Multi-Mycotoxin Contamination, Mold Incidence and Risk Assessment of Aflatoxin in Maize Kernels Originating from Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maize Samples
2.2. Quantitation of Mycotoxins
2.3. Assessing Mold Incidence in Maize Kernels and Molecular Identification
2.4. Study of Mycotoxin Production Potential of Selected Fungal Isolates
2.5. Risk and Exposure Assessment of Aflatoxin
2.6. Statistical Analysis
3. Results and Discussion
3.1. Mycotoxin Contamination
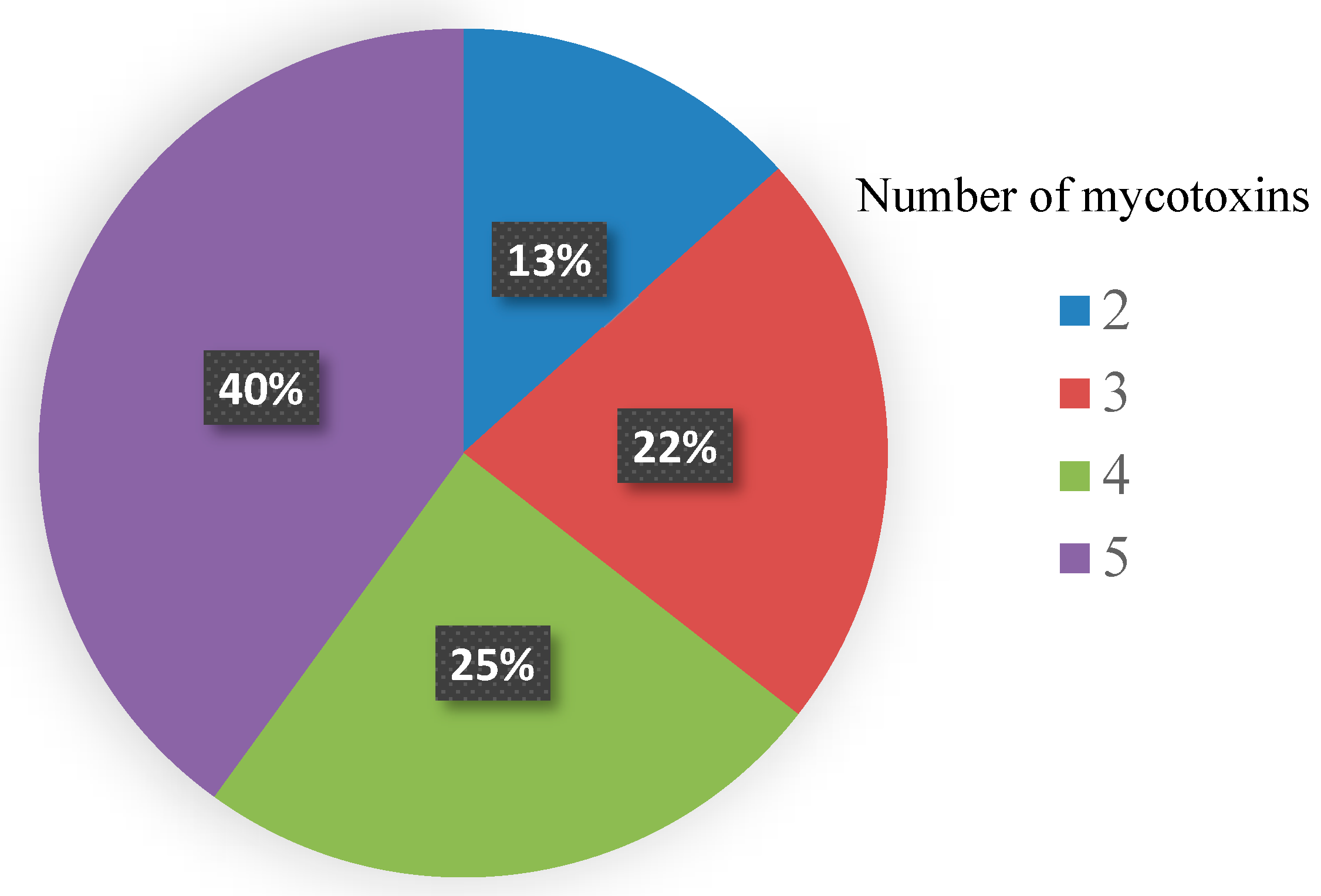
3.2. Co-Occurrence of Mycotoxins
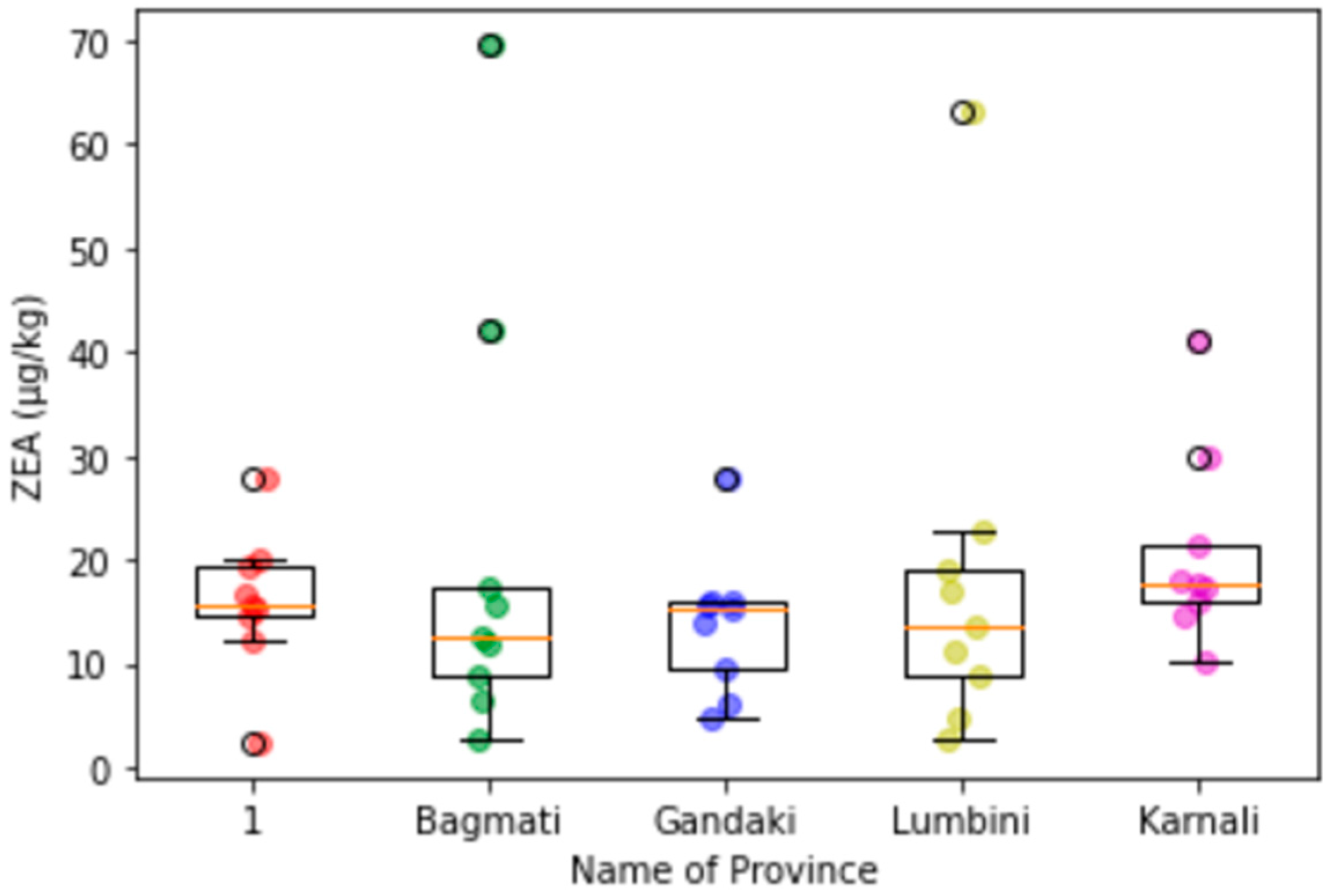
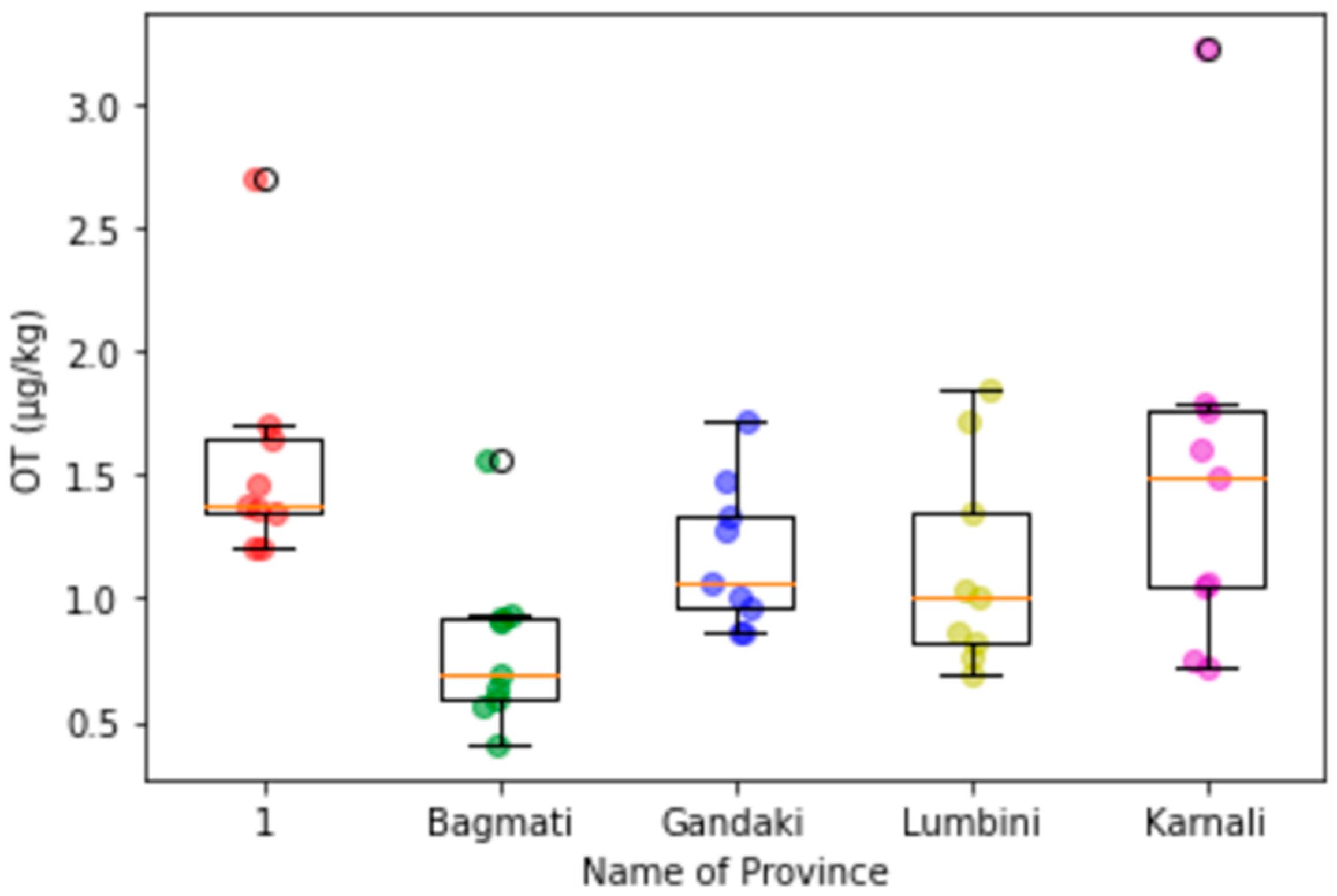
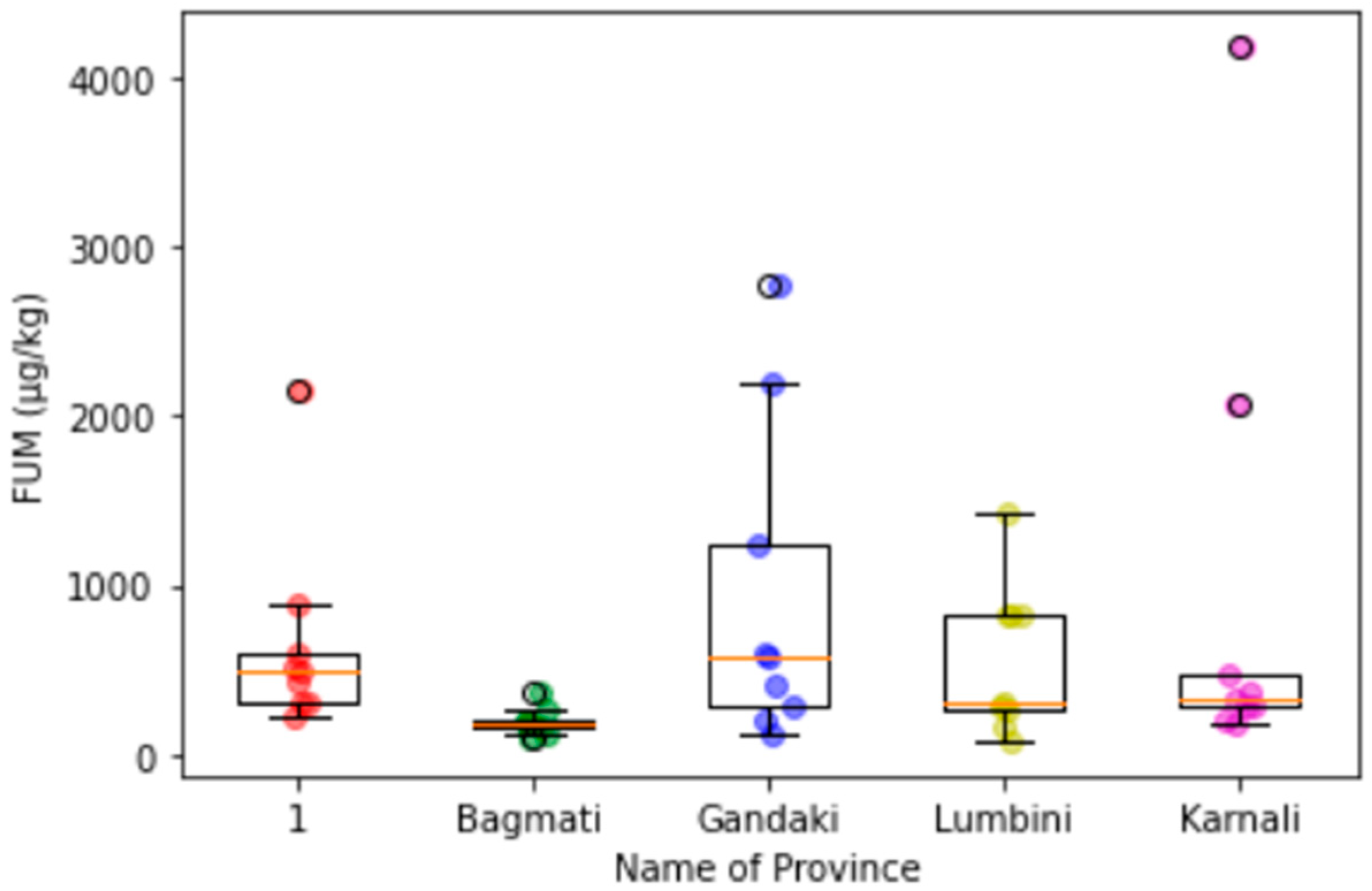
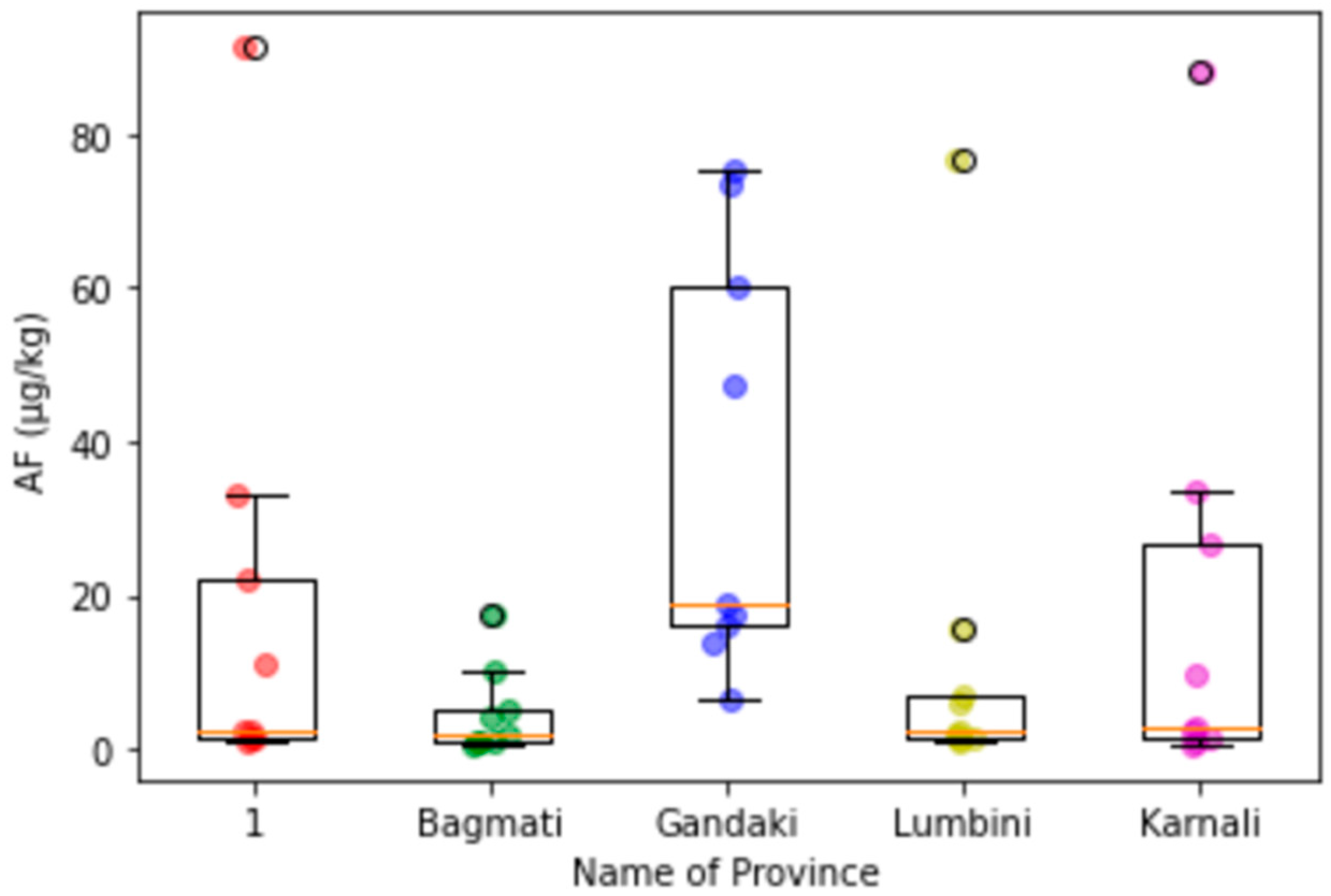
3.3. Provincial Distribution of Mycotoxins in Maize
3.4. Kernel Mold Infection
3.5. Mycotoxin-Producing Potential of Mold Isolated from Maize
3.6. Identification of Fungi Isolated from Maize
3.7. Dietary Exposure and Risk Assessment of Aflatoxin in Maize
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Heat Maps of Mycotoxin Occurrence in Nepalese Maize
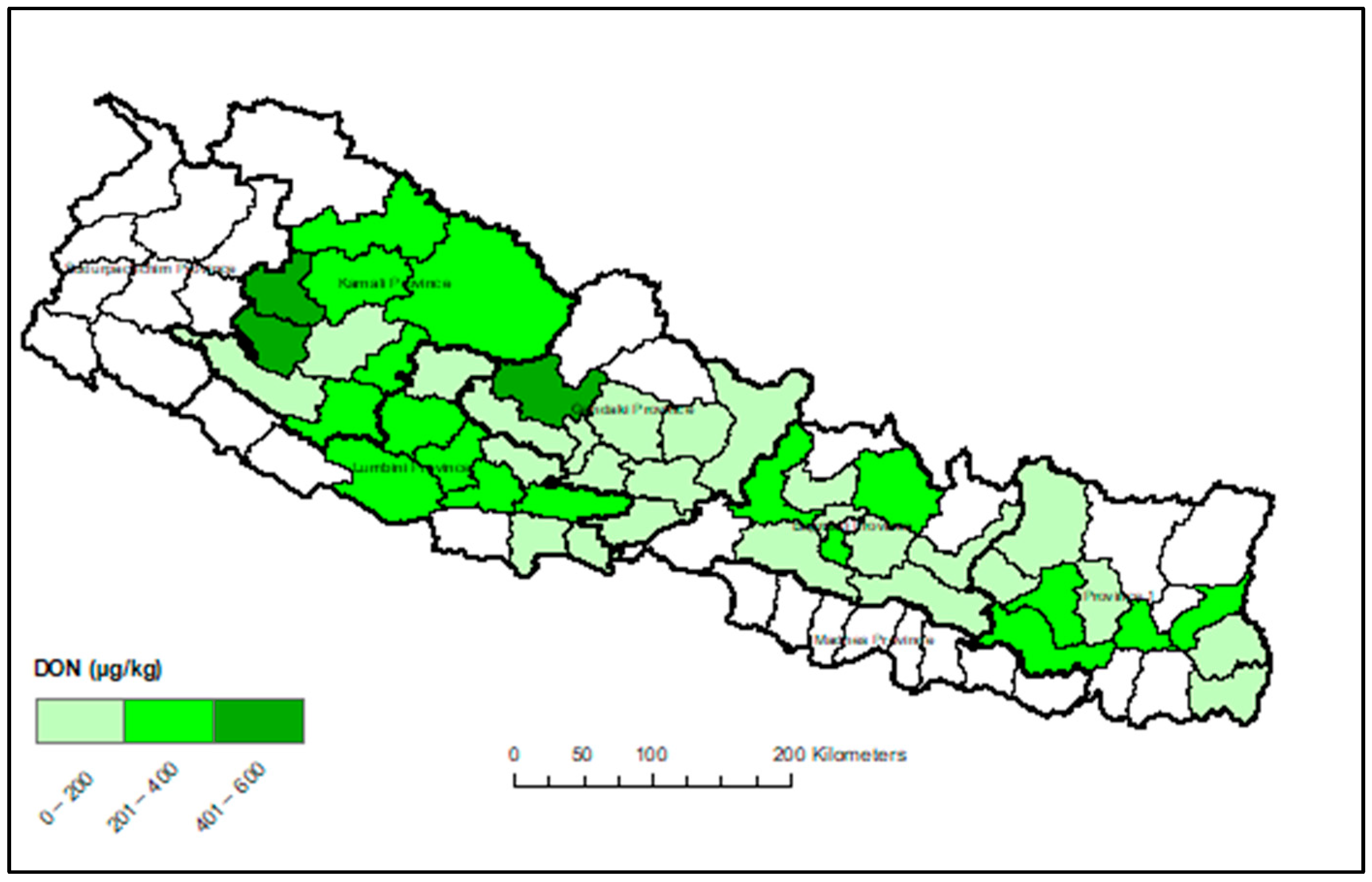
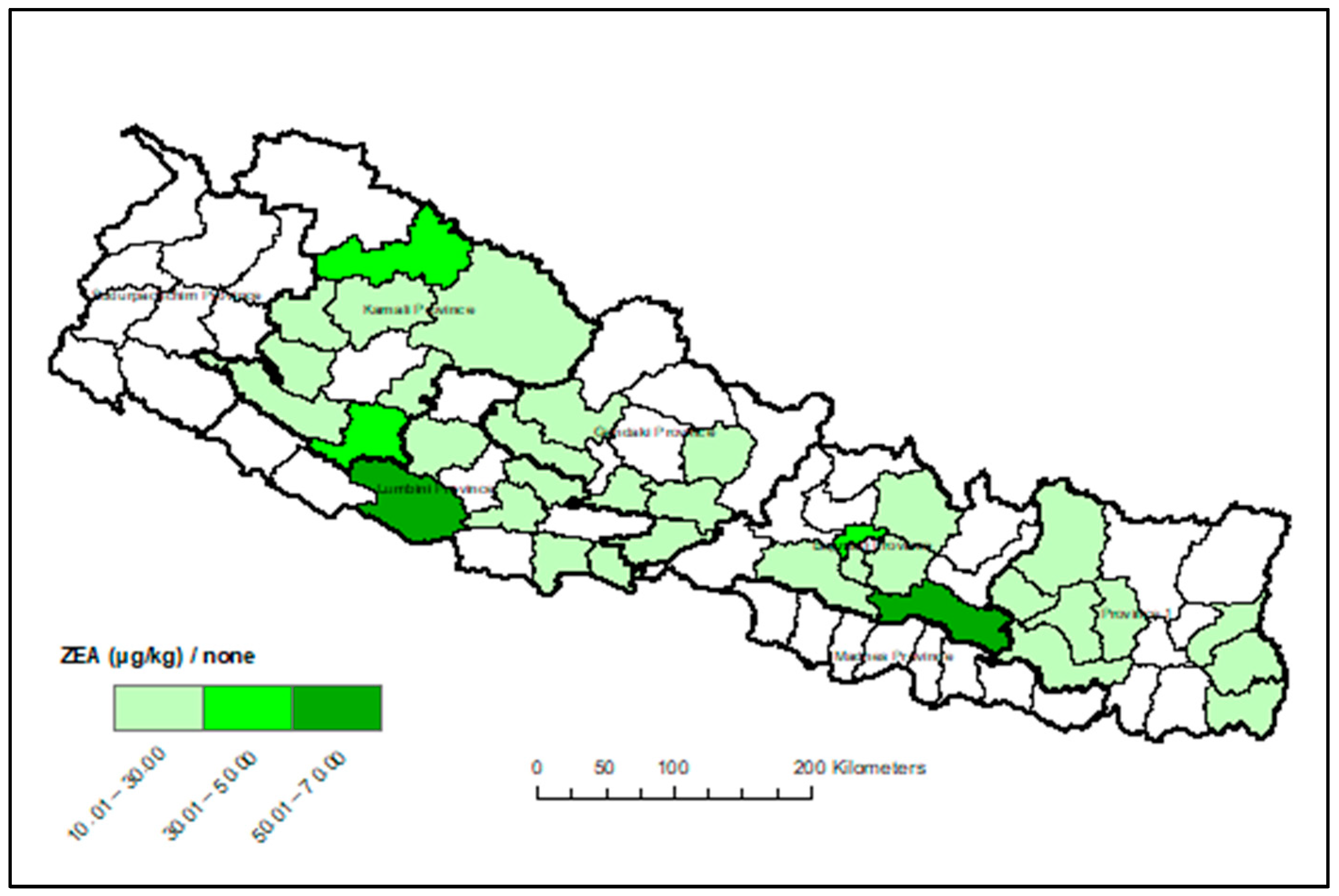
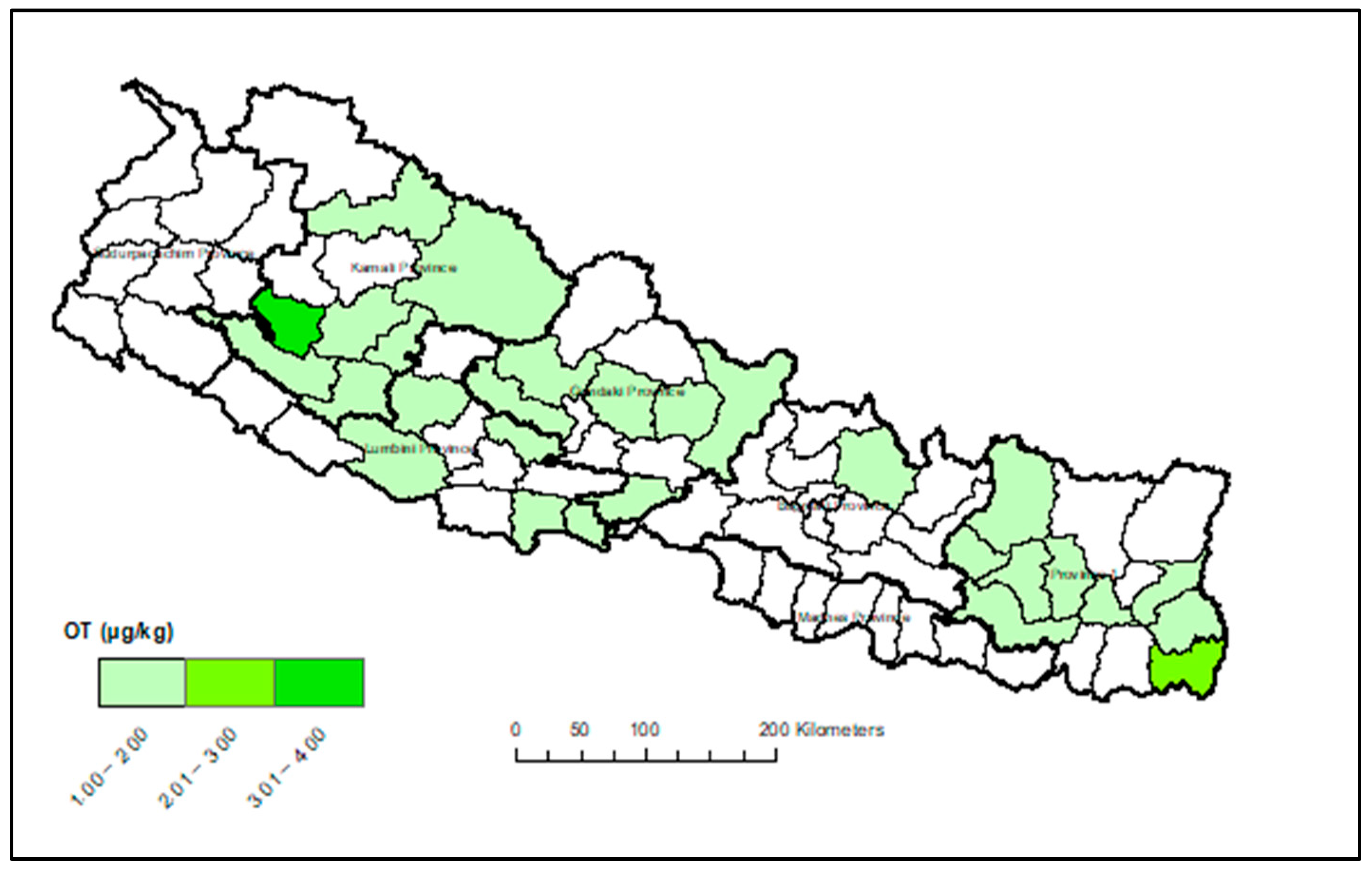

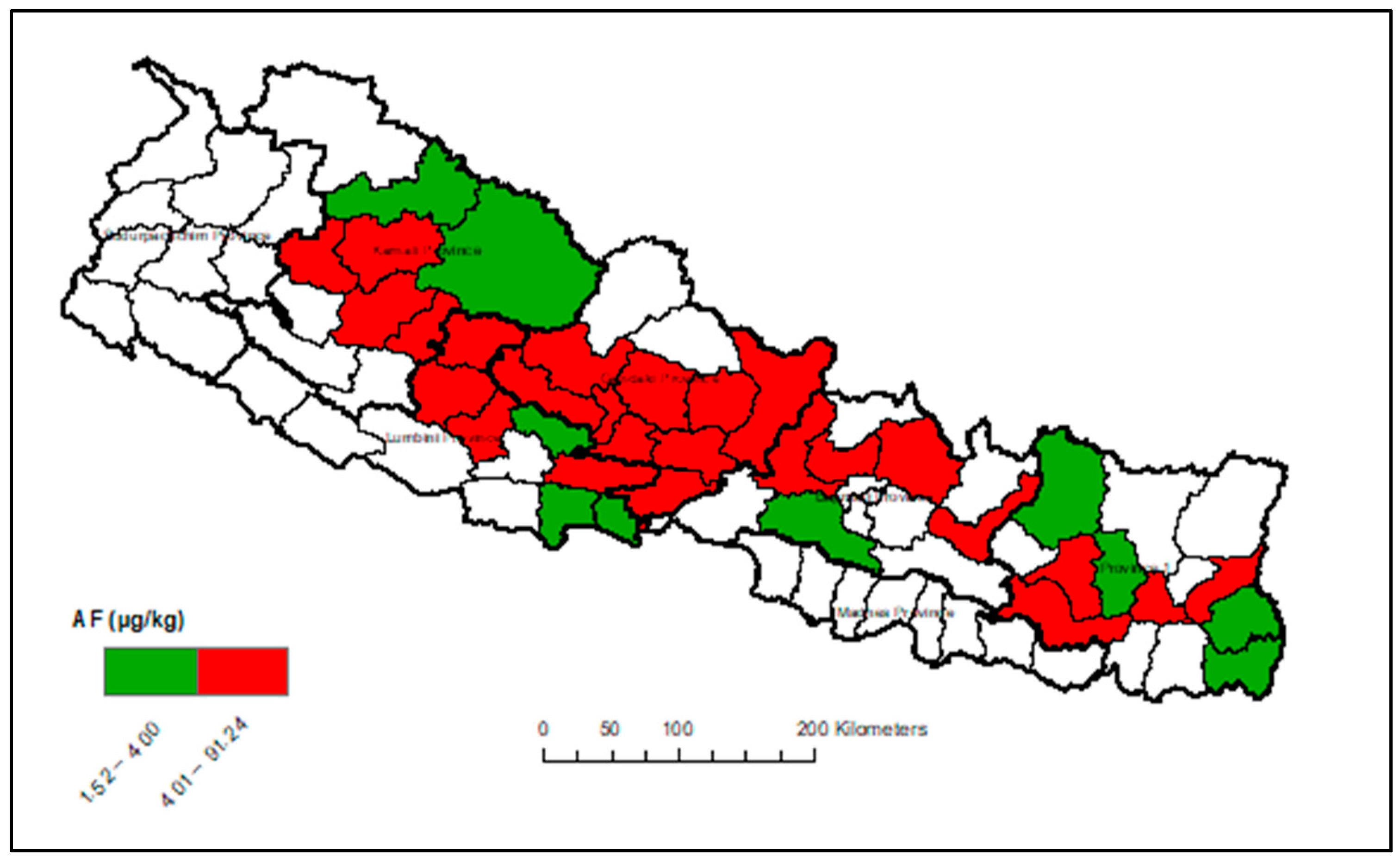
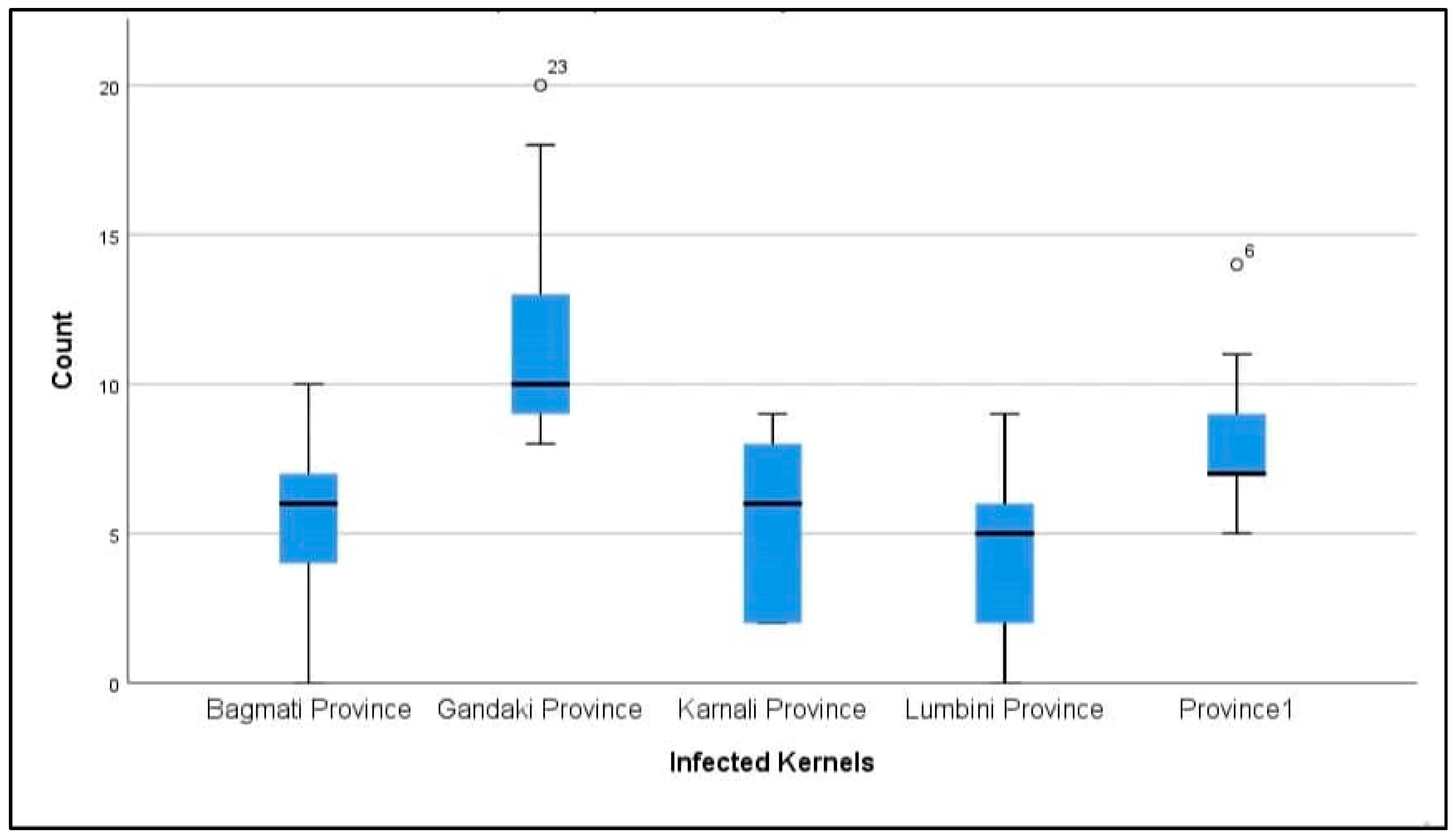
Appendix B. Box-Plot Diagram of Infected Maize Kernels
Appendix C. Box-Plot Diagrams with Scattering Dots for the Contamination Level of Mycotoxins in Maize in Different Provinces

References
- Wielogorska, E.; Mooney, M.; Eskola, M.; Ezekiel, C.N.; Stranska, M.; Krska, R.; Elliott, C. Occurrence and human-health impacts of mycotoxins in Somalia. J. Agric. Food Chem. 2019, 67, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cog. Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Rajapakse, N.; Kortenkamp, A. Something from “nothing”—Eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol. 2002, 36, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Gurung, D.B.; KC, D.; Ortiz Ferrara, G.; Gadal, N.; Pokhrel, S.; Bhandari, D.R.; Koirala, K.B.; Bhandari, B.R.; Tripathi, M. Maize value chains in Nepal. In Proceedings of the 11th Asian Maize Conference, Beijing, China, 7–11 November 2011. [Google Scholar]
- CBS. Nepal Living Standard Survey 2010/11, Statistical Report; Central Bureau of Statistics, Government of Nepal Kathmandu: Kathmandu, Nepal, 2011.
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Agriculture Organization of the United Nations. FAO. 2011. Available online: https://www.fao.org/faostat/en/#home (accessed on 11 February 2020).
- Pokhrel, P. Postharvest Handling and Prevalence of Aflatoxin Contamination in Nepalese Maize Produce. J. Food Sci. Technol. Nep. 2016, 9, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. 2010, 27, 651–657. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Busman, M. Mycotoxins in developing countries: A case study of maize in Nepal. Mycotoxin Res. 2006, 22, 92–95. [Google Scholar] [CrossRef]
- Qin, P.W.; Xu, J.; Jiang, Y.; Hu, L.; Van Der Lee, T.; Waalwijk, C.; Zhang, W.M.; Xu, X.D. Survey for toxigenic Fusarium species on maize kernels in China. World Mycotoxin J. 2020, 13, 213–224. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009; Volume 519, p. 388. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010. [Google Scholar]
- Zhou, J.; Bruns, M.A.; Tiedje, J.M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996, 62, 316–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food and Air Borne Fungi, 7th ed.; Fungal Biodiversity Centre CBS: Utrecht, The Netherlands, 2000; p. 389. [Google Scholar]
- Wee, J.; Day, D.M.; Linz, J.E. Effects of zinc chelators on aflatoxin production in Aspergillus parasiticus. Toxins 2016, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuaysrinule, C.; Maneeboon, T.; Roopkham, C.; Mahakarnchanakul, W. Occurrence of aflatoxin-and ochratoxin A-producing Aspergillus species in Thai dried chilli. J. Agric. Food Res. 2020, 2, 100054. [Google Scholar] [CrossRef]
- Rauová, D. Single laboratory-validated HPLC methods for determination of ochratoxin A, fumonisin B1 and B2, zearalenone and deoxynivalenol in cereals and cereal-based foods. J. Food. Nutr. Res. 2010, 49, 57–68. [Google Scholar]
- Etcheverry, M.; Torres, A.; Ramirez, M.L.; Chulze, S.; Magan, N. In vitro control of growth and fumonisin production by Fusarium verticillioides and F. proliferatum using antioxidants under different water availability and temperature regimes. J. Appl. Microbiol. 2002, 92, 624–632. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority; Food and Agriculture Organization; World Health Organization. Towards a harmonised Total Diet Study approach: A guidance document. EFSA J. 2011, 9, 2450. [Google Scholar]
- NHRC, Government of Nepal. Research Report on NCD Risk Factors Surveillance in Nepal; Research report; Nepal Health Research Council: Kathmandu, Nepal, 2003; pp. 1–50.
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 6040. [Google Scholar]
- EFSA Scientific Committee. Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA J. 2012, 10, 2578. [Google Scholar]
- Shrestha, S.M.; Shrestha, S. Chronic hepatitis B in Nepal: An Asian country with low prevalence of HBV infection. Trop. Gastroenterol. 2012, 33, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Manandhar, G.; Plattner, R.D.; Maragos, C.M.; Shrestha, K.; McCormick, S.P. Occurrence of Fusarium species and mycotoxins in Nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. J. Agric. Food Chem. 2000, 48, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Liu, Z.; Liu, S.; Yao, L.; Liu, Y.; Wu, Y.; Gong, Z. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.D.; Su, P.; Shan, H. Mycotoxin contamination of maize in China. Comp. Rev. Food Sci. Food Saf. 2017, 16, 835–849. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Ansari, K.M.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Occurrence of deoxynivalenol in cereals and exposure risk assessment in Indian population. Food Control 2013, 30, 549–555. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food. Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Milićević, D.; Jurić, V.; Stefanović, S.; Baltić, T.; Janković, S. Evaluation and validation of two chromatographic methods (HPLC-Fluorescence and LC–MS/MS) for the Determination and confirmation of ochratoxin A in pig tissues. Arch. Environ. Contam. Toxicol. 2010, 58, 1074–1081. [Google Scholar] [CrossRef]
- Jayarajavarma, B.; Kamalakannan, A.; Paranidharan, V. Occurrence of fumonisin B1 in maize kernels, poultry and livestock feeds in Tamil Nadu, India. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1792–1797. [Google Scholar] [CrossRef]
- Mudili, V.; Siddaih, C.N.; Nagesh, M.; Garapati, P.; Naveen Kumar, K.; Murali, H.S.; Yli Mattila, T.; Batra, H.V. Mould incidence and mycotoxin contamination in freshly harvested maize kernels originated from India. J. Sci. Food. Agric. 2014, 94, 2674–2683. [Google Scholar] [CrossRef]
- Gautam, D.N.; Bhatta, R.; Bhandary, M.R. Assessment of aflatoxin B1 level in chilli, maize and groundnut samples from Kathmandu Valley. J. Food Sci. Technol. Nep. 2008, 4, 57–60. [Google Scholar]
- Koirala, P.; Kumar, S.; Yadav, B.K.; Premarajan, K.C. Occurrence of aflatoxin in some of the food and feed in Nepal. Indian J. Med. Sci. 2005, 59, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robens, J.F.; Richard, J.L. Aflatoxins in animal and human health. Rev. Environ. Contam. Toxicol. 1992, 9, 69–94. [Google Scholar]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A. Co-occurrence of mycotoxins in maize food and maize-based feed from small-scale farms in Brazil: A pilot study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef]
- Kos, J.; Hajnal, E.J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Poschmaier, B.; Sulyok, M. Mycotoxins in maize harvested in Republic of Serbia in the period 2012–2015. Part 1: Regulated mycotoxins and its derivatives. Food Chem. 2020, 312, 126034. [Google Scholar] [CrossRef]
- Janardhana, G.R.; Raveesha, K.A.; Shetty, H.S. Mycotoxin contamination of maize grains grown in Karnataka (India). Food Chem. Toxicol. 1999, 37, 863–868. [Google Scholar] [CrossRef]
- Saleemi, M.K.; Khan, M.Z.; Khan, A.; Javed, I.; Ul Hasan, Z.; Hameed, M.R.; Hameed, S.; Mehmood, M.A. Occurrence of toxigenic fungi in maize and maize-gluten meal from Pakistan. Phytopathol. Mediterr. 2012, 219–224. [Google Scholar]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef]
- Wicklow, N.D.; McAlpin, C.E.; Platis, C.E. Characterization of the Aspergillus flavus population within an Illinois maize field. Mycol. Res. 1998, 102, 263–268. [Google Scholar] [CrossRef]
- Nelson, P.E.; Plattner, R.D.; Shackelford, D.D.; Desjardins, A. Production of fumonisins by Fusarium moniliforme strains from various substrates and geographic areas. Appl. Environ. Microbiol. 1991, 57, 2410–2412. [Google Scholar] [CrossRef] [Green Version]
- Susca, A.; Moretti, A.; Stea, G.; Villani, A.; Haidukowski, M.; Logrieco, A.; Munkvold, G. Comparison of species composition and fumonisin production in Aspergillus section Nigri populations in maize kernels from USA and Italy. Int. J. Food Microbiol. 2014, 188, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kankolongo, M.A.; Hell, K.; Nawa, I.N. Assessment for fungal, mycotoxin and insect spoilage in maize stored for human consumption in Zambia. J. Sci. Food. Agric. 2009, 89, 1366–1375. [Google Scholar] [CrossRef]
- Zulkifli, N.A.; Zakaria, L. Morphological and molecular diversity of Aspergillus from corn grain used as livestock feed. Hayati. J. Biosci. 2017, 24, 26–34. [Google Scholar] [CrossRef]
- Dobolyi, C.S.; Sebők, F.; Varga, J.; Kocsubé, S.; Szigeti, G.; Baranyi, N.; Szécsi, Á.; Tóth, B.; Varga, M.; Kriszt, B.; et al. Occurrence of aflatoxin producing Aspergillus flavus isolates in maize kernel in Hungary. Acta Aliment. 2013, 42, 451–459. [Google Scholar] [CrossRef]
- Thathana, M.G.; Murage, H.; Abia, A.L.K.; Pillay, M. Morphological characterization and determination of aflatoxin-production potentials of Aspergillus flavus isolated from maize and soil in Kenya. Agriculture 2017, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Frisvad, J.C.; Larsen, T.O.; Thrane, U.; Meijer, M.; Varga, J.; Samson, R.A.; Nielsen, K.F. Fumonisin and ochratoxin production in industrial Aspergillus niger strains. PLoS ONE 2011, 6, 23496. [Google Scholar] [CrossRef] [Green Version]
- Storari, M.; Bigler, L.; Gessler, C.; Broggini, G.A. Assessment of the ochratoxin A production ability of Aspergillus tubingensis. Food Addit. Contam. Part A 2012, 29, 1450–1454. [Google Scholar] [CrossRef]
- Medina, A.; Mateo, R.; López-Ocana, L.; Valle-Algarra, F.M.; Jiménez, M. Study of Spanish grape mycobiota and ochratoxin A production by isolates of Aspergillus tubingensis and other members of Aspergillus section Nigri. Appl. Environ. Microbiol. 2005, 71, 4696–4702. [Google Scholar] [CrossRef] [Green Version]
- Fotso, J.; Leslie, J.F.; Smith, J.S. Production of beauvericin, moniliformin, fusaproliferin, and fumonisins B1, B2, and B3 by fifteen ex-type strains of Fusarium species. Appl. Environ. Microbiol. 2002, 68, 5195–5197. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, A.E. Fusarium Mycotoxins: Chemistry, Genetics, and Biology; APS Press: St. Paul, MN, USA, 2006; Volume 531. [Google Scholar]
- Udovicki, B.; Djekic, I.; Gajdos Kljusuric, J.; Papageorgiou, M.; Skendi, A.; Djugum, J.; Rajkovic, A. Exposure assessment and risk characterization of aflatoxins intake through consumption of maize products in the adult populations of Serbia, Croatia and Greece. Food Addit. Contam. 2019, 36, 940–951. [Google Scholar] [CrossRef]
- Nugraha, A.; Khotimah, K.; Rietjens, I.M. Risk assessment of aflatoxin B1 exposure from maize and peanut consumption in Indonesia using the margin of exposure and liver cancer risk estimation approaches. Food. Chem. Toxicol. 2018, 113, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Liang, B.; Zhang, Y.; Zhong, X.; Luo, X.; Huang, J.; Wang, Y.; Cheng, W.; Chen, K. Probabilistic risk assessment of dietary exposure to aflatoxin B1 in Guangzhou, China. Sci. Rep. 2020, 10, 7973. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo, R.M.; Imungi, J.K.; Muiru, W.M.; Lamuka, P.O.; Njage, P.M.K. Household dietary exposure to aflatoxins from maize and maize products in Kenya. Food Addit. Contam Part A 2014, 31, 2055–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartus, A.; Schrenk, D. Current methods in risk assessment of genotoxic chemicals. Food. Chem. Toxicol. 2017, 106, 574–582. [Google Scholar] [CrossRef] [PubMed]
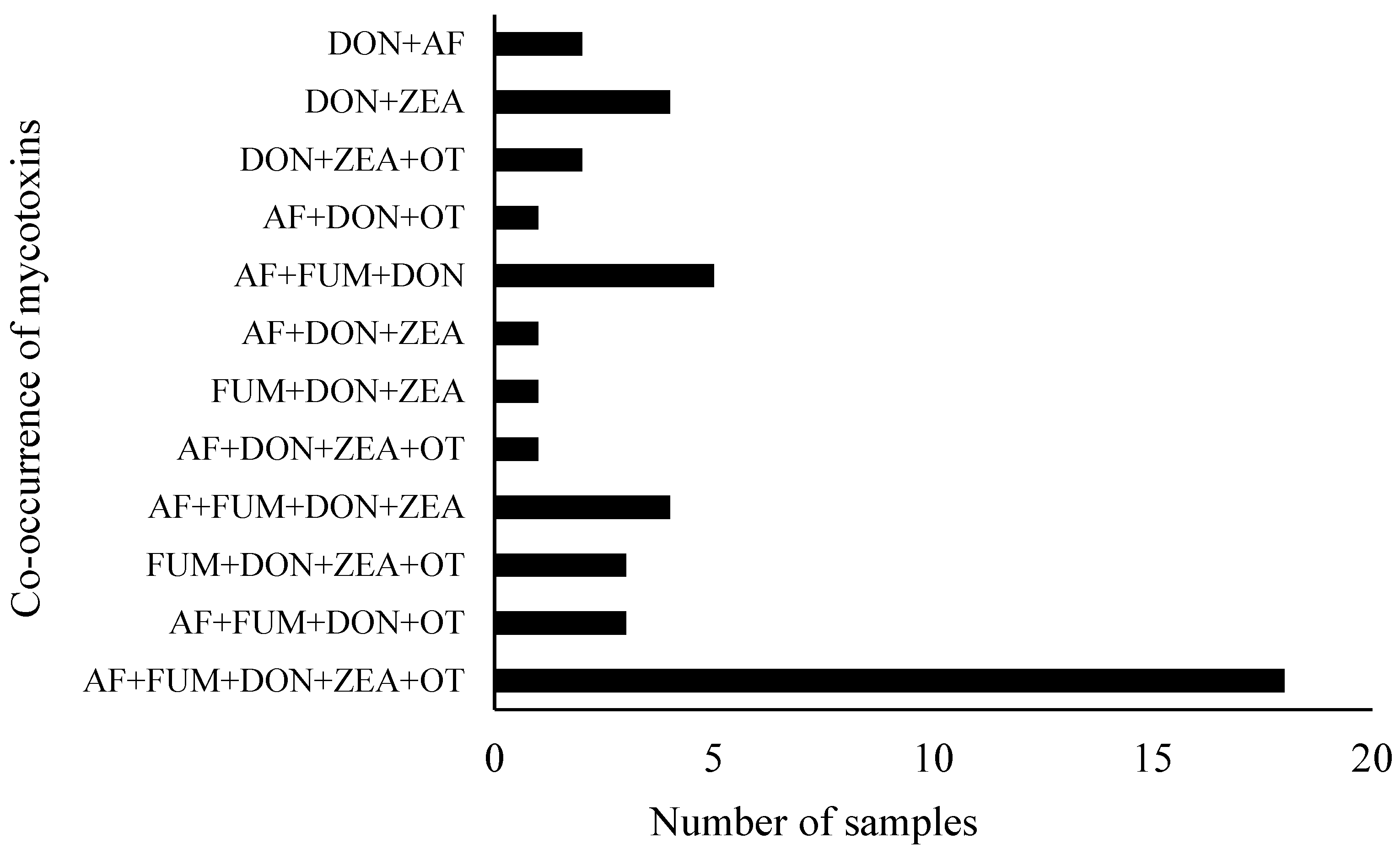
| Mycotoxin | Number of Positive Samples | % | Concentration (µg/kg) in Positive Samples (Mean ± SD) | Range | Median | MPL (µg/kg) (GoN/EU) |
|---|---|---|---|---|---|---|
| Aflatoxin | 35 | 78 | 23.04 ± 27.58 | 1.52–91.24 | 5.91 | 20/4 |
| Ochratoxin | 28 | 62 | 1.5125 ± 0.49 | 1–3.22 | 1.06 | NE/5 |
| Zearalenone | 34 | 76 | 21.72 ± 13.47 | 11.12–69.52 | 15.5 | NE/200 |
| Fumonisin | 34 | 76 | 816.76 ± 886.66 | 200–4180 | 310 | NE/2000 |
| Deoxynivalenol | 45 | 100 | 222.44 ± 104.8 | 110–520 | 190 | NE/1750 |
| Location | Number of Infected Kernels/Total Number of Investigated Kernels | % Infected Kernels ± SD |
|---|---|---|
| Province-1 | 74/180 | 41.11 ± 13.87 |
| Bagmati | 51/180 | 28.33 ± 16.01 |
| Gandaki | 108/180 | 60 ± 21.51 |
| Lumbini | 38/180 | 21.11 ± 17.28 |
| Karnali | 47/180 | 26.11 ± 14.09 |
| Isolate Code | Origin | Species Identified Using DNA Sequencing | Toxin Production/Quantity | NCBI Access Number |
|---|---|---|---|---|
| PJ 60 | Gandaki | Aspergillus flavus | AFB1/356.59 ng/g | MH864264 |
| PJ 84 | Gandaki | Aspergillus flavus | AFB1/3653.49 ng/g | MH864264 |
| PJ 58 | Gandaki | Aspergillus oryzae | ND | KJ175431 |
| PJ 76 | Province-1 | Aspergillus oryzae | ND | KJ175431 |
| PJ 119 | Bagmati | Aspergillus filifer | ND | NR137499 |
| PJ 66 | Province-1 | Aspergillus fumigatus | ND | MH865796 |
| PJ 04 | Lumbini | Aspergillus niger | OTA/631.17 ng/g | MH856565 |
| PJ 05 | Lumbini | Aspergillus niger | OTA/1402.12 ng/g | MH856565 |
| PJ 31 | Gandaki | Aspergillus tubingensis | OTA/871.38 ng/g | MH858714 |
| PJ 81 | Province-1 | Aspergillus niger | FB1/20,199 µg/kg | MH856565 |
| PJ 82 | Province-1 | Aspergillus niger | FB1/16,928 µg/kg | MH856565 |
| PJ 122 | Karnali | Aspergillus niger | FB1/5604 µg/kg | MH856565 |
| PJ 67 | Province-1 | Aspergillus niger | ND | MH856565 |
| PJ 77 | Province-1 | Aspergillus niger | ND | MH856565 |
| PJ 74 | Province-1 | Aspergillus niger | ND | MH856565 |
| PJ 39 | Province-1 | Fusarium pseudocircinatum | FUM/10,180 µg/kg | NR163683 |
| PJ 42 | Lumbini | Fusarium pseudocircinatum | FUM/10,270 µg/kg | NR163683 |
| PJ 41 | Province-1 | Fusarium pseudocircinatum | FUM/11,300 µg/kg | NR163683 |
| PJ 111 | Lumbini | Fusarium pseudocircinatum | FUM/11,120 µg/kg | NR163683 |
| PJ 52 | Gandaki | Fusarium pseudocircinatum | FUM/10,360 µg/kg | NR163683 |
| PJ 129 | Karnali | Fusarium pseudocircinatum | FUM/10,310 µg/kg | NR163683 |
| PJ 10 | Bagmati | Fusarium pseudocircinatum | FUM/5750 µg/kg | NR163683 |
| PJ 07 | Lumbini | Fusarium pseudocircinatum | FUM/15,670 µg/kg | NR163683 |
| PJ 19 | Lumbini | Fusarium pseudocircinatum | FUM/10,300 µg/kg | NR163683 |
| Parameter | Unit | Mean | 97.5th Percentile |
|---|---|---|---|
| AF concentration | µg/kg | 18.07 | 86.78 |
| EDI | ng/kg bw/day | 30.46 | 146.30 |
| MOE | - | 5.58 | 1.16 |
| Average potency | Cancer cases/year/100,000 population | 0.38 | 1.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, P.; Chauysrinule, C.; Mahakarnchanakul, W.; Maneeboon, T. Multi-Mycotoxin Contamination, Mold Incidence and Risk Assessment of Aflatoxin in Maize Kernels Originating from Nepal. Microbiol. Res. 2022, 13, 258-277. https://doi.org/10.3390/microbiolres13020021
Joshi P, Chauysrinule C, Mahakarnchanakul W, Maneeboon T. Multi-Mycotoxin Contamination, Mold Incidence and Risk Assessment of Aflatoxin in Maize Kernels Originating from Nepal. Microbiology Research. 2022; 13(2):258-277. https://doi.org/10.3390/microbiolres13020021
Chicago/Turabian StyleJoshi, Prateek, Chananya Chauysrinule, Warapa Mahakarnchanakul, and Thanapoom Maneeboon. 2022. "Multi-Mycotoxin Contamination, Mold Incidence and Risk Assessment of Aflatoxin in Maize Kernels Originating from Nepal" Microbiology Research 13, no. 2: 258-277. https://doi.org/10.3390/microbiolres13020021
APA StyleJoshi, P., Chauysrinule, C., Mahakarnchanakul, W., & Maneeboon, T. (2022). Multi-Mycotoxin Contamination, Mold Incidence and Risk Assessment of Aflatoxin in Maize Kernels Originating from Nepal. Microbiology Research, 13(2), 258-277. https://doi.org/10.3390/microbiolres13020021







