In Vitro Antibacterial Activity of Selected Palestinian Medicinal Plants against Chlamydia trachomatis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of HeLa Cells and Chlamydia trachomatis
2.2. Host Infection and Determination of Infectivity
2.3. Viability and Metabolic Activity of Host Cells
2.4. Selection and Collection of Medicinal Plants
2.5. Preparation of the Crude Extracts
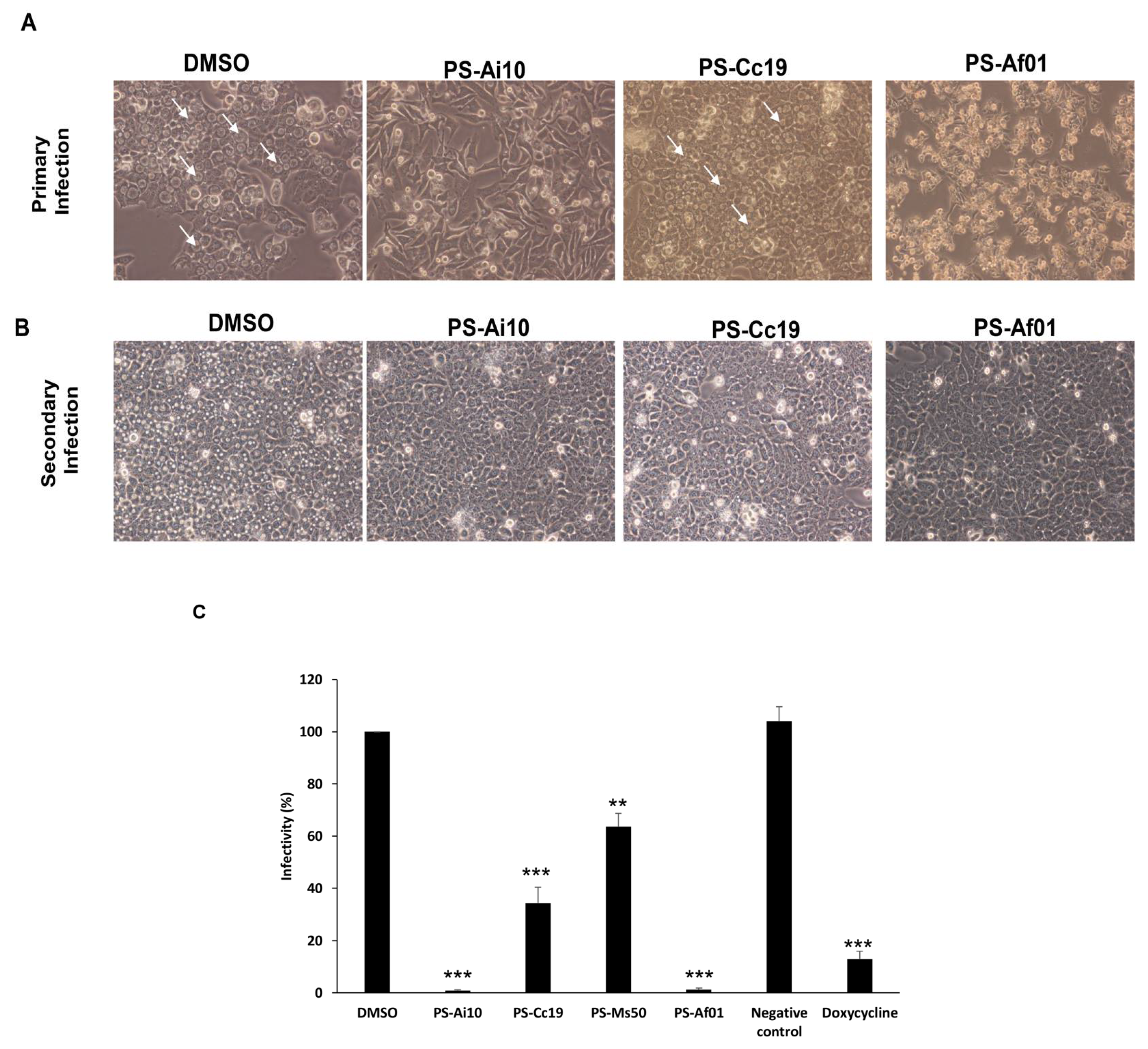
3. Results
Activity of Plant Extracts against Chlamydia trachomatis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hammerschlag, M.R. Chlamydia trachomatis and Chlamydia pneumoniae infections in children and adolescents. Pediatr. Rev. 2004, 25, 43–51. [Google Scholar] [CrossRef]
- Hammerschlag, M.R. Chlamydia pneumoniae and asthma in children: Diagnostic issues. Clin. Infect. Dis. 2004, 39, 1251–1252. [Google Scholar] [CrossRef]
- WHO. Global WHO alliance for the elimination of blinding trachoma by 2020. Wkly. Epidemiol. Rec. Relev. Épidémiol. Hebd. 2020, 87, 161–168. [Google Scholar]
- El Qouqa, I.A.; Shubair, M.E.; Al Jarousha, A.M.; Sharif, F.A. Prevalence of Chlamydia trachomatis among women attending gynecology and infertility clinics in Gaza, Palestine. Int. J. Infect. Dis. 2009, 13, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Darville, T. Recognition and treatment of chlamydial infections from birth to adolescence. Adv. Exp. Med. Biol. 2013, 764, 109–122. [Google Scholar] [CrossRef]
- Zar, H.J. Neonatal chlamydial infections. Pediatri. Drugs 2005, 7, 103–110. [Google Scholar] [CrossRef]
- Sandoz, K.M.; Rockey, D.D. Antibiotic resistance in Chlamydiae. Future Microbiol. 2010, 5, 1427–1442. [Google Scholar] [CrossRef]
- Kissinger, P.J.; White, S.; Manhart, L.E.; Schwebke, J.; Taylor, S.N.; Mena, L.; Khosropour, C.M.; Wilcox, L.; Schmidt, N.; Martin, D.H. Azithromycin Treatment Failure for Chlamydia trachomatis Among Heterosexual Men with Nongonococcal Urethritis. Sex. Transm. Dis. 2016, 43, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Hamarsheh, O.; Azmi, K.; Amro, A.; Schultheis, M.; Abdeen, Z.; Firdessa, R.; Sawalha, K.; Al-Rimawi, F.; Yaghmour, R.; Moll, H. Antileishmanial potential of crude plant extracts derived from medicinal plants in palestine. Ann. Clin. Cytol. Pathol. 2017, 3. [Google Scholar]
- Hamarsheh, O. Epidemiology of Scabies in Palestine. In Handbook of Healthcare in the Arab World; Laher, I., Ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Hamarsheh, O.; Amro, A. Epidemiology of Parasitic Infections in the West Bank and Gaza Strip, Palestine. Am. J. Trop. Med. Hyg. 2019, 102, 313–317. [Google Scholar] [CrossRef]
- Ahua, K.M.; Ioset, J.R.; Ioset, K.N.; Diallo, D.; Mauel, J.; Hostettmann, K. Antileishmanial activities associated with plants used in the Malian traditional medicine. J. Ethnopharmacol. 2007, 110, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.G.; Bouzada, M.L.; Fabri, R.L.; de Matos, O.M.; Moreira, F.O.; Scio, E.; Coimbra, E.S. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J. Ethnopharmacol. 2007, 111, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Abbasi, A.M.; Dastagir, G.; Nazir, A.; Shah, G.M.; Shah, M.M.; Shah, M.H. Ethnobotanical and antimicrobial study of some selected medicinal plants used in Khyber Pakhtunkhwa (KPK) as a potential source to cure infectious diseases. BMC Complement. Altern. Med. 2014, 14, 122. [Google Scholar] [CrossRef]
- Mathabe, M.C.; Nikolova, R.V.; Lall, N.; Nyazema, N.Z. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. J. Ethnopharmacol. 2006, 105, 286–293. [Google Scholar] [CrossRef]
- Tempone, A.G.; Sartorelli, P.; Teixeira, D.; Prado, F.O.; Calixto, I.A.; Lorenzi, H.; Melhem, M.S. Brazilian flora extracts as source of novel antileishmanial and antifungal compounds. Mem. Do Inst. Oswaldo Cruz 2008, 103, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Borris, R.P.; Carte, B.; Cordell, G.A.; Soejarto, D.D.; Cragg, G.M.; Gupta, M.P.; Iwu, M.M.; Madulid, D.R.; Tyler, V.E. Natural product drug discovery and development: New perspectives on international collaboration. J. Nat. Prod. 1995, 58, 1325–1357. [Google Scholar] [CrossRef]
- Fournet, A.; Munoz, V. Natural products as trypanocidal, antileishmanial and antimalarial drugs. Curr. Top. Med. Chem. 2002, 2, 1215–1237. [Google Scholar] [CrossRef]
- Izzo, A.A.; Ernst, E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs 2009, 69, 1777–1798. [Google Scholar] [CrossRef]
- Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural products as a source for treating neglected parasitic diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef] [PubMed]
- Al-Zeer, M.A.; Xavier, A.; Abu Lubad, M.; Sigulla, J.; Kessler, M.; Hurwitz, R.; Meyer, T.F. Chlamydia trachomatis Prevents Apoptosis Via Activation of PDPK1-MYC and Enhanced Mitochondrial Binding of Hexokinase II. EBioMedicine 2017, 23, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Sayanjali, B.; Christensen, G.J.M.; Al-Zeer, M.A.; Mollenkopf, H.J.; Meyer, T.F.; Bruggemann, H. Propionibacterium acnes inhibits FOXM1 and induces cell cycle alterations in human primary prostate cells. Int. J. Med. Microbiol. 2016, 306, 517–528. [Google Scholar] [CrossRef]
- Abu-Lubad, M.; Meyer, T.F.; Al-Zeer, M.A. Chlamydia trachomatis inhibits inducible NO synthase in human mesenchymal stem cells by stimulating polyamine synthesis. J. Immunol. 2014, 193, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.A.; Al-Ramahi, R.; Zaid, A.N.; Ayesh, O.I.; Eid, A.M. Ethnopharmacological survey of herbal remedies used for treatment of various types of cancer and their methods of preparations in the West Bank-Palestine. BMC Complement. Altern. Med. 2016, 16, 93. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Ayesh, O.I.; Anderson, C. Ethnopharmacological survey about medicinal plants utilized by herbalists and traditional practitioner healers for treatments of diarrhea in the West Bank/Palestine. J. Ethnopharmacol. 2016, 182, 57–66. [Google Scholar] [CrossRef]
- Jaradat, N.A. Medical plants utilized in Palestinian folk medicine for treatment of diabetes mellitus and cardiac diseases. J. Al-Aqsa Univ. 2005, 9, 1–28. [Google Scholar]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2002, 83, 251–265. [Google Scholar] [CrossRef]
- Duke, J.A.; Duke, P.-A.K.; Du Cellie, J.L. Duke’s Handbook of Medicinal Plants of the Bible; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Al-Shafie, J.H.; Elgharabah, W.A.; Kherfan, F.A.; Qarariah, K.H.; Isra’S, K.; Soos, I.M.; Musleh, A.A.; Isa, B.A. Traditional knowledge of wild edible plants used in Palestine (Northern West Bank): A comparative study. J. Ethnobiol. Ethnomed. 2008, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Munguia, J.; Nizet, V. Pharmacological Targeting of the Host-Pathogen Interaction: Alternatives to Classical Antibiotics to Combat Drug-Resistant Superbugs. Trends Pharmacol. Sci. 2017, 38, 473–488. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The artemisia L. Genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Al-Marzooky, M.A. Truffles in eye disease. Proc. Int. Islam. Med 1981, 1, 353–357. [Google Scholar]
- Semenya, S.S.; Potgieter, M.J. Sexually transmitted infections and their diagnoses: Bapedi experience. Afr. Health Sci. 2013, 13, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Semenya, S.S.; Potgieter, M.J.; Erasmus, L.J. Exotic and indigenous problem plants species used, by the Bapedi, to treat sexually transmitted infections in Limpopo Province, South Africa. Afr. Health Sci. 2013, 13, 320–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hossan, S.; Hanif, A.; Agarwala, B.; Sarwar, S.; Karim, M.; Taufiq-Ur-Rahman, M.; Jahan, R.; Rahmatullah, M. Traditional Use of Medicinal Plants in Bangladesh to Treat Urinary Tract Infections and Sexually Transmitted Diseases. Ethnobot. 2010, 8, 61–74. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; Geiser, I.; Rwangabo, P.C.; Sebikali, B. Rwandese herbal remedies used against gonorrhoea. J. Ethnopharmacol. 1983, 8, 279–286. [Google Scholar] [CrossRef]
- Bhengraj, A.R.; Dar, S.A.; Talwar, G.P.; Mittal, A. Potential of a novel polyherbal formulation BASANT for prevention of Chlamydia trachomatis infection. Int. J. Antimicrob. Agents 2008, 32, 84–88. [Google Scholar] [CrossRef]
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Rauha, J.-P.; Wennberg, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamarsheh, O.; Amro, A.; Al-Zeer, M.A. In Vitro Antibacterial Activity of Selected Palestinian Medicinal Plants against Chlamydia trachomatis. Microbiol. Res. 2021, 12, 656-662. https://doi.org/10.3390/microbiolres12030047
Hamarsheh O, Amro A, Al-Zeer MA. In Vitro Antibacterial Activity of Selected Palestinian Medicinal Plants against Chlamydia trachomatis. Microbiology Research. 2021; 12(3):656-662. https://doi.org/10.3390/microbiolres12030047
Chicago/Turabian StyleHamarsheh, Omar, Ahmad Amro, and Munir A. Al-Zeer. 2021. "In Vitro Antibacterial Activity of Selected Palestinian Medicinal Plants against Chlamydia trachomatis" Microbiology Research 12, no. 3: 656-662. https://doi.org/10.3390/microbiolres12030047
APA StyleHamarsheh, O., Amro, A., & Al-Zeer, M. A. (2021). In Vitro Antibacterial Activity of Selected Palestinian Medicinal Plants against Chlamydia trachomatis. Microbiology Research, 12(3), 656-662. https://doi.org/10.3390/microbiolres12030047







