31% in the control group receiving propranolol alone [122]. Another study reported the beneficial effect of VSL3 (two sachets/day) in a small group of 12 cirrhotic patients with ascites, resulting in a widespread improvement in systemic and portal hemodynamics, as suggested by the reduction in the HVPG, cardiac index, and heart rate, as well as the increase in systemic vascular resistance and mean arterial pressure [123].
6.3. Fecal Microbiota Transplantation and Other Agents
Fecal microbiota transplantation (FMT) can deeply modify the natural history of liver disease, especially acting on complications such as hepatic encephalopathy, being a unique tool able to completely reset the gut microbiome.
Although data on the effect on portal hypertension are limited, evidence exists regarding the effects of FMT on portal hypertension in mouse models. Fecal and terminal ileum microbiota transplantation in bile duct-ligated rats significantly reduced splanchnic flow, portosystemic shunt flow, and portal pressure in association with a decrease in splanchnic eNOS activity and an increase in Bifidobacteria [75]. In another study, FMT from rats on a control diet to rats with early steatohepatitis was associated with the restoration of intrahepatic vascular resistance through the normalization of endothelial dysfunction pathways, mainly involving phosphorylated protein kinase B and phosphorylated eNOS [76]. However, FMT from the steatohepatitis rat model in healthy animals did not cause a rise in portal pressure within the study time of 14 days. As suggested by the authors, the beneficial effects of FMT are likely to be fleeting, and therefore hardly usable in a clinical setting if not sustained over time. Perhaps prolonged treatment may achieve superior benefits, but further studies are needed to prove this effect.
Finally, recent data have demonstrated that obeticholic acid, an FXR-agonist, can decrease portal hypertension and improve the intestinal barrier, acting on mucus production and on the gut–vascular barrier, both in animal models of NASH and in a preliminary study involving patients with alcoholic cirrhosis [124,125,126]. Other FXR agonists have been investigated in liver disease [127,128], with promising effects on the modulation of liver fibrosis progression and of intrahepatic vascular resistance.
7. Effects of Portal Hypertension Lowering Agents on the Gut Microbiome
7.1. Non-Selective Beta-Blockers
Non-selective beta-blockers (NSBBs) are currently indicated in the primary and secondary prophylaxis of variceal bleeding, given their effects on the portal venous system, splanchnic blood flow, and heart rate [16,129,130]. In addition to the effects on hemodynamics, there is emerging evidence regarding the effects of NSBBs on the gut and the microbiota itself. Reinberger et al. [131] showed that the long-term response to NSBBs improved markers of intestinal permeability, bacterial translocation, and IL-6 serum levels in 50 patients with ACLD. Although this improvement was not exclusively observed in HVPG responders, but also in non-responders, patients with baseline HVPG >20 mmHg showed a poor improvement, likely suggesting that there also is a point of no return in the modulation of the gut–liver axis. In this regard, Reverter et al. identified several biomarkers predicting the acute HVPG response to NSBBs through metabolomic serum analysis. Sixty-six patients with cirrhosis and HVPG> = 10 mmHg underwent intravenous administration of propranolol during HVPG measurement; among the 177 metabolites analyzed, the serum levels of a phosphatidylcholine and a free fatty acid, combined in a two-cutoff system, were able to predict the acute response to NSBBs, with a positive predictive value of 84% and a negative predictive value of 82%.
A meta-analysis reported a reduced risk of SBP in NSBBs-treated patients both in hemodynamic responders and non-responders [132]. One possible explanation concerns the function of the sympathetic nervous system in this context, through the regulation of the enteric nerve plexus activity, but also of the intestinal mucosa and the GALT [133]. ACLD, among the above-mentioned mechanisms, is associated with the increased release of catecholamines in an attempt to counteract the vasodilation of the splanchnic venous system [51]. There is strong evidence suggesting that a high sympathetic tone in the gut is associated with a series of sustained alterations, starting with the reduction in intestinal peristalsis and impairing bacterial clearance. In addition, the increased sympathetic tone in the gut may be associated with SIBO and the overgrowth of specific bacteria, including Escherichia coli or other virulent strains [134,135,136], and also participates in interfering with phagocytosis and diapedesis, which are critical for gut immune homeostasis [137,138,139]. All these alterations seem to be, at least partially, counteracted by NSBBs [140,141].
Taken together, these data suggest that NSBBs, beyond their established effect on portal hemodynamics, may also act on the gut–liver axis through the modulation of the sympathetic nervous system, thereby improving the gut microbiome profile and restoring the integrity of the intestinal barrier [136].
7.2. Statins
In addition to their lipid-lowering property, statins are molecules with multiple pleiotropic effects, including anti-inflammatory and anti-fibrotic effects [142]. In particular, simvastatin and atorvastatin have been shown to exert a beneficial role in ACLD, reducing portal pressure and fibrosis, and improving liver sinusoidal and microvascular dysfunction [143].
A recent study in mice with NASH demonstrated statins’ ability to decrease portal pressure by reverting SECs’ transition to capillarization and HSCs’ abnormal activation [144]. The effects of statins have also been studied in patients with cirrhosis and portal hypertension.
A randomized controlled trial including 59 patients with portal hypertension evaluated the effect of simvastatin administration, initially at a dose of 20 mg/day that then increased to 40 mg/day on day 15, compared with a placebo for one month. Simvastatin significantly decreased HVPG and improved liver perfusion without inducing arterial hypotension [145]. Another study with a similar design enrolled 158 patients with variceal bleeding who were followed over 2 years [146]. The addition of simvastatin to NSBBs and band ligation was not associated with a significant reduction in the re-bleeding rate, but it achieved a significant survival benefit.
Currently, there are no published studies linking the effect of statins on portal hypertension to the modulation of the gut microbiome. However, some evidence showed that statins can influence the composition of the gut microbiome, especially in patients with cardiovascular disorders [147,148]. Indeed, a study including patients with acute coronary syndrome demonstrated that statins could reduce potentially pathogenic bacteria and increase beneficial bacteria, shifting the gut microbiome toward a healthier condition [149]. This might result in an improved profile of circulating metabolites and reduced metabolic risk.
Based on these findings, the LIVERHOPE project is trying to assess if the combination of simvastatin plus rifaximin can prevent the progression to ACLF in patients with decompensated cirrhosis, with the analysis of the gut microbiome planned as a secondary endpoint (NCT03780673).
8. Conclusions
Our understanding of the role of the gut microbiota in human diseases has rapidly advanced in recent years. The increasing accessibility of complex methods of integrated metagenomics and metabolomics analysis provides a broader perspective, aiding in the identification of new molecular targets that can change the disease story from diagnosis to treatment. Metabolomics shed light on the importance of functional diversity in gut microbiome enzymatic activities, distancing from a point of view based solely on compositional analysis. However, there is still much to learn about the role of the gut–liver axis in the development and progression of ACLD and portal hypertension. There is growing evidence that the disruption of the gut–liver axis leads to the development of CSPH, especially through dysbiosis, damage to the intestinal barrier resulting in increased permeability, and alterations in the enterohepatic circulation of bile acids. The analysis of the microbial components crossing the intestinal barrier could be a shortcut to stratify patients according to the systemic inflammatory and hemodynamic conditions. The identification of the gut–liver-axis-related metabolic and molecular pathways that can be involved in this process is an unmet need that may serve to not only clarify pathogenesis and to define prognosis, but also as a target for new therapeutic strategies. The modulation of the intestinal environment with FMT is a very promising tool for the treatment of portal hypertension, with interesting results in animal models; however, randomized controlled trials in humans are needed to demonstrate its efficacy and to elucidate its mechanisms of action. Finally, the interaction between the gut microbiome and the different available pharmacological treatments could be a useful tool to monitor treatment efficacy as a noninvasive predictor of the hemodynamic response, shifting to a personalized therapy approach and thus having a considerable impact on the prognosis and survival of these patients.
Author Contributions
F.S., G.C., L.G. and F.R.P. developed the concept, revised the literature, and wrote the manuscript; A.G. developed the concept, supervised the research, and revised the manuscript. All investigators participated in data interpretation and contributed to the revision of the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Fondazione Roma for the continuous support to our scientific research. Figure 1 was created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schnabl, B.; Brenner, D.A. Interactions between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Caetano M Antunes, L.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Seo, Y.S.; Shah, V.H. The Role of Gut-Liver Axis in the Pathogenesis of Liver Cirrhosis and Portal Hypertension. Clin. Mol. Hepatol. 2012, 18, 337–346. [Google Scholar] [CrossRef]
- Wiest, R.; Garcia-Tsao, G. Bacterial Translocation (BT) in Cirrhosis. Hepatology 2005, 41, 422–433. [Google Scholar] [CrossRef]
- Goel, A.; Gupta, M.; Aggarwal, R. Gut Microbiota and Liver Disease. J. Gastroenterol. Hepatol. 2014, 29, 1139–1148. [Google Scholar] [CrossRef]
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut–Liver Axis, Cirrhosis and Portal Hypertension: The Chicken and the Egg. Hepatol. Int. 2017, 12, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G. Potential Mechanisms Linking Gut Microbiota and Portal Hypertension. Liver Int. 2019, 39, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G. Portal Hypertension. Curr. Opin. Intern. Med. 2006, 5, 399–407. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII—Renewing Consensus in Portal Hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Berzigotti, A.; Garcia-Pagan, J.C.; Abraldes, J.G. The Management of Portal Hypertension: Rational Basis, Available Treatments and Future Options. J. Hepatol. 2008, 48, S68–S92. [Google Scholar] [CrossRef]
- Bloom, S.; Kemp, W.; Lubel, J. Portal Hypertension: Pathophysiology, Diagnosis and Management. Intern. Med. J. 2015, 45, 16–26. [Google Scholar] [CrossRef]
- Mauro, E.; Gadano, A. What’s New in Portal Hypertension? Liver Int. 2020, 40, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Gunarathne, L.S.; Rajapaksha, H.; Shackel, N.; Angus, P.W.; Herath, C.B. Cirrhotic Portal Hypertension: From Pathophysiology to Novel Therapeutics. World J. Gastroenterol. 2020, 26, 6111–6140. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines on Prevention and Management of Bleeding and Thrombosis in Patients with Cirrhosis. J. Hepatol. 2022, 76, 1151–1184. [CrossRef] [PubMed]
- Wanless, I.R.; Wong, F.; Blendis, L.M.; Greig, P.; Heathcote, E.J.; Levy, G. Hepatic and Portal Vein Thrombosis in Cirrhosis: Possible Role in Development of Parenchymal Extinction and Portal Hypertension. Hepatology 1995, 21, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Turco, L.; Garcia-Tsao, G. Portal Hypertension: Pathogenesis and Diagnosis. Clin. Liver Dis. 2019, 23, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Marrone, G.; Fernández-Iglesias, A. Hepatic Microcirculation and Mechanisms of Portal Hypertension. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Cammà, C.; Marietta, M.; Luongo, M.; Critelli, R.; Colopi, S.; Tata, C.; Zecchini, R.; Gitto, S.; Petta, S.; et al. Enoxaparin Prevents Portal Vein Thrombosis and Liver Decompensation in Patients with Advanced Cirrhosis. Gastroenterology 2012, 143, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Turco, L.; Schepis, F.; Villa, E. The Role of Anticoagulation in Treating Portal Hypertension. Curr. Hepatol. Rep. 2018, 17, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- van Itallie, C.M.; Holmes, J.; Bridges, A.; Gookin, J.L.; Coccaro, M.R.; Proctor, W.; Colegio, O.R.; Anderson, J.M. The Density of Small Tight Junction Pores Varies among Cell Types and Is Increased by Expression of Claudin-2. J. Cell Sci. 2008, 121, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gautreaux, M.D.; Dietch, E.A.; Berg, R.D. T Lymphocytes in Host Defense against Bacterial Translocation from the Gastrointestinal Tract. Infect. Immun. 1994, 62, 2874–2884. [Google Scholar] [CrossRef]
- Gautreaux, M.D.; Gelder, F.B.; Deitch, E.A.; Berg, R.D. Adoptive Transfer of T Lymphocytes to T-Cell-Depleted Mice Inhibits Escherichia Coli Translocation from the Gastrointestinal Tract. Infect. Immun. 1995, 63, 3827–3834. [Google Scholar] [CrossRef]
- Hapfelmeier, S.; Lawson, M.A.E.; Slack, E.; Kirundi, J.K.; Stoel, M.; Heikenwalder, M.; Cahenzli, J.; Velykoredko, Y.; Balmer, M.L.; Endt, K.; et al. Reversible Microbial Colonization of Germ-Free Mice Reveals the Dynamics of IgA Immune Responses. Science 2010, 328, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological Bacterial Translocation in Liver Cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Duparc, T.; Plovier, H.; Marrachelli, V.G.; van Hul, M.; Essaghir, A.; Ståhlman, M.; Matamoros, S.; Geurts, L.; Pardo-Tendero, M.M.; Druart, C.; et al. Hepatocyte MyD88 Affects Bile Acids, Gut Microbiota and Metabolome Contributing to Regulate Glucose and Lipid Metabolism. Gut 2017, 66, 620–632. [Google Scholar] [CrossRef]
- Lorenzo-Zúñiga, V.; Bartolí, R.; Planas, R.; Hofmann, A.F.; Viñado, B.; Hagey, L.R.; Hernández, J.M.; Mañé, J.; Alvarez, M.A.; Ausina, V.; et al. Oral Bile Acids Reduce Bacterial Overgrowth, Bacterial Translocation, and Endotoxemia in Cirrhotic Rats. Hepatology 2003, 37, 551–557. [Google Scholar] [CrossRef]
- Bertók, L. Bile Acids in Physico-Chemical Host Defence. Pathophysiology 2004, 11, 139–145. [Google Scholar] [CrossRef]
- di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut Microbiome and Liver Diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.U. Microbiota in T-Cell Homeostasis and Inflammatory Diseases. Exp. Mol. Med. 2017, 49, e340. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Zocco, M.A.; Cerrito, L.; Gasbarrini, A.; Pompili, M. Bacterial Translocation in Patients with Liver Cirrhosis: Physiology, Clinical Consequences, and Practical Implications. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 641–656. [Google Scholar] [CrossRef]
- Duffield, J.S.; Forbes, S.J.; Constandinou, C.M.; Clay, S.; Partolina, M.; Vuthoori, S.; Wu, S.; Lang, R.; Iredale, J.P. Selective Depletion of Macrophages Reveals Distinct, Opposing Roles during Liver Injury and Repair. J. Clin. Investig. 2005, 115, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; de Minicis, S.; Österreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 Enhances TGF-β Signaling and Hepatic Fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Brandl, K.; Kumar, V.; Eckmann, L. MINI-REVIEW Microbiome and Host Interactions Gut-Liver Axis at the Frontier of Host-Microbial Interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.S.; Thomas, P.; Broitman, S.A. Clearance of Gut-Derived Endotoxins by the Liver. Release and Modification of 3H, 14C-Lipopolysaccharide by Isolated Rat Kupffer Cells. Gastroenterology 1989, 96, 456–461. [Google Scholar] [CrossRef]
- Seki, E.; Schnabl, B. Role of Innate Immunity and the Microbiota in Liver Fibrosis: Crosstalk between the Liver and Gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Pirlich, M. Gastrointestinal Tract in Liver Disease: Which Organ Is Sick? Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Tranah, T.H.; Edwards, L.A.; Schnabl, B.; Shawcross, D.L. Targeting the Gut-Liver-Immune Axis to Treat Cirrhosis. Gut 2021, 70, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Macnaughtan, J.; Schnabl, B.; Shawcross, D.L.; Bajaj, J.S. The Microbiota in Cirrhosis and Its Role in Hepatic Decompensation. J. Hepatol. 2021, 75, S67–S81. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Poluektova, E.; Shirokova, E.; Maslennikov, R.; Poluektova, E.; Maslennikov, R. Gut-Liver Axis in Cirrhosis: Are Hemodynamic Changes a Missing Link? World J. Clin. Cases 2021, 9, 9320–9332. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Møller, S.; Ring-Larsen, H.; Christensen, N.J. The Sympathetic Nervous System in Liver Disease. J. Hepatol. 1998, 29, 328–341. [Google Scholar] [CrossRef]
- Lin, R.S.; Lee, F.Y.; Lee, S.D.; Tsai, Y.T.; Lin, H.C.; Rei-Hwa, L.; Wan-Ching, H.; Cheng-Chun, H.; Sun-Sang, W.; Kwang-Juei, L. Endotoxemia in Patients with Chronic Liver Diseases: Relationship to Severity of Liver Diseases, Presence of Esophaegeal Varices, and Hyperdynamic Circulation. J. Hepatol. 1995, 22, 165–172. [Google Scholar] [CrossRef]
- Bellot, P.; García-Pagán, J.C.; Francés, R.; Abraldes, J.G.; Navasa, M.; Pérez-Mateo, M.; Such, J.; Bosch, J. Bacterial DNA Translocation Is Associated with Systemic Circulatory Abnormalities and Intrahepatic Endothelial Dysfunction in Patients with Cirrhosis. Hepatology 2010, 52, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Lee, F.Y.; Barden, G.E.; Cartun, R.; Brian West, A. Bacterial Translocation to Mesenteric Lymph Nodes Is Increased in Cirrhotic Rats with Ascites. Gastroenterology 1995, 108, 1835–1841. [Google Scholar] [CrossRef]
- Cirera, I.; Martin Bauer, T.; Miguel, N.; Vila, J.; Grande, L.; Taurá, P.; Fuster, J.; García-Valdecasas, J.C.; Lacy, A.; María Jesús, S.; et al. Bacterial Translocation of Enteric Organisms in Patients with Cirrhosis. J. Hepatol. 2001, 34, 32–37. [Google Scholar] [CrossRef]
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal Permeability in the Pathogenesis of Liver Damage: From Non-Alcoholic Fatty Liver Disease to Liver Transplantation. World J. Gastroenterol. 2019, 25, 4814–4834. [Google Scholar] [CrossRef]
- Mcdermott, A.J.; Huffnagle, G.B. The Microbiome and Regulation of Mucosal Immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef]
- Marrone, G.; Shah, V.H.; Gracia-Sancho, J. Sinusoidal Communication in Liver Fibrosis and Regeneration. J. Hepatol. 2016, 65, 608–617. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Mehta, G.; Gustot, T.; Mookerjee, R.P.; Garcia-Pagan, J.C.; Fallon, M.B.; Shah, V.H.; Moreau, R.; Jalan, R. Inflammation and Portal Hypertension—The Undiscovered Country. J. Hepatol. 2014, 61, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G. Origins of Portal Hypertension in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2018, 63, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Puoti, C.; Bellis, L. Steatosis and Portal Hypertension. Eur. Rev. Med. Pharmacol. Sci. 2005, 9, 285–290. [Google Scholar] [PubMed]
- Zhang, W.; Wu, Y.; Mu, D.; Gong, J.; Wu, C.; Huang, C. Kupffer Cells: Increasingly Significant Role in Nonalcoholic Fatty Liver Disease. Ann. Hepatol. 2014, 13, 489–495. [Google Scholar] [CrossRef]
- Heymann, F.; Tacke, F. Immunology in the Liver-from Homeostasis to Disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Bosch, J.; García-Pagán, J.C. Complications of Cirrhosis. I. Portal Hypertension. J. Hepatol. 2000, 32 (Suppl. 1), 141–156. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Shah, V.; Rockey, D.C. Vascular Pathobiology in Chronic Liver Disease and Cirrhosis—Current Status and Future Directions. J. Hepatol. 2014, 61, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Groszmann, R.J. The Paradox of Nitric Oxide in Cirrhosis and Portal Hypertension: Too Much, Not Enough. Hepatology 2002, 35, 478–791. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Groszmann, R.J. Vascular Endothelial Dysfunction in Cirrhosis. J. Hepatol. 2007, 46, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Francés, R.; Muñoz, C.; Zapater, P.; Uceda, F.; Gascón, I.; Pascual, S.; Pérez-Mateo, M.; Such, J. Bacterial DNA Activates Cell Mediated Immune Response and Nitric Oxide Overproduction in Peritoneal Macrophages from Patients with Cirrhosis and Ascites. Gut 2004, 53, 860–864. [Google Scholar] [CrossRef]
- Paratore, M.; Santopaolo, F.; Cammarota, G.; Pompili, M.; Gasbarrini, A.; Ponziani, F.R. Fecal Microbiota Transplantation in Patients with Hbv Infection or Other Chronic Liver Diseases: Update on Current Knowledge and Future Perspectives. J. Clin. Med. 2021, 10, 2605. [Google Scholar] [CrossRef] [PubMed]
- Gedgaudas, R.; Bajaj, J.S.; Skieceviciene, J.; Varkalaite, G.; Jurkeviciute, G.; Gelman, S.; Valantiene, I.; Zykus, R.; Pranculis, A.; Bang, C.; et al. Circulating Microbiome in Patients with Portal Hypertension. Gut Microbes 2022, 14, 2029674. [Google Scholar] [CrossRef]
- Virseda-Berdices, A.; Brochado-Kith, O.; Díez, C.; Hontañon, V.; Berenguer, J.; González-García, J.; Rojo, D.; Fernández-Rodríguez, A.; Ibañez-Samaniego, L.; Llop-Herrera, E.; et al. Blood Microbiome Is Associated with Changes in Portal Hypertension after Successful Direct-Acting Antiviral Therapy in Patients with HCV-Related Cirrhosis. J. Antimicrob. Chemother. 2022, 77, 719–726. [Google Scholar] [CrossRef]
- Yokoyama, K.; Tsuchiya, N.; Yamauchi, R.; Miyayama, T.; Uchida, Y.; Shibata, K.; Fukuda, H.; Umeda, K.; Takata, K.; Tanaka, T.; et al. Exploratory Research on the Relationship between Human Gut Microbiota and Portal Hypertension. Intern. Med. 2020, 59, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, A.; Gómez-Hurtado, I.; Moya-Pérez, Á.; Zapater, P.; Peiró, G.; González-Navajas, J.M.; Gómez Del Pulgar, E.M.; Such, J.; Sanz, Y.; Francés, R. Bifidobacterium Pseudocatenulatum CECT7765 Promotes a TLR2-Dependent Anti-Inflammatory Response in Intestinal Lymphocytes from Mice with Cirrhosis. Eur. J. Nutr. 2016, 55, 197–206. [Google Scholar] [CrossRef]
- García-Lezana, T.; Raurell, I.; Bravo, M.; Torres-Arauz, M.; Salcedo, M.T.; Santiago, A.; Schoenenberger, A.; Manichanh, C.; Genescà, J.; Martell, M.; et al. Restoration of a Healthy Intestinal Microbiota Normalizes Portal Hypertension in a Rat Model of Nonalcoholic Steatohepatitis. Hepatology 2018, 67, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, L.; Farre, R.; Trebicka, J.; Komuta, M.; Roskams, T.; Klein, S.; Elst, I.V.; Windmolders, P.; Vanuytsel, T.; Nevens, F.; et al. Obeticholic Acid, a Farnesoid X Receptor Agonist, Improves Portal Hypertension by Two Distinct Pathways in Cirrhotic Rats. Hepatology 2014, 59, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Vizzutti, F.; Garcia-Pagan, J.C.; Rodes, J.; Bosch, J. Anti-VEGF Receptor-2 Monoclonal Antibody Prevents Portal-Systemic Collateral Vessel Formation in Portal Hypertensive Mice. Gastroenterology 2004, 126, 886–894. [Google Scholar] [CrossRef]
- Fernández, M.; Semela, D.; Bruix, J.; Colle, I.; Pinzani, M.; Bosch, J. Angiogenesis in Liver Disease. J. Hepatol. 2009, 50, 604–620. [Google Scholar] [CrossRef]
- Moghadamrad, S.; Mccoy, K.D.; Geuking, M.B.; Sägesser, H.; Kirundi, J.; Macpherson, A.J.; de Gottardi, A. Attenuated Portal Hypertension in Germ-Free Mice: Function of Bacterial Flora on the Development of Mesenteric Lymphatic and Blood Vessels. Hepatology 2015, 61, 1685–1695. [Google Scholar] [CrossRef]
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Distrutti, E.; Mencarelli, A.; Santucci, L.; Renga, B.; Orlandi, S.; Donini, A.; Shah, V.; Fiorucci, S. The Methionine Connection: Homocysteine and Hydrogen Sulfide Exert Opposite Effects on Hepatic Microcirculation in Rats. Hepatology 2008, 47, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Antonelli, E.; Mencarelli, A.; Orlandi, S.; Renga, B.; Rizzo, G.; Distrutti, E.; Shah, V.; Morelli, A. The Third Gas: H2S Regulates Perfusion Pressure in Both the Isolated and Perfused Normal Rat Liver and in Cirrhosis. Hepatology 2005, 42, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, N.; Guo, J.; Qian, G.; Fang, D.; Shi, D.; Xu, M.; Yang, F.; He, Z.; van Nostrand, J.D.; et al. Functional Gene Arrays-Based Analysis of Fecal Microbiomes in Patients with Liver Cirrhosis. BMC Genom. 2014, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The Severity of Nonalcoholic Fatty Liver Disease Is Associated with Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Peleman, C.; Camilleri, M. Rifaximin, Microbiota Biology, and Hepatic Encephalopathy. Clin. Transl. Gastroenterol. 2016, 7, e195. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Mitten, E.K.; Baffy, G. Microbiota Transplantation in Portal Hypertension: Promises and Pitfalls. Clin. Sci. 2022, 136, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Author Correction: Bile Acid Metabolites Control TH17 and Treg Cell Differentiation (Nature, (2019), 576, 7785, (143-148), 10.1038/S41586-019-1785-z). Nature 2020, 576, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, V.; di Gregorio, V.; Iebba, V.; Giusto, M.; Schippa, S.; Merli, M.; Thalheimer, U. Microbiota and the Gut-Liver Axis: Bacterial Translocation, Inflammation and Infection in Cirrhosis. World J. Gastroenterol. 2014, 20, 16795–16810. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.; Olde Damink, S.W.M.; von Bergen, M.; Schaap, F.G. Interactions between Bile Salts, Gut Microbiota, and Hepatic Innate Immunity. Immunol. Rev. 2017, 279, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Kakiyama, G.; Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Heuman, D.M.; Kang, D.J.; Takei, H.; Nittono, H.; Ridlon, J.M.; Fuchs, M.; et al. Colonic Inflammation and Secondary Bile Acids in Alcoholic Cirrhosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 306, G929–G937. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Zong, X.; Fu, J.; Xu, B.; Wang, Y.; Jin, M. Interplay between gut microbiota and antimicrobial peptides. Anim. Nutr. 2020, 6, 389–396. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Abrudan, M.I.; Smakman, F.; Grimbergen, A.J.; Westhoff, S.; Miller, E.L.; van Wezel, G.P.; Rozen, D.E. Socially mediated induction and suppression of antibiosis during bacterial coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 11054–11059. [Google Scholar] [CrossRef]
- Wang, L.; Fouts, D.E.; Stärkel, P.; Hartmann, P.; Chen, P.; Llorente, C.; DePew, J.; Moncera, K.; Ho, S.B.; Brenner, D.A.; et al. Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell Host Microbe. 2016, 19, 227–239. [Google Scholar] [CrossRef]
- Teltschik, Z.; Wiest, R.; Beisner, J.; Nuding, S.; Hofmann, C.; Schoelmerich, J.; Bevins, C.L.; Stange, E.F.; Wehkamp, J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology 2012, 55, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, F.; Guo, J.; Shi, D.; Fang, D.; Li, L. Dysbiosis of Small Intestinal Microbiota in Liver Cirrhosis and Its Association with Etiology. Sci. Rep. 2016, 6, 34055. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Zocco, M.A.; D’Aversa, F.; Pompili, M.; Gasbarrini, A. Eubiotic Properties of Rifaximin: Disruption of the Traditional Concepts in Gut Microbiota Modulation. World J. Gastroenterol. 2017, 23, 4491–4499. [Google Scholar] [CrossRef]
- Gillis, J.C.; Brogden, R.N. Rifaximin: A Review of Its Antibacterial Activity, Pharmacokinetic Properties and Therapeutic Potential in Conditions Mediated by Gastrointestinal Bacteria. Drugs 1995, 49, 467–484. [Google Scholar] [CrossRef]
- Vlachogiannakos, J.; Saveriadis, A.S.; Viazis, N.; Theodoropoulos, I.; Foudoulis, K.; Manolakopoulos, S.; Raptis, S.; Karamanolis, D.G. Intestinal Decontamination Improves Liver Haemodynamics in Patients with Alcohol-Related Decompensated Cirrhosis. Aliment. Pharmacol. Ther. 2009, 29, 992–999. [Google Scholar] [CrossRef]
- Vlachogiannakos, J.; Viazis, N.; Vasianopoulou, P.; Vafiadis, I.; Karamanolis, D.G.; Ladas, S.D. Long-Term Administration of Rifaximin Improves the Prognosis of Patients with Decompensated Alcoholic Cirrhosis. J. Gastroenterol. Hepatol. 2013, 28, 450–455. [Google Scholar] [CrossRef]
- Lim, Y.L.; Kim, M.Y.; Jang, Y.O.; Baik, S.K.; Kwon, S.O. Rifaximin and Propranolol Combination Therapy Is More Effective than Propranolol Monotherapy for the Reduction of Portal Pressure: An Open Randomized Controlled Pilot Study. Gut Liver 2017, 11, 702–710. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Gerardi, V.; Pecere, S.; D’Aversa, F.; Lopetuso, L.; Zocco, M.A.; Pompili, M.; Gasbarrini, A. Effect of Rifaximin on Gut Microbiota Composition in Advanced Liver Disease and Its Complications. World J. Gastroenterol. 2015, 21, 12322–12333. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Sanyal, A.J.; Hylemon, P.B.; Sterling, R.K.; Stravitz, R.T.; Fuchs, M.; Ridlon, J.M.; Daita, K.; Monteith, P.; et al. Modulation of the Metabiome by Rifaximin in Patients with Cirrhosis and Minimal Hepatic Encephalopathy. PLoS ONE 2013, 8, e60042. [Google Scholar] [CrossRef]
- Yukawa-Muto, Y.; Kamiya, T.; Fujii, H.; Mori, H.; Toyoda, A.; Sato, I.; Konishi, Y.; Hirayama, A.; Hara, E.; Fukuda, S.; et al. Distinct Responsiveness to Rifaximin in Patients with Hepatic Encephalopathy Depends on Functional Gut Microbial Species. Hepatol. Commun. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zou, L.; Jagavelu, K.; Simonetto, D.A.; Huebert, R.C.; Jiang, Z.D.; Dupont, H.L.; Shah, V.H. Intestinal Decontamination Inhibits TLR4 Dependent Fibronectin-Mediated Cross-Talk between Stellate Cells and Endothelial Cells in Liver Fibrosis in Mice. J. Hepatol. 2012, 56, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shah, Y.M.; Guo, G.L.; Wang, T.; Krausz, K.W.; Idle, J.R.; Gonzalez, F.J. Rifaximin Is a Gut-Specific Human Pregnane X Receptor Activator. J. Pharmacol. Exp. Ther. 2007, 322, 391–398. [Google Scholar] [CrossRef]
- Langmann, T.; Moehle, C.; Mauerer, R.; Scharl, M.; Liebisch, G.; Zahn, A.; Stremmel, W.; Schmitz, G. Loss of Detoxification in Inflammatory Bowel Disease: Dysregulation of Pregnane X Receptor Target Genes. Gastroenterology 2004, 127, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Renga, B.; Palladino, G.; Claudio, D.; Ricci, P.; Distrutti, E.; Barbanti, M.; Baldelli, F.; Fiorucci, S. Inhibition of NF-ΚB by a PXR-Dependent Pathway Mediates Counter-Regulatory Activities of Rifaximin on Innate Immunity in Intestinal Epithelial Cells. Eur. J. Pharmacol. 2011, 668, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kemp, W.; Colman, J.; Thompson, K.; Madan, A.; Vincent, M.; Chin-Dusting, J.; Kompa, A.; Krum, H.; Roberts, S. Norfloxacin Treatment for Clinically Significant Portal Hypertension: Results of a Randomised Double-Blind Placebo-Controlled Crossover Trial. Liver Int. 2009, 29, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Rasaratnam, B.; Kaye, D.; Jennings, G.; Dudley, F.; Chin-Dusting, J.P.F. The Effect of Selective Intestinal Decontamination on the Hyperdynamic Circulatory State in Cirrhosis: A Randomized Trial. Ann. Intern. Med. 2003, 139, 186–193. [Google Scholar] [CrossRef]
- Moratalla, A.; Gómez-Hurtado, I.; Santacruz, A.; Moya, Á.; Peiró, G.; Zapater, P.; González-Navajas, J.M.; Giménez, P.; Such, J.; Sanz, Y.; et al. Protective Effect of Bifidobacterium Pseudocatenulatum CECT7765 against Induced Bacterial Antigen Translocation in Experimental Cirrhosis. Liver Int. 2014, 34, 850–858. [Google Scholar] [CrossRef]
- Gómez-Hurtado, I.; Zapater, P.; Portune, K.; Juanola, O.; Fernández-Iglesias, A.; González-Navajas, J.M.; Gracia-Sancho, J.; Sanz, Y.; Francés, R. Improved Hemodynamic and Liver Function in Portal Hypertensive Cirrhotic Rats after Administration of B. Pseudocatenulatum CECT 7765. Eur. J. Nutr. 2019, 58, 1647–1658. [Google Scholar] [CrossRef]
- Jayakumar, S.; Carbonneau, M.; Hotte, N.; Befus, A.D.; St. Laurent, C.; Owen, R.; Mccarthy, M.; Madsen, K.; Bailey, R.J.; Ma, M.; et al. VSL#3® Probiotic Therapy Does Not Reduce Portal Pressures in Patients with Decompensated Cirrhosis. Liver Int. 2013, 33, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Kumar, A.; Sharma, P.; Garg, V.; Sharma, B.C.; Sarin, S.K. Effects of the Adjunctive Probiotic VSL#3 on Portal Haemodynamics in Patients with Cirrhosis and Large Varices: A Randomized Trial. Liver Int. 2013, 33, 1148–1157. [Google Scholar] [CrossRef]
- Rincón, D.; Vaquero, J.; Hernando, A.; Galindo, E.; Ripoll, C.; Puerto, M.; Salcedo, M.; Francés, R.; Matilla, A.; Catalina, M.V.; et al. Oral Probiotic VSL#3 Attenuates the Circulatory Disturbances of Patients with Cirrhosis and Ascites. Liver Int. 2014, 34, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Tsai, M.H.; Chang, C.C.; Pun, C.K.; Huang, Y.H.; Hou, M.C.; Lee, F.Y.; Hsu, S.J. Microbiota Transplants from Feces or Gut Content Attenuated Portal Hypertension and Portosystemic Collaterals in Cirrhotic Rats. Clin. Sci. 2021, 135, 2709–2728. [Google Scholar] [CrossRef]
- Mookerjee, R.; Rosselli, M.; Pieri, G.; Beecher-Jones, T.; Hooshmand-Rad, R.; Chouhan, M.; Mehta, G.; Jalan, R.; Shapiro, D. O15 effects of the fxr agonist obeticholic acid on hepatic venous pressure gradient (hvpg) in alcoholic cirrhosis: A proof of concept phase 2a study. J. Hepatol. 2014, 60, S7–S8. [Google Scholar] [CrossRef]
- Sorribas, M.; Jakob, M.O.; Yilmaz, B.; Li, H.; Stutz, D.; Noser, Y.; de Gottardi, A.; Moghadamrad, S.; Hassan, M.; Albillos, A.; et al. FXR Modulates the Gut-Vascular Barrier by Regulating the Entry Sites for Bacterial Translocation in Experimental Cirrhosis. J. Hepatol. 2019, 71, 1126–1140. [Google Scholar] [CrossRef]
- Schwabl, P.; Hambruch, E.; Seeland, B.A.; Hayden, H.; Wagner, M.; Garnys, L.; Strobel, B.; Schubert, T.L.; Riedl, F.; Mitteregger, D.; et al. The FXR Agonist PX20606 Ameliorates Portal Hypertension by Targeting Vascular Remodelling and Sinusoidal Dysfunction. J. Hepatol. 2017, 66, 724–733. [Google Scholar] [CrossRef]
- Li, J.; Kuruba, R.; Wilson, A.; Gao, X.; Zhang, Y.; Li, S. Inhibition of Endothelin-1-Mediated Contraction of Hepatic Stellate Cells by FXR Ligand. PLoS ONE 2010, 5, e13955. [Google Scholar] [CrossRef]
- Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Grace, N.D.; Burroughs, A.K.; Planas, R.; Escorsell, A.; Garcia-Pagan, J.C.; Patch, D.; Matloff, D.S.; et al. Beta-Blockers to Prevent Gastroesophageal Varices in Patients with Cirrhosis. New Engl. J. Med. 2005, 353, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Abraldes, J.G.; Berzigotti, A.; Garcia-Pagan, J.C.; Bosch, J. Renin-Angiotensin-Aldosterone Inhibitors in the Reduction of Portal Pressure: A Systematic Review and Meta-Analysis. J. Hepatol. 2010, 53, 273–282. [Google Scholar] [CrossRef]
- Reiberger, T.; Ferlitsch, A.; Payer, B.A.; Mandorfer, M.; Heinisch, B.B.; Hayden, H.; Lammert, F.; Trauner, M.; Peck-Radosavljevic, M.; Vogelsang, H. Non-Selective Betablocker Therapy Decreases Intestinal Permeability and Serum Levels of LBP and IL-6 in Patients with Cirrhosis. J. Hepatol. 2013, 58, 911–921. [Google Scholar] [CrossRef]
- Senzolo, M.; Cholongitas, E.; Burra, P.; Leandro, G.; Thalheimer, U.; Patch, D.; Burroughs, A.K. β-Blockers Protect against Spontaneous Bacterial Peritonitis in Cirrhotic Patients: A Meta-Analysis. Liver Int. 2009, 29, 1189–1193. [Google Scholar] [CrossRef]
- Straub, R.H.; Wiest, R.; Strauch, U.G.; Härle, P.; Schölmerich, J. The Role of the Sympathetic Nervous System in Intestinal Inflammation. Gut 2006, 55, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Alverdy, J.; Holbrook, C.; Rocha, F.; Seiden, L.; Licheng, R.; Wu; Musch, M.; Chang, E.; Ohman, D.; Suh, S. Gut-Derived Sepsis Occurs When the Right Pathogen with the Right Virulence Genes Meets the Right Host: Evidence for in Vivo Virulence Expression in Pseudomonas Aeruginosa. Ann. Surg. 2000, 232, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.P.; Williams, P.H.; Haigh, R.D.; Maggs, A.F.; Neal, C.P.; Lyte, M. Growth Stimulation of Intestinal Commensal Escherichia Coli by Catecholamines: A Possible Contributory Factor in Trauma-Induced Sepsis. Shock 2002, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.P.E.; Sandrini, S.M.; Haigh, R.D.; Lyte, M. Microbial Endocrinology: How Stress Influences Susceptibility to Infection. Trends Microbiol. 2008, 16, 55–64. [Google Scholar] [CrossRef]
- McIntyre, A.S.; Thompson, D.G.; Day, S.; Burnham, W.R.; Walker, E.R. Modulation of Human Upper Intestinal Nutrient Transit by a Beta Adrenoreceptor Mediated Pathway. Gut 1992, 33, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Shilov, J.I.; Orlova, E.G. Role of Adrenergic Mechanisms in Regulation of Phagocytic Cell Functions in Acute Stress Response. Immunol. Lett. 2003, 86, 229–233. [Google Scholar] [CrossRef]
- García, J.J.; del Carmen Sáez, M.; de la Fuente, M.; Ortega Rincón, E. Regulation of Phagocytic Process of Macrophages by Noradrenaline and Its End Metabolite 4-Hydroxy-3-Metoxyphenyl-Glycol. Role of α- and β- Adrenoreceptors. Mol. Cell. Biochem. 2003, 254, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Senzolo, M.; Fries, W.; Buda, A.; Pizzuti, D.; Nadal, E.; Sturniolo, G.C.; Burroughs, A.K.; D’Inc, R. Oral Propranolol Decreases Intestinal Permeability in Patients with Cirrhosis: Another Protective Mechanism against Bleeding. Am. J. Gastroenterol. 2009, 104, 3115–3116. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Paramo, M.; Munoz, J.; Albillos, A.; Freile, I.; Portero, F.; Santos, M.; Ortiz-Berrocal, J. Effect of Propranolol on the Factors Promoting Bacterial Translocation in Cirrhotic Rats with Ascites. Hepatology 2000, 31, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Pose, E.; Trebicka, J.; Mookerjee, R.P.; Angeli, P.; Ginès, P. Statins: Old Drugs as New Therapy for Liver Diseases? J. Hepatol. 2019, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Gracia-Sancho, J.; Abraldes, J.G. Cirrhosis as New Indication for Statins. Gut 2020, 69, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.; Raurell, I.; Hide, D.; Fernández-Iglesias, A.; Gil, M.; Barberá, A.; Salcedo, M.T.; Augustin, S.; Genescà, J.; Martell, M. Restoration of Liver Sinusoidal Cell Phenotypes by Statins Improves Portal Hypertension and Histology in Rats with NASH. Sci. Rep. 2019, 9, 20183–12. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Albillos, A.; Bañares, R.; Turnes, J.; González, R.; García-Pagán, J.C.; Bosch, J. Simvastatin Lowers Portal Pressure in Patients With Cirrhosis and Portal Hypertension: A Randomized Controlled Trial. Gastroenterology 2009, 136, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Villanueva, C.; Aracil, C.; Turnes, J.; Hernandez-Guerra, M.; Genesca, J.; Rodriguez, M.; Castellote, J.; García-Pagán, J.C.; Torres, F.; et al. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients with Cirrhosis. Gastroenterology 2016, 150, 1160–1170.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, W.; Guo, M.; Hua, Y.; Zhou, B.; Li, X.; Zhang, X.; Dong, J.; Yang, X.; Wang, Y.; et al. The Gut Microbiota Is Associated with Clinical Response to Statin Treatment in Patients with Coronary Artery Disease. Atherosclerosis 2021, 325, 16–23. [Google Scholar] [CrossRef]
- Vieira-Silva, S.; Falony, G.; Belda, E.; Nielsen, T.; Aron-Wisnewsky, J.; Chakaroun, R.; Forslund, S.K.; Assmann, K.; Valles-Colomer, M.; Nguyen, T.T.D.; et al. Statin Therapy Is Associated with Lower Prevalence of Gut Microbiota Dysbiosis. Nature 2020, 581, 310–315. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Zhao, X.; Zhou, R.; Liu, H.; Sun, Y.; Fan, Y.; Shi, Y.; Qiao, S.; Liu, S.; et al. Multi-Omics Study Reveals That Statin Therapy Is Associated with Restoration of Gut Microbiota Homeostasis and Improvement in Outcomes in Patients with Acute Coronary Syndrome. Theranostics 2021, 11, 5778–5793. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sheng, X.; Wang, P.Q.; Xin, H.G.; Guo, Y.-B.; Lin, Y.; Zhong, J.W.; He, C.Z.; Yin, J.; Liu, T.T.; et al. Low-Dose Rifaximin Prevents Complications and Improves Survival in Patients with Decompensated Liver Cirrhosis. Hepatol. Int. 2021, 15, 155–165. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).