Abstract
Flaxseed (Linum usitatissimum L.) displays functional properties and contains α-linolenic acid (omega-3). It also contains soluble and insoluble fiber, lignans, phenolic acids, flavonoids, phytic acid, vitamins, and minerals. However, its microbiota can cause fungal contaminations, drastically reducing its quality. The objective of this work was to identify the fungi present in bulk flaxseed through the internal transcribed spacer (ITS1) intergenic region using a metataxonomics approach. Fungal identification was performed via high-performance sequencing of the ITS1 region using ITS1 (GAACCWGCGGARGGATCA) and ITS2 (GCTGCGTTCTTCATCGATGC) as primers with 300 cycles and single-end sequencing in the MiSeq Sequencing System equipment (Illumina Inc., San Diego, CA, USA). Six genera and eight species of fungi were found in the sample. The genus Aspergillus stood out with three xerophilic species found, A. cibarius, A. Appendiculatus, and A. amstelodami, the first being the most abundant. The second most abundant genus was Wallemia, with the species W. muriae. This is one of the fungi taxa with great xerophilic potential, and some strains can produce toxins. Metataxonomics has proved to be a complete, fast, and efficient method to identify different fungi. Furthermore, high-performance genetic sequencing is an important ally in research, helping to develop novel technological advances related to food safety.
1. Introduction
Fungi are natural biodegrading agents of paramount importance to humanity. They have contributed to the food industry for many years, helping in the production of many foods such as cheeses and breads, and in the pharmaceutical industry, especially in the production of antibiotics. However, fungi can also cause severe economic losses due to contamination and deterioration of various foodstuffs [1,2]. Food contamination by fungi occurs in several ways; however, the most worrying factor is that some genera of fungi can produce secondary metabolites, which may present human toxicity. These metabolites are called mycotoxins and, when present in food, cause adverse effects to consumers, called mycotoxicosis [3]. In this context, according to the Food and Agriculture Organization (FAO), it is estimated that around 25% of the world’s food is contaminated with mycotoxins, which may lead to a public health problem [4].
Fungal development depends on several factors such as substrate composition, temperature, moisture content, water activity, air humidity, redox potential, and pH [5]. In addition, inappropriate harvesting and post-harvest practices, insect attacks, drought periods, poor fertilization, and competition with other crops can also favor the growth of mycotoxin-producing fungi [6]. The main toxigenic fungi genera Aspergillus, Fusarium, and Penicillium, which can contaminate a large variety of cereals through the soil or even during planting, harvesting, drying, transport, and storage [7]. These fungi develop exceptionally well on nutrient-rich substrates such as peanuts, corn, barley, rice, sorghum, wheat, and flaxseed. Their development and toxin production can occur during all stages from field to storage [8].
Flaxseed is considered a functional food due to its high concentration of α-linolenic acid (ALA), fibers, and lignans. Flaxseed is also frequently used as food, animal feed, or industrial material and can be sold directly or after processing [9,10]. There are two variations of flaxseed: brown and golden. The first is cultivated in regions with a hot climate, such as Brazil, while the second thrives in colder climates, such as the north of the United States and Canada. However, there are few studies that identify the species of fungi present in flaxseed samples [11].
Many fungal species are difficult to be cultivated in laboratory cultures as they require specific growth conditions. The use of sophisticated techniques to better understand microorganisms is becoming increasingly common. In this sense, samples amplified through a metataxonomics approach, with the direct extraction of genomic deoxyribonucleic acid (DNA), reveal new species never found by traditional cultivation techniques [12]. To identify fungi through their genome, specific primers are applied in certain regions, which ensures an extremely reliable identification. The ITS (internal transcribed spacer) regions of the ribosomal ribonucleic acid (RNA) are continuous regions of DNA that aid in the phylogenetic organization and distinction of fungal species and are the most used technique for fungal identification via metataxonomics [13]. With the advances in microbial community research, the need for higher resolution in the tools used becomes apparent. Polymerase chain reaction (PCR) cloning followed by traditional culture techniques and sequencing via the Sanger method has been replaced by next-generation sequencing technology (NGS). Some authors used the 454-pyrosequencing platform where amplicons were sequenced by the fungi’s ITS1 intergenic region. This was the first study where NGS was used to discover fungi present in cereal grains. Researchers surmise that a much more diverse microbial community can be identified through NGS when compared to other cultivation methods [14]. In this sense, the objective of this work was to identify fungi present in bulk flaxseed using the ITS intergenic region in a metataxonomic approach.
2. Material and Methods
A bulk sample of golden flaxseed (500 g) was acquired from a local seller in Florianópolis, SC, Brazil (27°35′49″ S 48°32′56″ W). The sample was kept in its original packaging, at room temperature, until the moment of analysis. Fungi identification was performed using high-performance sequencing of the ITS1 region. Library preparation followed a proprietary protocol (Neoprospecta Microbiome Technologies, Brazil). Amplification was performed using primers for the ITS1 region (GAACCWGCGGARGGATCA) [15], as well as ITS2 (GCTGCGTTCTTCATCGATGC). The libraries were sequenced using the MiSeq Sequencing System (Illumina Inc., San Diego, CA, USA) and the V2 kit, with 300 cycles and single-end sequencing. The sequences were analyzed using the pipeline Sentinel.
Using the Sentinel pipeline, fastq files were evaluated for Phred (QP) quality using FastQC v.0.11.8. Then, the fastq files were submitted to primers and low-quality sequences screening (Phred < 20). The proprietary software used for this purpose was built in Python v.3.6, which was inspired by the features of the BioPython project [16].
Clusters with an abundance lower than 2 were removed from the analysis, for such structures are usually related to chimeral sequences [17]. Taxonomic identifications were performed with blastn v.2.6.0+ [18], using a proprietary database as a reference. As for the definition of a species, among the 20 hits returned for each cluster, a Python instruction evaluated if one of three requirements were met by the hits: (1) highest bit-score; (2) lower e-value; and (3) taxonomies with greater representation.
The hits that met at least one of the previous items were chosen as the representative species. These analyses were carried out on Amazon’s computational platform, where Neoprospecta’s bioinformatics structure is hosted. DMD (digital molecular diagnostics) fungi analyses were performed against references for the ITS intergenic region in the proprietary database. The sequence bank for the ITS intergenic region has (mostly) complete sequences, which contain sequences retrieved from unambiguous genomes and filtered for chimeral sequences.
3. Results and Discussion
Table 1 presents the taxonomic data of the fungi found in golden flaxseed via high-performance sequencing of the ITS intergenic region. All species found belonged to the Fungi kingdom, with seven species belonging to the Ascomycota and one to the Basidiomycota phyla. Mamede [19] reports that the Ascomycota phylum encompasses a vast number of yeasts, which are indispensable in the food industry. The Basidiomycota phylum is composed of more evolved fungi, including mushrooms (about 20% of the total), which can produce sexual spores classified as basidiospores. Recent research shows that there are between 17,500 and 20,000 species of lichen-forming fungi, 40% of which belong to the Ascomycota phylum [20]. Lichens are known for their secondary compounds, widely used in the production of antiviral and antibacterial products [20]. The secondary metabolites produced by fungi provide them significant survival advantages, displaying antiproliferative, catabolic, and antibiotic properties. Furthermore, numerous Ascomycetes produce secondary metabolites, which include mycotoxins, phytotoxins, and other compounds that can enhance pathogenesis and virulence [21].

Table 1.
Taxonomic data of fungi found in golden flaxseed via high-performance sequencing of the ITS intergenic region.
Illumina platform sequencing generated a total of 355 sequences (reads). According to Souza [22], reads are thousands of small intervals that exhibit homology during the transcription of DNA into RNA. Short reads are used to remove adapters and poor-quality sequences. Long reads, on the other hand, are faster, deal with large volumes of sequencing data, and offer long-range spatial information [23]. Using this approach, metataxonomics allows access to a vast and yet unexplored range of genetic and metabolic diversity [24].
The fungi found during the metataxonomic analysis were grouped into four classes: Dothideomycetes, Eurotiomycetes, Saccharomycetes, and Wallemiomycetes. The Eurotiomycetes group was the most prominent in the analyzed sample (54.37%). The family Aspergillaceae and the genus Aspergillus were also included in this group. The Eurotiomycetes are considered to be one of the most numerous and are also known as enzymes and essential secondary metabolites producers [25]. Steenwyk et al. [26] point out that the evolution of members of the Aspergillaceae family requires a more developed phylogenetic structure as there are species from other genera, such as Penicillium present in this family.
The second most abundant class were the Wallemiomycetes (11.83%), containing the Wallemiales order and the genus Wallemia. In third place was the Dothideomycetes (6.48%), encompassing the Pleosporales order, Pleosporaceae family, and the Alternaria genus, as well as the Cladosporiales order, Cladosporiaceae family, and Cladosporium genus. Finally, the Saccharomycetes (1.41%) was represented by the Saccharomycetales order, Saccharomycodaceae family, and two distinct fungal genera, Hanseniaspora and Zygosaccharomyces. However, the sequencing of the ITS1 and ITS2 region was not able to identify 25.92% of the sequences found. Thus, it is possible that other regions, such as ITS3, ITS4, or ITS5, must be sequenced together, in a multilocus approach, in order to be able to identify the remaining sequences.
The total number of sequences identified for different fungal species found in golden flaxseed is presented in Table 2. The identification analysis showed that Aspergillus cibarius was the most prominent species of fungi (45.63%) among the eight species found in the sample (Figure 1). Bal et al. [18] have found 33.157 sequences of the ITS intergenic region in Nuruk wheat, which is dominated by the Aspergillus and Mucoralles genera. During a 30-day fermentation process using Nuruk wheat, the population of A. cibarius increased 10 times more after 10 days of fermentation when compared to the population found after 6 days, being one of the most viable species for this kind of fermentation. Aspergillus are known for their ability to grow on substrates with low water activity. They produce a wide range of secondary metabolites, but there is some controversy regarding the production of aflatoxins, ochratoxins, sterigmatocystin, and gliotoxins. Currently, there are few studies focusing on taxonomically isolated species of Aspergillus. However, in one such study, A. flavus, A. cibarius, Gibberella fujikuroi, Lasiodiplodia theobromae, and Rhizopus arrhizus were the dominant species found in sorghum-based Kisra [27]. A. flavus and A. parasiticus are known producers of secondary metabolites (mycotoxins). They produce aflatoxins, compounds with hepatotoxic and carcinogenic potential. However, A. cibarius strains found in sorghum and pumpkin samples did not produce any mycotoxins [28]. The quantification, in reads, of this species in the golden flaxseed sample was the highest among the eight found. Thus it is possible to infer that there are no toxic secondary metabolites to humans produced by this specific Aspergillus species. Furthermore, A. amstelodami and A. appendiculatus were also identified in the sample. A. appendiculatus was recorded for the first time in sheep manure, smoked sausage, and stored grains, which in turn may cause significant economic losses due to spoilage during storage [29]. Until now, these species have not been reported to produce mycotoxins; however, more studies are necessary in order to confirm or deny this [3,30,31,32].

Table 2.
Fungal species identified and quantified (reads) in golden flaxseed via high-performance sequencing of the ITS intergenic region.
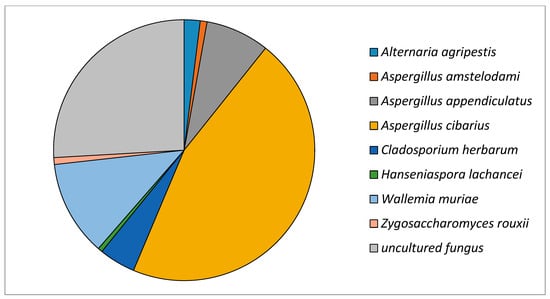
Figure 1.
Relative presence (%) of fungal species identified in golden flaxseed via high-performance sequencing of the ITS intergenic region.
The species with the second-highest relative presence in the sample was Wallemia muriae, representing 11.83% of the total. Resistance to low water activity conditions is rare in the Basidiomycota phylum, to which W. muriae belongs, but tests have indicated Wallemia as a fungal class with xerophilic characteristics. To characterize its morphology and xerotolerance, a new class was proposed, the Wallemiomycetes [33]. According to Zajc and Gunde-Cimerman [34], the Wallemia genus encompasses the most xerotolerant, xerophilic, and halophilic known species of fungi. Wallemia spp. can be found in a variety of osmotically challenged environments, such as foods with high salt or sugar content, dry foods (such as seeds), hypersaline waters, salt crystals, and agricultural aerosols. W. muriae has recently been recognized as belonging to the Wallemia genus and is commonly associated with food [34]. Some Wallemia strains, including W. muriae, produce walleminol and walleminon, toxins that can cause subcutaneous infections and allergic complications in humans [33]. However, this is the first time that the presence of this species in golden flaxseed has been observed. Zajc and Gunde-Cimerman [34] report that this species is difficult to be isolated via traditional culture means, as it has a low demand for Aw, and they only grow in media supplemented with additional solutes such as salts and sugars. Thus, molecular identification may greatly assist in the study of this genus of fungi and their impact on different foods. The presence of Alternaria agripestis (1.97%) and Cladosporium herbarum (4.51%) from the Pleosporaceae and Cladosporiaceae families, respectively, was also observed.
Pitt [32] states that Cladosporium herbarum causes the deterioration of fresh fruits and vegetables, stored apples, passion fruit, peanuts, walnuts, soy, and other cereals. Cladosporium species were found by them during a two-year analysis of the mycoflora present in barley, oats, and wheat, with C. herbarum being the most prominent (85% in barley, 95% in oats, and 77% in wheat). Meanwhile, Gruzdeviene et al. [35] analyzed the infection of flaxseed at harvest and during storage. They have found that most of the fungi found belonged to Alternaria and Fusarium genii, which are usually related to contamination in the field. Finally, two species of fungi belonging to the Saccharomycodaceae family were found in the flaxseed sample, Hanseniaspora opuntiae (0.56%) and Zygosaccharomyces rouxii (0.85%).
The presence of yeasts in flaxseed is generally not reported, as these species are more frequently related to fermentation processes. For example, Hanseniaspora is the most abundant genus in grapes, with H. opuntiae being present in large quantities at the beginning of the must fermentation [36]. Meanwhile, strains of Zygosaccharomyces rouxii, Saccharomyces cerevisiae, Saccharomyces kluyveri, Debaryomyces hansenii, and Pichia burtonii were isolated from Sel roti samples (a Nepali rice bread). Yeasts are also usually responsible for the production of gas that causes the dough to expand [37]. Z. rouxii is usually found in jams, fruit concentrates, plums, and dried dates. This species proliferates best in low Aw and high sugar content substrates [32]. Yonzan [37] also points out that the presence of this yeast in Sel roti bread would likely be caused due to the addition of sugars and honey to the dough.
It should be noted that identifying and controlling fungal contamination in flaxseed and consequently preventing the occurrence of toxic metabolites such as mycotoxins is paramount to the food industry. In this context, Hermann and Trigo-Stockli [38] suggest that the factors that influence the production of these toxins are mostly related to storage conditions, being humidity and temperature the major factors, as they affect both fungal growth and toxins production. Moisture requirements vary wildly between fungal species regarding the minimum moisture necessary as well as the optimal range in which they will predominate [39]. Temperature is another factor that affects grain quality during storage, with deterioration being exacerbated or reduced due to the interaction of many biotic and abiotic factors [40]. Thus, the knowledge and understanding of techniques to control these variables during storage are extremely important. These conditions directly impact the safety and quality of foods, which may be easily compromised by fungal development and mycotoxin production.
Several authors who have been studying the influence of temperature and water activity (Aw) on fungal growth and mycotoxin production have concluded that these two factors are mutually dependent [41]. Furthermore, through these two factors, it is possible to guarantee a longer storage time for grains with a lower incidence of fungi and the presence of mycotoxins [31,41]. Depending on the fungal species in question, either the temperature or Aw will be a factor of greater influence, and several studies have been carried out seeking to ensure the microbiological quality of products such as grains, cereals, and oilseeds [31,41,42]. In general, it is known that the higher the temperature and moisture content of grains, the shorter is their shelf life regarding fungal proliferation [31,43]. However, when moderate temperatures and reduced moisture conditions are used, the product’s shelf life increases considerably [3].
According to Midio and Martins [3], grains, cereals, and oilseeds that are stored under conditions close to 25 °C and 26% relative humidity are suitable for consumption for up to one week. However, if the humidity were to be reduced to 18% and the temperature lowered to 5 °C, the product’s stability during storage would increase significantly, reaching up to 50 weeks, showing the high correlation between these two factors [3,31].
Mycotoxins have been detected in several cereals, oilseeds, and processed products of both plant and animal origin. Many of these foods can be monitored for fungal contamination, but in thermo-processed foods, monitoring must be carried out while the product is still raw. High-risk raw materials such as peanuts and corn should always be evaluated for fungal contamination and the presence of toxins. Studies demonstrate a moderate risk of mycotoxin contamination in corn-based breakfast cereals by aflatoxins and in wheat-based breakfast cereals by trichothecenes [5].
Therefore, the control of fungal growth is extremely important in order to obtain products suitable for human consumption. According to studies conducted in recent years, it is evident that this control must be carried out during all stages, from planting, harvesting, shipping, and storage. The latter is one of the main stages where fungal development can be controlled. In this sense, the monitoring of some factors, such as temperature and Aw, is of fundamental importance to ensure that flaxseed remains a safe food that poses no risk when consumed by humans.
4. Conclusions
Among the fungal species found in golden flaxseed sample, three xerophilic species of Aspergillus stand out (A. cibarius, A. amstelodami, and A. appendiculatus), with A. cibarius being the most abundant. Wallemia spp. was the second most abundant in the sample, with W. muriae. Some strains of this genus may be potential mycotoxin producers. The identification of these fungi directly reflects on food safety, for many are producers of mycotoxins or otherwise harmful to human/animal health. The prevention of fungal contamination in flaxseed occurs mainly by controlling some factors such as temperature and Aw during storage. Furthermore, monitoring the product’s quality during all stages, from harvesting to storage, is essential. Metataxonomics proved to be a complete, fast, and efficient method to identify fungi in flaxseed samples, especially when compared to traditional fungal cultivation methods, being an important ally in evaluating the safety of foods that are consumed by the population at large.
Author Contributions
Conceptualization, N.d.C.R. and S.V.; Data curation, M.D.P. and S.V.; Formal analysis, N.d.C.R. and G.d.S.H.; Investigation, G.d.S.H.; Methodology, B.M.M.; Project administration, S.V.; Supervision, S.V.; Validation, F.M.D.N. and S.V.; Writing—original draft, N.d.C.R. and B.M.M.; Writing—review and editing, G.d.S.H., M.D.P., B.M.M., F.M.D.N. and S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sidrim, J.J.C.; Rocha, M.F.G. Micologia Médica à luz de Autores Contemporâneos, 1st ed.; Guanabara Koogan: Rio De Janeiro, Brazil, 2004. [Google Scholar]
- Putzke, J.; Putzek, M.T.L. Os Reinos dos Fungos, 2nd ed.; Edunisc: Santa Cruz do Sul, Brazil, 2002. [Google Scholar]
- Midio, A.F.; Martins, D.I. Toxicologia de Alimentos, 1st ed.; Livraria Varela: São Paulo, Brazil, 2000. [Google Scholar]
- FAO (Food and Agriculture Organization). Mycotoxins. Food Safety and Quality. Available online: http://www.fao.org/food/food-safety-quality/a-z-index/mycotoxins/en/ (accessed on 10 April 2021).
- Iamanaka, B.T.; Oliveira, I.S.; Taniwaki, M.H. Micotoxinas em alimentos. Anais Acad. Pernambucana Ciência Agronômica 2010, 7, 138–161. [Google Scholar]
- Manu, N.; Opit, G.P.; Osekre, E.A.; Arthur, F.H.; Mbata, G.; Armstrong, P.; Danso, J.K.; McNeill, S.G.; Campbell, J.F. Moisture content, insect pest infestation and mycotoxin levels of maize in markets in the northern region of Ghana. J. Stored Prod. Res. 2019, 80, 10–20. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef] [Green Version]
- Giordano, B.N.E. Efeito do Ozônio Sobre a Micoflora e Aflatoxinas Durante a Armazenagem de Castanha-do-Brasil com Casca (Bertholettia Excelsa H.B.K.). Master’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2009. [Google Scholar]
- Bechlin, T.R.; Granella, S.J.; Christ, D.; Coelho, S.R.M.; Viecelli, C.A. Evaluation of grain and oil quality of packaged and ozonized flaxseed. J. Stored Prod. Res. 2019, 83, 311–316. [Google Scholar] [CrossRef]
- Singh, K.K.; Mridula, D.; Barnwal, P.; Rehal, J. Flaxseed: A Potential Souce of Food, Feed and Fiber. Food Sci. Nut. 2011, 51, 210–222. [Google Scholar]
- Novello, D.; Pollonio, M.A.R. Caracterização físico-química e microbiológica da linhaça dourada e marrom (Linum Usitatissimum L.). Rev. Inst. Adolfo Lutz. 2012, 71, 2. [Google Scholar]
- Lorenz, P.; Schleper, C. Metagenome—A challenging source of enzyme discovery. J. Mol. Catal. B Enzym. 2002, 19–20, 13–19. [Google Scholar] [CrossRef]
- Andrade, H.F. Caracterização Molecular de Fungos da Micoteca/UFPE e Screening da Produção de Taxol. Bachelor’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2015. [Google Scholar]
- Nicolaisen, M.; Justesen, A.; Knorr, K.; Wang, J.; Pinnschmidt, H. Fungal communities in wheat grain show significant co-existence patterns among species. Fungal Ecol. 2014, 11, 145–153. [Google Scholar] [CrossRef]
- Schmidt, P.-A.; Bálint, M.; Greshake, B.; Bandow, C.; Römbke, J.; Schmitt, I. Illumina metabarcoding of a soil fungal community. Soil Biol. Biochem. 2013, 65, 128–132. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Antao, T.; Chang, J.T.; Chapman, B.; Cox, C.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-B.; Lee, M.; Kim, D.-H.; Meijer, M.; Majoor, E.; Vankuyk, P.A.; Samson, R.A. Aspergillus cibarius sp. nov., from traditional meju in Korea. J. Microbiol. 2012, 50, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Bal, J.; Yun, S.-H.; Yeo, S.-H.; Kim, J.-M.; Kim, D.-H. Metagenomic analysis of fungal diversity in Korean traditional wheat-based fermentation starter nuruk. Food Microbiol. 2016, 60, 73–83. [Google Scholar] [CrossRef]
- Mamede, A.C.P.B. Avaliação da Atividade Antibacteriana de Fungos do Filo Ascomycota e Basidiomycota sobre Staphylococcus Aureus e Escherichia Coli. Completion of Course Work; Universidade Federal de Santa Catarina: Florianópolis, Brazil, 2012. [Google Scholar]
- Wang, Y.-Y.; Liu, B.; Zhang, X.-Y.; Zhou, Q.-M.; Zhang, T.; Li, H.; Yu, Y.-F.; Zhang, X.-L.; Hao, X.-Y.; Wang, M.; et al. Genome characteristics reveal the impact of lichenization on lichen-forming fungus Endocarpon pusillum Hedwig (Verrucariales, Ascomycota). BMC Genom. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagee, D.; Hardan, A.A.; Botero, J.; Arnone, J.T. Genomic clustering whitin functionally related gene families in Ascomycota fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F. Análise de Expressão Diferencial em Transcriptomas. Curso de Introdução à Bioinformática Aplicada a Genômica. Available online: http://www.uel.br/laboratorios/lbi/pages/arquivos/curso/01_curso_transcriptoma.pdf (accessed on 7 March 2021).
- Dilthey, A.T.; Jain, C.; Koren, S.; Phillippy, A.M. Strain-level metagenomic assignment and compositional estimation for long reads with MetaMaps. Nat. Commun. 2019, 10, 3066. [Google Scholar] [CrossRef] [Green Version]
- Demain, A.L.; Martens, E. Production of valuable compounds by molds and yeasts. J. Antibiot. 2017, 70, 347–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, N.; Rawat, R.; Sharma, R.; Oberoi, H.S.; Srivastava, M.; Singh, J. Effect of Nickel–Cobaltite Nanoparticles on Production and Thermostability of Cellulases from Newly Isolated Thermotolerant Aspergillus fumigatus NS (Class: Eurotiomycetes). Appl. Biochem. Biotechnol. 2014, 174, 1092–1103. [Google Scholar] [CrossRef]
- Steenwyk, J.L.; Shen, X.X.; Lind, A.L.; Goldman, G.H.; Rokas, A. A robust phylogenomic timetree by biotechnologically and medically important fungi from Aspergillaceae (Eurotiomycetes, Ascomycota). BioRxiv 2018, 370429. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, M.M.; Eltigani, S.A.; Taniguchi, T. Pyrosequencing scrutiny of bacterial and fungal communities in two Sudanese sorghum-based fermented foods. Ann. Microbiol. 2020, 70, 53. [Google Scholar] [CrossRef]
- Forwood, D.L.; Caro, E.; Holman, D.B.; Meale, S.J.; Chaves, A.V. Ensiling sorghum with unsalable pumpkin improves feed digestibility with minimal influence on the rumen microbial population using the rumen simulation technique. Appl. Microbiol. Biotechnol. 2021, 105, 3289–3300. [Google Scholar] [CrossRef]
- Hubka, V.; Kolarik, M.; Kubatova, A.; Peterson, S.W. Taxonomic revision of Eurotium and transfer of species to Aspergillus. Mycologia 2013, 105, 912–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klich, M.A.; Thomas, S.H.; Mellon, J.E. Field Studies on the Mode of Entry of Aspergillus flavus into Cotton Seeds. Mycologia 1984, 76, 665. [Google Scholar] [CrossRef]
- Pitt, J.I. Fungal ecology and the occurrence of mycotoxins. In Mycotoxins and Phycotoxins: Advances in Determination, Toxicology and Exposure Management; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 33–41. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Zalar, P.; De Hoog, G.S.; Schroers, H.-J.; Frank, J.M.; Gunde-Cimerman, N. Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie Leeuwenhoek 2005, 87, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Gunde-Cimerman, N. The Genus Wallemia—From Contamination of Food to Health Threat. Microorganisms 2018, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Gruzdeviene, E.; Mankeviciene, A.; Lugauskas, A.; Repeckiene, J. The effect of environmental conditions on the variation of fungi and mycotoxin contents in oil flax seed. Ekologija 2006, 3, 64–70. [Google Scholar]
- Bovo, B.; Andrighetto, C.; Carlot, M.; Corich, V.; Lombardi, A.; Giacomini, A. Yeast population dynamics during pilot-scale storage of grape marcs for the production of Grappa, a traditional Italian alcoholic beverage. Int. J. Food Microbiol. 2009, 129, 221–228. [Google Scholar] [CrossRef]
- Yonzan, H.; Tamang, J.P. Microbiology and Nutritional Value ofSelroti, an Ethnic Fermented Cereal Food of the Himalayas. Food Biotechnol. 2010, 24, 227–247. [Google Scholar] [CrossRef]
- Herman, T.; Trigo-Stockli, D. Mycotoxins in Infeed Grain and Ingredients; Kansas State University: Manhattan, KS, USA, 2008. [Google Scholar]
- Dias, I.E. Crescimento Micelar e Produção de Toxinas por Fungos de Armazenamento Associados a Grãos de Milho sob Diferentes Níveis de Restrição Hídrica. Ph.D. Thesis, Universidade Federal de Lavras, Minas Gerais, Brazil, 2012. [Google Scholar]
- D’Aarce, M.A.B.R. Pós-colheita e Armazenamento de grãos. Material Didático. Departamento de Agroindústria, Alimentos e nutrição, ESALQ/USP. Available online: http://sinueloagropecuaria.com.br/wp-content/uploads/2016/09/armazenamento-de-graos-1.pdf (accessed on 18 April 2020).
- Tsai, G.-J.; Yu, S.-C. Detecting Aspergillus parasiticus in cereals by an enzyme-linked immunosorbent assay. Int. J. Food Microbiol. 1999, 50, 181–189. [Google Scholar] [CrossRef]
- Garcia, D.M. Análise de Atividade de Água em Alimentos Armazenados no Interior de Granjas de Integração Avícola. Master’s Thesis, Universidade Federal do Rio Grande, Rio Grande do Sul, Brazil, 2004. [Google Scholar]
- Sibamoto, T.; Bjeldanes, L.F. Introduction to Food Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).