Abstract
Sepsis frequently leads to multiple organ failure and is a major cause of morbidity and mortality in critically ill patients. Although intensive care protocols and antibiotic therapy have improved sepsis treatment, specific management is lacking with respect to efficient protection from tissue damage and long-term outcomes. Probiotics are live microbes that modulate the immune system and inflammation and colonize the gut. In this narrative review, we have traced the evolution of the administration of probiotics in an animal model of sepsis and treatment alternatives in the intensive care unit setting. First, probiotics are categorized by species before describing their modulation of the microbiota, repair of tissue-specific damage, immune response, and molecular pathways to prevent complications. The impact on therapy for infant and adult patients is also addressed. Finally, we have emphasized the challenges and gaps in current studies as well as future perspectives for further investigation. The present review can open up avenues for new strategies that employ promising probiotic strains for the treatment of sepsis and discusses their ability to prevent disease-associated long-term complications.
1. Introduction
Sepsis is a life-threatening condition that poses a public health risk. Despite persistent socioeconomic differences throughout the world, levels of morbidity and mortality due to sepsis remain high, imposing a burden on healthcare systems worldwide. Access to and quality of healthcare contribute to lower rates of complications during sepsis. However, underreporting of sepsis cases negatively impacts treatment and impedes the delivery of healthcare services [1,2,3].
Inflammation triggered by host defense mechanisms against infectious agents, when not properly kept in check by immunoregulatory mechanisms, can lead to tissue damage and multiple organ failure [4,5]. Even with vasopressor and intravenous fluid interventions, early and aggressive antibiotic administration has been administered during the hyperinflammatory phase to prevent complications and death due to sepsis [6]. Unfortunately, antibiotic therapy affects the intestinal microbiome by promoting dysbiosis and might contribute to gastrointestinal tract (gut) damage. In this regard, intestinal microbiota can be associated with the long-term progression in the ICU environment, being a trend of clinical research. Microbiota alteration can predict the risk of sepsis and its mortality based on the abundance of certain harmful gut microbes [7]. Antimicrobial resistance is another factor that impedes recovery despite therapy [8]. In addition, the lack of resources to perform interventions in individuals with sepsis might contribute to the high mortality rates observed in middle-income countries [9,10]. Furthermore, sepsis survivors develop post-sepsis syndrome with physical and/or psychological long-term effects, necessitating re-hospitalization due to new bacterial infections [11].
Intestinal epithelial integrity, as well as interactions between the immune system and intestinal microbiota, protect the host and maintain gut homeostasis. However, sepsis disrupts this epithelial barrier by triggering enterocyte apoptosis and reducing cell proliferation, which compromises the local microbiome after immune response activation [12,13,14]. The gut microbiota composition and the microbial metabolite profiles are different in healthy patients than in individuals with sepsis. Patients with sepsis have enteric dysbiosis associated with organ injury [15]. Microbial growth has been associated with inflammation, and loss of microbial diversity can be used to predict the risk of sepsis in an intensive care unit (ICU) environment by evaluating the presence and growth of harmful gut microbes [7]. Although it is known that the gut is involved in sepsis, its exact roles in the progression of organ dysfunction and the therapeutic modulation of dysbiosis are still not clear. Studies have demonstrated that treatment is required to restore mucosal integrity, ameliorate the local immune response, and reverse dysbiosis in critically ill patients, including those with sepsis [12,13,16,17].
In order to promote gut homeostasis by neutralizing the growth of harmful microorganisms and/or reinforcing local immunoregulatory networks, probiotics have been developed as supplements with health benefits. In recent years, the biological effects of probiotics have been explored to increase their spectrum of prophylactic and therapeutic modulation in humans and animals [18,19]. If administered in adequate amounts, these live microorganisms can provide measurable physiological benefits to ailing and immunocompromised individuals [20]. Furthermore, probiotics could play important roles in attenuating sepsis and enterocolitis [21,22].
Based on the potential colonization of probiotics and their ability to prevent tissue dysfunction triggered by inflammatory mediators, it is necessary to clarify the mechanisms involved in the restoration of homeostasis by probiotics during sepsis. In this review, we will describe the effects of probiotic administration on a cecal ligation and puncture (CLP) model, a type of polymicrobial sepsis that mimics the complexity of human disease. Finally, we will emphasize the clinical aspects of probiotics in sepsis management among ICU patients.
Between January 2020 and June 2020, a literature search was conducted on the PubMed database. For an experimental model, the following search terms were used in combination: “CLP”, “polymicrobial sepsis”, “probiotic”, and treatment”. For human studies, we selected previous studies that demonstrated the probiotic therapy in the hospital environment. The following search terms were used in combination: “bacteremia”, “ICU”, “human”, “hospital”, “probiotic”, and “sepsis”. All studies published in the English language were considered, and a date restriction was not applied. In addition, authors reviewed the studies and selected those based on relevance to the topic. Additional articles were identified by manually searching reference lists of included articles.
2. Lactobacillus Rhamnosus GG (LGG)
LGG is a common microbial strain used in basic and applied research. It is characterized as a Gram-positive bacterium that can tolerate different oxygen levels and is found in healthy human intestines. Furthermore, LGG has been consumed as a probiotic supplement for a normal diet and a healthy balance of gut bacteria [23,24]. Many studies have reported the beneficial effects of LGG administration in gastrointestinal [25], allergy [26], and lung [27] diseases. As a result, LGG is a promising agent for modulatory activity in inflammatory disorders, including sepsis.
The prophylactic effect of LGG in mice subjected to sepsis was first addressed by Khailova et al. [28]. The oral administration (p.o.) of LGG immediately before CLP-induced sepsis protects mice from lung damage through the inhibition of neutrophil migration. Importantly, the lung expression of inflammatory markers, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and cyclooxygenase (COX)-2, is abrogated. Furthermore, the expression of Toll-like receptor (TLR)-2, its proximal protein myeloid differentiation primary response 88 (MyD88), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) genes, downstream of TLR-2, is inhibited by LGG treatment.
To demonstrate the protective effect of LGG on sepsis, Chen et al. administered LGG (p.o.) daily to mice for 1 month and induced sepsis via CLP. Consequently, the time of death was delayed by several days after probiotic prophylaxis. In addition, LGG attenuated mucosal damage to the ileum, enhanced gut barrier integrity, and normalized lysophosphatidylcholine metabolite levels, which are known to be proinflammatory markers [29]. However, the mechanisms involved in these processes are not known. Based on the same condition of probiotic pre-administration, the group focused on modulation of the gut microbiome, mucosal barrier homeostasis, and inflammatory markers [30]. After colonization, LGG reversed the dysbiosis that was associated with sepsis and improved bacterial diversity, both of which have been proposed as therapeutic targets [31]. Although LGG inhibited the release of IL-2 and IL-22, the levels of TNFα and IL-6 were similar to those in septic mice without probiotic supplementation. Furthermore, Chen et al. [30] reported that LGG prevented epithelial cell apoptosis and improved epithelial tight junctions after the onset of sepsis. Recently, the group demonstrated that LGG was able to reduce bacteremia and restore colon microbiome homeostasis [32].
The impact of LGG administration on polymicrobial sepsis was also evaluated by Ding et al. [33], who determined whether this probiotic could modulate sepsis-related liver injury. Rats that received LGG (p.o.) once immediately prior to insult were protected from death caused by sepsis. The modulatory activities of LGG treatment in alleviating liver damage were associated with inhibition of the expression and/or release of IL-1β, IL-6, NLRP3 inflammasomes, TNFα, vascular endothelial growth factor, and monocyte chemoattractant protein 1, as well as the reduction of oxidative stress and lipid peroxidation. Furthermore, LGG downregulated NF-κB and hypoxia-inducible factor 1-α, indicating an important mechanism by which the probiotic alleviates a cytokine storm.
3. Bifidobacterium Longum
Bifidobacterium longum is another probiotic used in basic and clinical studies. This Gram-positive microorganism has shown positive effects on modulation of the gut [34,35,36], obesity [37], anxiety, stress, depression [38,39,40], and cytokines [41,42]. Khailova et al. [28] demonstrated that the previous administration of B. longum could protect septic mice from lung injury, decreasing TNFα and IL-6 levels, along with levels of COX-2, TLR-2, and MyD88. However, probiotic prophylaxis did not affect TLR-4 and NF-κB expression in the lung tissue.
4. Escherichia Coli
In general, E. coli is a facultative anaerobic Gram-negative bacterium that adapts to diverse environments. Most strains colonize the gut of warm-blooded animals as part of the normal microbiota. Furthermore, E. coli has an extraordinary ability to survive in soil, water, sediment, and food environments [43,44]. Although this bacterium is beneficial to the healthy microbiome, some strains can be extremely virulent in both extraintestinal and intestinal environments. These disorders are caused by the contamination of food products, water, and milk [43,45,46]. However, E. coli has been used by the biotechnology industry as a recombinant therapeutic resource [47]. E. coli Nissle 1917 (EcN) is a probiotic and the most promising strain that exhibits protective activities against different inflammatory disorders, such as allergies [48,49], obesity [50], intestinal inflammation [51,52,53], tumor growth [54,55], and autoimmune dysfunction [56]. Hence, it may be an excellent therapeutic agent for sepsis.
Recently, Guo et al. [57] reported the protective effect of EcN on the intestinal barrier functions of septic mice. An EcN suspension was administered p.o. 2 weeks before the CLP procedure. In the small intestine, the treatment reversed the loss of tight junction proteins, such as zona occludens-1 (ZO-1) and claudin-1, and it slightly downregulated claudin-2. These markers are involved in maintenance of the correct architecture and intestinal barrier permeability. Furthermore, these researchers demonstrated that the supernatant growth medium of EcN was also able to regulate tight junction proteins and inhibit NF-κB and myosin light-chain kinase activation in vitro.
5. Zymomonas Mobilis
Zymomonas mobilis is an anaerobic Gram-negative bacterium used in the metabolic engineering of alcohol production [58,59,60]. This ethanogenic strain produces secondary metabolites with antileukemic properties [61] and is able to abolish Schistosoma mansoni infections [62]. Furthermore, Z. mobilis has been used as a dietary supplement in animal husbandry [63] as it also normalizes intestinal transit and has antilipidemic properties, attenuating cholesterol, and lipoprotein fractions [64].
Campos et al. [65] described the role of Z. mobilis on an experimental model of sepsis. These researchers demonstrated that pretreatment or its association with post-treatment (p.o.) protected mice from severe sepsis. In addition, lung and spleen damage were attenuated due to a decrease in myeloperoxidase levels and apoptotic cells. Bacterial growth and neutrophil migration to local injury were inhibited with the administration of Z. mobilis. The upregulation of IL-10 was found to have a protective role, whereas TNF-α levels, also downmodulated by Z. mobilis, were found to be responsible for organ dysfunction and an increase in the rate of mortality during sepsis.
6. Probiotic Combinations
Several studies have focused on the benefits of probiotic strains and their combinations that can colonize the intestine and modulate immunological responses in gut disorders and inflammatory diseases [66,67,68,69,70,71,72]. Bacillus subtilis and Enterococcus faecium, both Gram-positive microorganisms, have been used as probiotic supplements and regulate microbiota [73,74] and obesity [75,76], have antioxidant and antimicrobial properties [77,78], repair disruptions in the intestinal barrier [79,80], attenuate elevated cholesterol levels [81], and modulate intestinal mucosal immune responses [82]. The oral administration of a mixture of B. subtilis and E. faecium was found to improve the host response of mice challenged with the CLP procedure [83]. A once-daily treatment for 1 week delayed animal death and improved the survival rate. These probiotics upregulate ZO-1, claudin-1, and occludin proteins and attenuated proinflammatory macrophage activation in the ileum. Furthermore, levels of IL-6 and TNF-α were found to be decreased in the serum and ileum tissue. Levels of histamine, a marker associated with mast cell activation, were also attenuated in the serum and the peritoneum. Interestingly, the combination of probiotics did not modulate the levels of anti-inflammatory markers such as TGF-β, IL-10, and type 2 macrophage. The phosphorylation of the serine/threonine-specific protein kinase AKT was also found to be increased with treatment [83].
Biological activities reported previously herein, emphasizing the immunomodulatory and anti-inflammatory activities and the mechanisms of actions of probiotics, are summarized in Table 1 and Figure 1.

Table 1.
Experimental designs and immunomodulatory activities of probiotics on cecal ligation and puncture model.
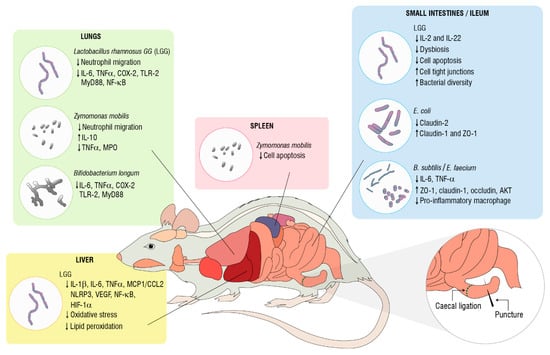
Figure 1.
Biological activities of probiotics in cecal ligation and puncture-induced sepsis. Cyclooxygenase (COX)-2. Hypoxia-inducible factor (HIF)-1-α. Interleukin (IL). Monocyte chemoattracctant protein-1/C-C motif chemokine 2 (MCP1/CCL2). Myeloid differentiation primary response 88 (MYD88). Myeloperoxidase (MPO). Toll-like receptor (TLR). Nod-like receptor NACHT, LRR, and PYD domain-containing protein 3 (NLRP3). Nuclear factor-kappaB (NF-κB). Tumor necrosis factor (TNF)-α. V-akt murine thymoma viral oncogene homolog 1 (AKT1). Vascular endothelial growth factor (VEGF). Zonula occludens (ZO)-1.
It is interesting to note that LGG administration can attenuate multiple organ dysfunction, pro-inflammatory markers, and cell death and regulate imbalances and epithelial disruption in the gut. Despite these findings, four out of five of these studies did not find an efficient response in terms of survival rate. Further research into LGG is required to explore its systemic effects and to establish preclinical protocols, which would allow for the complete recovery from sepsis. Whereas Z. mobilis has functional activity in reducing sepsis-induced lung and spleen injury and mortality, knowledge of the molecular mechanisms and signaling pathways involved in this process are limited. Therefore, this live microorganism could be a promising candidate to prevent an immunosuppressive state because of its ability to abrogate cell death in the spleen, which has a crucial role in sepsis severity. The modulatory effect of Z. mobilis on the long-term complications of sepsis might be addressed in future studies, along with B. longum, which protects the lung tissue during the inflammatory response to systemic infection. Another suggestion addressed in experimental sepsis models involving E. coli or B. subtilis plus E. faecium probiotics, which recover gut barrier functions via the maintenance of tight junction proteins, is to verify if bacterial diversity can be preserved or improved with probiotic supplementation. These preclinical studies suggest that selective probiotics or their combinations might be applied as possible adjuvants for the treatment of sepsis to ameliorate the immune response and regulate inflammation. In this context, further studies on clinical efficacy, safety, costs, and benefits should be conducted.
7. Probiotics in the Pediatric ICU
In addition to animal models, the potentially therapeutic and anti-inflammatory properties of probiotics have been investigated in humans, but their effects on sepsis in clinical settings remain unclear. In 2002, Dani et al. [84] reported that prophylactic LGG administration does not protect newborn and low-birth-weight infants from bacterial sepsis, which has been shown recently with Bacillus clausii treatment [85]. These findings corroborated those of other studies showing that Bifidobacterium breve BBG-001 [86], as well as Lactobacillus and Bifidobacterium, combined with oligosaccharides and lactoferrin [87], were not effective against necrotizing enterocolitis (NEC), sepsis, or mortality in neonates and infants. However, a probiotic combination of Bifidobacterium infantis, Bifidobacterium lactis, and Streptococcus thermophilus was found to protect infants from NEC without positive results for late-onset sepsis [88]. In addition, the adjuvant potential of Saccharomyces spp. in inducing a protective response against sepsis remains unclear because of controversial studies about the efficacy of this treatment [89,90,91].
In contrast, beneficial outcomes were observed with respect to the incidence of late-onset sepsis and inhibition of colonization by Candida strains in the gut with L. rhamnosus and L. reuteri supplementation [92]. Another positive effect of the probiotic combination was a reduction in hospitalization days of low-birth-weight infants [93]. Furthermore, L. plantarum, combined with fructooligosaccharide, promotes a significant reduction in the incidence of culture-positive and culture-negative sepsis and in lower respiratory tract infections in infants [94]. Fortmann et al. [95] recently reported the efficient supplementation of L. acidophilus and B. infantis plus human milk, which protected infants from sepsis. A combination of Lactobacillus and Bifidobacterium strains was found to lower TNF-α, IL-6, IL-12p70, and IL-17 levels and increase IL-10 and TGF-β levels in children with severe sepsis, protecting them from organ failure [96]. Recently, the benefits of probiotic use in preterm infants between 30 and 37 weeks revealed an increase in the feeding capacity and growth and improved gut functions, in addition to reducing the hospital stay [97].
8. Probiotics for Adult Patients in the ICU
The administration of Lactobacillus, Bifidobacterium, and Streptococcus microorganisms along with oligofructose was able to alter the microbiota in the upper gut of adult septic patients, but it had no effect on intestinal permeability or mortality [98]. B. longum, L. bulgaricus, and S. thermophilus increase IL-12p70 and IFN-γ levels in blood and decrease IL-4 and IL-10 levels in patients with severe traumatic-brain injury, reducing the incidence of nosocomial infections and long stays in the ICU [99]. Furthermore, B. breve strain Yakult, L. casei strain Shirota, and galactooligosaccharides administered to septic patients alter the gut microbiota composition and attenuate respiratory complications [100]. However, the mortality rate was not affected in these studies. Finally, some studies suggested that probiotics could be used to reduce sepsis and infectious complications in patients with colorectal liver metastases who had undergone local resection [101] or in post-injury infections in patients with multiple injuries [102] and abdominal surgery [103]. Table 2 shows the outcomes in sepsis of pediatric and adult patients admitted in the ICU who received probiotic administration.

Table 2.
Administration of probiotics in children and adults admitted in ICU.
9. Probiotic Side Effects in Clinical Practice
Even though probiotics have been considered safe to balance the intestinal microbiota, several isolated cases demonstrated that the same strain used to treat gut dysfunctions can translocate into the blood, resulting in septicemia. Saccharomyces cerevisiae and associated variations were mainly reported to be a source of fungemia from newborns to elderly patients [104,105,106,107,108,109,110,111,112,113,114,115,116,117,118]. In addition, bloodstream infections of Lactobacillus spp. [119,120,121,122,123,124,125,126,127,128,129,130,131,132,133], Bifidobacterium spp. [134,135,136,137,138,139], E. coli Nissle 1977 [140], P. pentosaceus [141], and B. clausii [142] have been reported in septic patients following probiotic administration. Interestingly, many of these patients were previously diagnosed with comorbidities [105,112,118,122,125,142], inflammatory disorders [108,113,123,130,140,141], congenital malformations [109,121,127,134,138,139], or acquired immunodeficiency syndrome [124,126,139] before starting probiotic therapy. These relationships can be interpreted as challenging for the use of probiotics in sepsis, since the complexity of the disease itself and its treatment can lead to a similar state of intense inflammation or immunosuppression. Moreover, it is possible that due to underlying diseases, even commensal bacteria or adjuvant supplementation can result in host infection.
10. Conclusions and Future Perspectives
Probiotic supplementary therapy or enteral administration to abolish dysbiosis-related diseases can carefully be evaluated in critically ill patients. First, microbiota composition and establishment are challenged during life. The infant gut microbiota is less stable, more variable, and can be altered by maternal microbiota, lifestyle, health status, complementary food, and duration of lactation. In this period of life, gut microbiota play a key role in immunological network impacting on human health. In the same way, dietary patterns, microbial infections, and clinical interventions can modify adult microbiota composition and increase the risk of diseases [143,144]. In addition, due to the lack of guidelines for probiotic usage in the ICU environment, randomized placebo-controlled trials will help to clarify the efficacy and safety of probiotics to establish a pattern that indicates a cause–effect correlation based on infections and probiotics.
This literature review discussed the potential effects of probiotics as targets for sepsis therapy. Although the majority of the studies reviewed here demonstrated the possibility of using Lactobacillus, Bacillus, and Bifidobacterium strains to manage the complications of sepsis in experimental animal models, the aforementioned clinical trials have focused on testing mainly combinations of probiotics with positive or negative outcomes through partial protection and by modulating cytokines and changing the microbiota composition. Implications regarding the animal model also are addressed. CLP has been used as a preclinical model to clarify the pathophysiology of sepsis and its therapeutic direction. However, different murine strains display distinct susceptibilities to the procedure that can impact on the replication and consistency of results [145,146]. Therefore, the heterogeneous methodology of probiotic administration and their dose ranges, as well as the use of different animal strains and diverse patient profiles, limit our understanding of the possible benefits of clinical probiotic administration.
Prospective multicenter studies with different probiotic strains, alone or in combination, need to be conducted to clarify the effects of probiotics in the hospital environment. Interestingly, many studies have investigated the ability of probiotics to modulate the gut and the hyperinflammatory phase in sepsis. However, the mechanisms of these actions have not been fully elucidated. Additionally, exploring the importance of probiotic treatment to long-term consequences of sepsis in specific tissues, such as lung, kidney, brain, liver, and gut, or to patient readmission to the hospital due to illnesses after recovery from sepsis should reveal strategies that promote physiological homeostasis and protect against post-sepsis syndrome. Finally, further research is necessary to understand whether probiotics could act as immunologic adjuvants that regulate the immune response and sepsis-induced immunosuppression.
Author Contributions
Conceptualization, J.B.N.F.S.; methodology, V.R.d.S., G.O.d.C. and J.B.N.F.S.; formal analysis, V.R.d.S., G.O.d.C. and J.B.N.F.S.; investigation, V.R.d.S., G.O.d.C. and J.B.N.F.S.; writing—original draft preparation, V.R.d.S., G.O.d.C. and J.B.N.F.S.; writing—review and editing, V.R.d.S., G.O.d.C. and J.B.N.F.S.; visualization, V.R.d.S., G.O.d.C. and J.B.N.F.S.; supervision, J.B.N.F.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Leonardo Arcanjo Franco for the help with Figure.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tiru, B.; DiNino, E.K.; Orenstein, A.; Mailloux, P.T.; Pesaturo, A.; Gupta, A.; McGee, W.T. The economic and humanistic burden of severe sepsis. Pharmacoeconomics 2015, 33, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Kissoon, N.; Limmathurotsakul, D.; Bory, S.; Mutahunga, B.; Seymour, C.W.; Angus, D.C.; West, T.E. The global burden of sepsis: Barriers and potential solutions. Crit. Care 2018, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Salomão, R.; Ferreira, B.; Salomão, M.; Santos, S.; Azevedo, L.; Brunialti, M. Sepsis: Evolving concepts and challenges. Braz. J. Med. Biol. Res. 2019, 52, e8595. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Barboza, C.L.; Valete, C.O.S.; da Silva, A.R.A. Bundle adherence of intravenous antibiotic fluid resuscitation and vasopressor in children with severe sepsis or septic shock. Indian J. Crit. Care Med. 2020, 24, 128–132. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Valdés-Duque, B.E.; Giraldo-Giraldo, N.A.; Jaillier-Ramírez, A.M.; Giraldo-Villa, A.; Acevedo-Castaño, I.; Yepes-Molina, M.A.; Barbosa-Barbosa, J.; Benítez-Paéz, A. Gut microbiota profiles in critically ill patients; potential biomarkers and risk variables for sepsis. Gut Microb. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, H. Sepsis: Mechanisms of bacterial injury to the patient. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 19. [Google Scholar] [CrossRef]
- Quintano Neira, R.A.; Hamacher, S.; Japiassú, A.M. Epidemiology of sepsis in Brazil: Incidence, lethality, costs, and other indicators for Brazilian unified health system hospitalizations from 2006 to 2015. PLoS ONE 2018, 13, e0195873. [Google Scholar] [CrossRef]
- Taniguchi, L.U.; De Azevedo, L.C.P.; Bozza, F.A.; Cavalcanti, A.B.; Ferreira, E.M.; Carrara, F.S.A.; Sousa, J.L.; Salomão, R.; Machado, F.R. Availability of resources to treat sepsis in Brazil: A random sample of Brazilian institutions. Rev. Bras. Ter. Intensiva 2019, 31, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mostel, Z.; Perl, A.; Marck, M.; Mehdi, S.F.; Lowell, B.; Bathija, S.; Santosh, R.; Pavlov, V.A.; Chavan, S.S.; Roth, J. Post-sepsis syndrome—An evolving entity that afflicts survivors of sepsis. Mol. Med. 2019, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Coopersmith, C.M. Redefining the gut as the motor of critical illness. Trends Mol. Med. 2014, 20, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Klingensmith, N.J.; Coopersmith, C.M. New insights into the gut as the driver of critical illness and organ failure. Curr. Opin. Crit. Care 2017, 23, 143–148. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Triantos, C.; Thomopoulos, K.; Fligou, F.; Maroulis, I.; Marangos, M.; Gogos, C.A. Gut-origin sepsis in the critically ill patient: Pathophysiology and treatment. Infection 2018, 46, 751–760. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Fang, H.; Chen, X.; Guo, Y.; Gong, S.; Niu, M.; Zhou, H.; Jiang, Y.; Chang, P.; et al. Enteric dysbiosis is associated with sepsis in patients. FASEB J. 2019, 33, 12299–12310. [Google Scholar] [CrossRef]
- Haak, B.W.; Wiersinga, W.J. The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2017, 2, 135–143. [Google Scholar] [CrossRef]
- Brüssow, H. Probiotics and prebiotics in clinical tests: An update. F1000Res. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Probiotics: Definition, scope and mechanisms of action. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 17–25. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Huys, G.; Daube, G. Probiotics: An update. J. Pediatr. (Rio J.) 2015, 91, 6–21. [Google Scholar] [CrossRef]
- Sun, J.; Marwah, G.; Westgarth, M.; Buys, N.; Ellwood, D.; Gray, P.H. Effects of probiotics on necrotizing enterocolitis, sepsis, intraventricular hemorrhage, mortality, length of hospital stay, and weight gain in very preterm infants: A meta-analysis. Adv. Nutr. 2017, 8, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Underwood, M.A. Probiotics and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 39–46. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell Fact. 2014, 13, 1–16. [Google Scholar] [CrossRef]
- Basturk, A.; Isik, I.; Atalay, A.; Yılmaz, A. Investigation of the efficacy of Lactobacillus rhamnosus GG in infants with cow’s milk protein allergy: A randomised double-blind placebo-controlled trial. Probiotics Antimicrob. Proteins 2020, 12, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Pace, F.; Pace, M.; Quartarone, G. Probiotics in digestive diseases: Focus on Lactobacillus GG. Minerva Gastroenterol. Dietol. 2015, 61, 273–292. [Google Scholar]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R. Probiotics as treatment for food allergies among pediatric patients: A meta-analysis. World Allergy Organ. J. 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Morrow, L.E.; Kollef, M.H.; Casale, T.B. Probiotic prophylaxis of ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2010, 182, 1058–1064. [Google Scholar] [CrossRef]
- Khailova, L.; Petrie, B.; Baird, C.H.; Rieg, J.D.; Wischmeyer, P.E. Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis. PLoS ONE 2014, 9, e97861. [Google Scholar] [CrossRef]
- Chen, L.; Xu, K.; Gui, Q.; Chen, Y.; Chen, D.; Yang, Y. Probiotic pre-administration reduces mortality in a mouse model of cecal ligation and puncture-induced sepsis. Exp. Ther. Med. 2016, 12, 1836–1842. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Li, J.; Chen, Y.; Yang, Y. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int. J. Mol. Med. 2019, 43, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Haak, B.W.; Prescott, H.C.; Wiersinga, W.J. Therapeutic potential of the gut microbiota in the prevention and treatment of sepsis. Front. Immunol. 2018, 9, 2042. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Chen, Y.; Yang, Y. Probiotic Lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutrition 2020, 78, 110863. [Google Scholar] [CrossRef]
- Ding, L.; Gong, Y.; Yang, Z.; Zou, B.; Liu, X.; Zhang, B.; Li, J. Lactobacillus rhamnosus GG ameliorates liver injury and hypoxic hepatitis in rat model of CLP-induced sepsis. Dig. Dis. Sci. 2019, 64, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, H.; Odamaki, T.; Fukuda, S.; Kato, T.; Xiao, J.-Z.; Abe, F.; Kikuchi, J.; Ohno, H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci. Rep. 2015, 5, 13548. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef]
- Bonfrate, L.; Di Palo, D.M.; Celano, G.; Albert, A.; Vitellio, P.; De Angelis, M.; Gobbetti, M.; Portincasa, P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020, 50, e13201. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Li, Z.; Ji, G.E. Anti-Obesity effects of a mixture of fermented ginseng Bifidobacterium longum BORI, and Lactobacillus paracasei CH88 in high-fat diet-fed mice. J. Microbiol. Biotechnol. 2018, 28, 688–696. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.F.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, J.-P.; Deng, K.; Li, X.; Yuan, Y.; Xuan, Q.; Xie, J.; He, X.-M.; Wang, Q.; Li, J.-J.; et al. Prophylactic effects of Bifidobacterium adolescentis on anxiety and depression-like phenotypes after chronic stress: A role of the gut microbiota-inflammation axis. Front. Behav. Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Volti, G.L.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non-alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef]
- Makioka, Y.; Tsukahara, T.; Ijichi, T.; Inoue, R. Oral supplementation of Bifidobacterium longum strain BR-108 alters cecal microbiota by stimulating gut immune system in mice irrespectively of viability. Biosci. Biotechnol. Biochem. 2018, 82, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Leimbach, A.; Hacker, J.; Dobrindt, U.E. coli as an all-rounder: The thin line between commensalism and pathogenicity. Curr. Top. Microbiol. Immunol. 2013, 358, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Hur, H.-G.; Sadowsky, M.; Byappanahalli, M.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.D.; Dobrindt, U. What defines extraintestinal pathogenic Escherichia coli? Int. J. Med. Microbiol. 2011, 301, 642–647. [Google Scholar] [CrossRef]
- Figler, H.M.; Dudley, E.G. The interplay of Escherichia coli O157:H7 and commensal E. coli: The importance of strain-level identification. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 415–417. [Google Scholar] [CrossRef]
- Huang, C.J.; Lin, H.; Yang, X. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J. Ind. Microbiol. Biotechnol. 2012, 39, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Weise, C.; Zhu, Y.; Ernst, D.; Kühl, A.A.; Worm, M. Oral administration of Escherichia coli Nissle 1917 prevents allergen-induced dermatitis in mice. Exp. Dermatol. 2011, 20, 805–809. [Google Scholar] [CrossRef]
- Secher, T.; Maillet, I.; Mackowiak, C.; Le Bérichel, J.; Philippeau, A.; Panek, C.; Boury, M.; Oswald, E.; Saoudi, A.; Erard, F.; et al. The probiotic strain Escherichia coli Nissle 1917 prevents papain-induced respiratory barrier injury and severe allergic inflammation in mice. Sci. Rep. 2018, 8, 11245. [Google Scholar] [CrossRef]
- Ma, J.; Li, C.; Wang, J.; Gu, J. Genetically engineered Escherichia coli Nissle 1917 secreting GLP-1 analog exhibits potential antiobesity effect in high-fat diet-induced obesity mice. Obesity (Silver Spring) 2020, 28, 315–322. [Google Scholar] [CrossRef]
- Schultz, M.; Strauch, U.G.; Linde, H.-J.; Watzl, S.; Obermeier, F.; Göttl, C.; Dunger, N.; Grunwald, N.; Schölmerich, J.; Rath, H.C. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin. Diagn. Lab. Immunol. 2004, 11, 372–378. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Fernández-Caballero, J.A.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. The administration of Escherichia coli Nissle 1917 ameliorates development of DSS-induced colitis in mice. Front. Pharmacol. 2018, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C.; Maleki, B.H.; Pilat, C.; Ringseis, R.; Eder, K.; Teschler, M.; Krüger, K. Effects of Escherichia coli strain Nissle 1917 on exercise-induced disruption of gastrointestinal integrity. Eur. J. Appl. Physiol. 2020, 120, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Helbig, L.; Fu, J.; Bian, X.; Herrmann, J.; Baumann, M.; Stewart, A.F.; Müller, R.; Li, A.; Zips, D.; et al. Expressing cytotoxic compounds in Escherichia coli Nissle 1917 for tumor-targeting therapy. Res. Microbiol. 2019, 170, 74–79. [Google Scholar] [CrossRef]
- Yu, X.; Lin, C.; Yu, J.; Qi, Q.; Wang, Q. Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy. Microb. Biotechnol. 2019, 13, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Secher, T.; Kassem, S.; Benamar, M.; Bernard, I.; Boury, M.; Barreau, F.; Oswald, E.; Saoudi, A. Oral administration of the probiotic strain Escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front. Immunol. 2017, 8, 1096. [Google Scholar] [CrossRef]
- Guo, S.; Chen, S.; Ma, J.; Ma, Y.; Zhu, J.; Ma, Y.; Liu, Y.; Wang, P.; Pan, Y. Escherichia coli Nissle 1917 protects intestinal barrier function by inhibiting NF-κB-mediated activation of the MLCK-P-MLC signaling pathway. Mediat. Inflamm. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, L.C.A.; Mendes, T.D.C.D.; Dos Santos, B.S.; Filho, V.D.M.S.; Lima, G.M.D.S.; De Araújo, J.M.; Correia, M.T.D.S.; De Oliveira, M.B.M.; Júnior, M.A.M.; Da Silva, M.V. Molecular identification and physiological characterization of Zymomonas mobilis strains from fuel-ethanol production plants in north-east Brazil. Lett. Appl. Microbiol. 2018, 67, 54–63. [Google Scholar] [CrossRef]
- Gao, X.; Gao, Q.; Bao, J. Improving cellulosic ethanol fermentability of Zymomonas mobilis by overexpression of sodium ion tolerance gene ZMO0119. J. Biotechnol. 2018, 282, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Shen, W.; Yan, X.; He, Q.; Cai, D.; Chen, S.; Wei, H.; Knoshaug, E.P.; Zhang, M.; Himmel, M.E.; et al. Metabolic engineering of Zymomonas mobilis for anaerobic isobutanol production. Biotechnol. Biofuels 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Karen, E.; Baptista, I.C.; Pereira, J.C.C.V.; Costa-Amaral, I.C.; da Costa, E.S.; Ribeiro, M.C.M.; Land, M.G.P.; Alves, T.L.M.; Larentis, A.L.; Almeida, R.V. Recombinant L-asparaginase from Zymomonas mobilis: A potential new antileukemic agent produced in Escherichia coli. PLoS ONE 2016, 11, e0156692. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.F.M.; Vasconcelos, J.; De Souza, J.R.; Coutinho, E.D.M.; Montenegro, S.M.L.; Azevedo-Ximenes, E. The effect of Zymomonas mobilis culture on experimental Schistosoma mansoni infection. Rev. Soc. Bras. Med. Trop. 2004, 37, 502–504. [Google Scholar] [CrossRef]
- Alade, A.A.; Bamgbose, A.M.; Oso, A.O.; Adewumi, B.A.; Jegede, A.V.; Anigbogu, N.M.; Agwunobi, L.N.; Ogunsola, I.A. Effects of dietary inclusion of Zymomonas mobilis degraded cassava sifting on growth performance, apparent nutrient digestibility, ileal digesta viscosity, and economy of feed conversion of broiler chickens. Trop. Anim. Health Prod. 2020, 52, 1413–1423. [Google Scholar] [CrossRef]
- Silva, A.T.D.A.; Cavalcanti, I.D.L.; Fernandes, M.A.D.L.; Coimbra, C.G.D.O.; Lima, G.M.D.S. Effect of Zymomonas mobilis probiotic on cholesterol and its lipoprotein fractions and the intestinal regulation. Clin. Nutr. 2020, 39, 3750–3755. [Google Scholar] [CrossRef] [PubMed]
- Campos, I.A.; Ximenes, E.A.; Júnior, C.H.R.C.; De Mesquita, A.R.C.; Silva, J.B.N.; Maia, M.B.S.; Franco, E.S.; Medeiros, P.L.; Peixoto, C.A.; Da Silva, T.G. Zymomonas mobilis culture protects against sepsis by modulating the inflammatory response, alleviating bacterial burden and suppressing splenocyte apoptosis. Eur. J. Pharm. Sci. 2013, 48, 1–8. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Development of new probiotics by strain combinations: Is it possible to improve the adhesion to intestinal mucus? J. Dairy Sci. 2007, 90, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef] [PubMed]
- Al-Muzafar, H.M.; Amin, K.A. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complement. Altern. Med. 2017, 17, 43. [Google Scholar] [CrossRef]
- Mohammadi, G.; Dargahi, L.; Naserpour, T.; Mirzanejad, Y.; Alizadeh, S.A.; Peymani, A.; Nassiri-Asl, M. Probiotic mixture of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 attenuates hippocampal apoptosis induced by lipopolysaccharide in rats. Int. Microbiol. 2019, 22, 317–323. [Google Scholar] [CrossRef]
- Trallero, G.O.; Serrano, H.L.; Inglés, B.M.; Vallés, D.R.; Rodríguez, A.M. Effect of the administration of a probiotic with a combination of Lactobacillus and Bifidobacterium strains on antibiotic-associated diarrhea. Rev. Esp. Quimioter. 2019, 32, 268–272. [Google Scholar]
- Deng, X.; Zheng, C.; Wang, S.; Yang, R.; Liu, Z.; Chen, T. Treatment with a probiotic combination reduces abdominal adhesion in rats by decreasing intestinal inflammation and restoring microbial composition. Oncol. Rep. 2020, 43, 986–998. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.-Y.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar] [CrossRef]
- Hu, C.; Xing, W.; Liu, X.; Zhang, X.; Li, K.; Liu, J.; Deng, B.; Deng, J.; Li, Y.; Tan, C. Effects of dietary supplementation of probiotic Enterococcus faecium on growth performance and gut microbiota in weaned piglets. AMB Express 2019, 9, 33. [Google Scholar] [CrossRef]
- Aljumaah, M.R.; Alkhulaifi, M.M.; Abudabos, A.M.; Aljumaah, R.S.; Alsaleh, A.N.; Stanley, D. Bacillus subtilis PB6 based probiotic supplementation plays a role in the recovery after the necrotic enteritis challenge. PLoS ONE 2020, 15, e0232781. [Google Scholar] [CrossRef] [PubMed]
- Do, H.J.; Chung, J.H.; Hwang, J.W.; Kim, O.Y.; Lee, J.-Y.; Shin, M.-J. 1-Deoxynojirimycin isolated from Bacillus subtilis improves hepatic lipid metabolism and mitochondrial function in high-fat–fed mice. Food Chem. Toxicol. 2015, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Q.; Li, Z.; Wang, H.; Li, J.; Wan, X. Fermented soybean powder containing Bacillus subtilis SJLH001 protects against obesity in mice by improving transport function and inhibiting angiogenesis. J. Funct. Foods 2019, 59, 60–70. [Google Scholar] [CrossRef]
- Algburi, A.; Al-Hasani, H.M.; Ismael, T.K.; Abdelhameed, A.; Weeks, R.; Ermakov, A.M.; Chikindas, M.L. Antimicrobial activity of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 against Staphylococcus aureus biofilms isolated from wound infection. Probiotics Antimicrob. Proteins 2020, 13, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bouallegue, A.; Casillo, A.; Chaari, F.; La Gatta, A.; Lanzetta, R.; Corsaro, M.M.; Bachoual, R.; Ellouz-Chaabouni, S. Levan from a new isolated Bacillus subtilis AF17: Purification, structural analysis and antioxidant activities. Int. J. Biol. Macromol. 2020, 144, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhen, W.; Geng, Y.; Wang, Z.; Guo, Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019, 9, 10256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Guo, C.; Geng, M.; Gai, S.; Qi, W.; Li, Z.; Song, Y.; Luo, X.; Zhang, T.; et al. Bacillus subtilis RZ001 improves intestinal integrity and alleviates colitis by inhibiting notch signaling pathway and activating ATOH-1. Pathog. Dis. 2020, 78, ftaa016. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, F.; Xu, D.; Zhang, Z.; Xu, F.; Tao, X.; Qiu, L.; Wei, H. Enterococcus faecium WEFA23 from infants lessens high-fat-diet-induced hyperlipidemia via cholesterol 7-alpha-hydroxylase gene by altering the composition of gut microbiota in rats. J. Dairy Sci. 2018, 101, 7757–7767. [Google Scholar] [CrossRef]
- Wu, C.; Ouyang, M.; Guo, Q.; Jia, J.; Liu, R.; Jiang, Y.; Wu, M.; Shen, S. Changes in the intestinal microecology induced by Bacillus subtilis inhibit the occurrence of ulcerative colitis and associated cancers: A study on the mechanisms. Am. J. Cancer Res. 2019, 9, 872–886. [Google Scholar] [PubMed]
- Guo, L.; Meng, M.; Wei, Y.; Lin, F.; Jiang, Y.; Cui, X.; Wang, G.; Wang, C.; Guo, X. Protective effects of live combined B. subtilis and E. faecium in polymicrobial sepsis through modulating activation and transformation of macrophages and mast cells. Front. Pharmacol. 2019, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Biadaioli, R.; Bertini, G.; Martelli, E.; Rubaltelli, F.F. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. Biol. Neonate 2002, 82, 103–108. [Google Scholar] [CrossRef]
- Tewari, V.V.; Dubey, S.K.; Gupta, G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: A randomized controlled trial. J. Trop. Pediatr. 2015, 61, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar] [CrossRef]
- Pehlevan, O.S.; Benzer, D.; Gursoy, T.; Karatekin, G.; Ovali, F. Synbiotics use for preventing sepsis and necrotizing enterocolitis in very low birth weight neonates: A randomized controlled trial. Clin. Exp. Pediatr. 2020, 63, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.; Garland, S.M.; the ProPrems Study Group. Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef]
- Demirel, G.; Celik, I.H.; Erdeve, O.; Saygan, S.; Dilmen, U.; Canpolat, F.E. Prophylactic Saccharomyces boulardii versus nystatin for the prevention of fungal colonization and invasive fungal infection in premature infants. Eur. J. Pediatr. 2013, 172, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.; Wang, Y.; Fu, J.; Sun, M.; Mao, Z.; Vandenplas, Y. A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCM I-745 in formula-fed preterm infants. J. Pediatr. (Rio J.) 2016, 92, 296–301. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Shi, L.; Feng, W.; Yi, K. Effect and Safety of Saccharomyces boulardii for Neonatal Necrotizing Enterocolitis in Pre-term Infants: A Systematic Review and Meta-Analysis. J. Trop. Pediatr. 2020, fmaa22. [Google Scholar] [CrossRef]
- Romeo, M.G.; Romeo, D.; Trovato, L.; Oliveri, S.; Palermo, F.; Cota, F.; Betta, P. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J. Perinatol. 2010, 31, 63–69. [Google Scholar] [CrossRef]
- Sinha, A.; Gupta, S.S.; Chellani, H.; Maliye, C.; Kumari, V.; Arya, S.; Garg, B.S.; Gaur, S.D.; Gaind, R.; Deotale, V.; et al. Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: A randomised controlled trial. BMJ Open 2015, 5, e006564. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Sailajanandan, P.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [Google Scholar] [CrossRef]
- Fortmann, I.; Marißen, J.; Siller, B.; Spiegler, J.; Humberg, A.; Hanke, K.; Faust, K.; Pagel, J.; Eyvazzadeh, L.; Brenner, K.; et al. Lactobacillus Acidophilus/Bifidobacterium infantis probiotics are beneficial to extremely low gestational age infants fed human milk. Nutrients 2020, 12, 850. [Google Scholar] [CrossRef]
- Angurana, S.K.; Bansal, A.; Singhi, S.; Aggarwal, R.; Jayashree, M.; Salaria, M.; Mangat, N.K. Evaluation of effect of probiotics on cytokine levels in critically Ill children with severe sepsis. Crit. Care Med. 2018, 46, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Cavicchiolo, M.E.; Magnani, M.; Calgaro, S.; Bonadies, L.; Castagliulo, I.; Morelli, L.; Verlato, G.; Baraldi, E. Neonatal sepsis associated with Lactobacillus supplementation. J. Perinat. Med. 2019, 48, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; McNaught, C.E.; Anderson, A.D.; MacFie, J.; Mitchell, C.J. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: A randomised controlled trial. Clin. Nutr. 2004, 23, 467–475. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, J.-C.; Du, J.; Zhang, L.-M.; Yin, H.-H. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: A prospective randomized pilot study. Crit. Care 2011, 15, R290. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Yamada, T.; Ogura, H.; Mohri, T.; Kiguchi, T.; Fujimi, S.; Asahara, T.; Ojima, M.; Ikeda, M.; Shimazu, T. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: A randomized controlled trial. Crit. Care 2018, 22, 239. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Huang, M.; Tong, C.; Zhang, X.; Wang, L.; Peng, H.; Lan, P.; Zhang, P.; Huang, N.; et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: A double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015, 15, 34. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Bengmark, S.; Kanellakopoulou, K.; Kotzampassi, K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J. Trauma 2009, 67, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.H.; Adiamah, A.; Kushairi, A.; Varadhan, K.K.; Krznaric, Z.; Kulkarni, A.D.; Neal, K.R.; Lobo, D.N. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. Ann. Surg. 2020, 271, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Rijnders, B.J.; Van Wijngaerden, E.; Verwaest, C.; Peetermans, W.E. Saccharomyces fungemia complicating Saccharomyces boulardii treatment in a non-immunocompromised host. Intensive Care Med. 2000, 26, 825. [Google Scholar] [CrossRef]
- Cherifi, S.; Robberecht, J.; Miendje, Y. Saccharomyces cerevisiae fungemia in an elderly patient with Clostridium difficile colitis. Acta Clin. Belg. 2004, 59, 223–224. [Google Scholar] [CrossRef]
- Nikolaos, L.; Dimitrios, V.; Hellen, M.; Sofia, K.; Aristea, V.; Charis, T.; Chronis, T.; Angellos, P.; Ioannis, P. Saccharomyces boulardii fungaemia in an intensive care unit patient treated with caspofungin. Crit. Care 2008, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Stefanatou, E.; Paridou, A.; Koutsodimitropoulos, I.; Giannopoulou, P.; Markou, N.; Kalofonou, M.; Tsidemiadou, F.; Trikka-Graphakos, E.; Kompoti, M. Probiotic sepsis due to Saccharomyces fungaemia in a critically ill burn patient. Mycoses 2011, 54, e643–e646. [Google Scholar] [CrossRef]
- Thygesen, J.B.; Glerup, H.; Tarp, B. Saccharomyces boulardii fungemia caused by treatment with a probioticum. BMJ Case Rep. 2012, bcr0620114412. [Google Scholar] [CrossRef]
- Chioukh, F.; Ben Hmida, H.; Ben Ameur, K.; Toumi, A.; Monastiri, K. Septicémie à Saccharomyces cerevisiae chez un prématuré traité par Ultra-Levure® [Saccharomyces cerevisiae fungemia in a premature neonate treated receiving probiotics]. Med. Mal. Infect. 2013, 43, 359–360. [Google Scholar] [CrossRef]
- Appel-Da-Silva, M.C.; Narvaez, G.A.; Perez, L.R.; Drehmer, L.; Lewgoy, J. Saccharomyces cerevisiae var. boulardii fungemia following probiotic treatment. Med. Mycol. Case Rep. 2017, 18, 15–17. [Google Scholar] [CrossRef]
- Atıcı, S.; Soysal, A.; Cerit, K.K.; Yılmaz, Ş.; Aksu, B.; Kıyan, G.; Bakır, M. Catheter-related Saccharomyces cerevisiae Fungemia Following Saccharomyces boulardii Probiotic Treatment: In a child in intensive care unit and review of the literature. Med. Mycol. Case Rep. 2017, 15, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.W.; Tonner, R.; Trivedi, J.; Miller, H.; Lee, R.; Liang, X.; Rotello, L.; Isenbergh, E.; Anderson, J.; Perl, T.; et al. Saccharomyces boulardii probiotic-associated fungemia: Questioning the safety of this preventive probiotic’s use. Diagn. Microbiol. Infect. Dis. 2017, 87, 286–288. [Google Scholar] [CrossRef]
- Krahenbühl, T.; De Fátima Guimarães, R.; De Azevedo Barros Filho, A.; Gonçalves, E.M. Saccharomyces cerevisiae fungemia in a pediatric patient after treatment with probiotics. Rev. Paul. Pediatr. 2017, 35, 361–364. [Google Scholar] [CrossRef]
- Roy, U.; Jessani, L.G.; Rudramurthy, S.M.; Gopalakrishnan, R.; Dutta, S.; Chakravarty, C.; Jillwin, J.; Chakrabarti, A. Seven cases of Saccharomyces fungaemia related to use of probiotics. Mycoses 2017, 60, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Fadhel, M.; Patel, S.; Liu, E.; Levitt, M.; Asif, A. Saccharomyces cerevisiae fungemia in a critically ill patient with acute cholangitis and long term probiotic use. Med. Mycol. Case Rep. 2018, 23, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Kara, I.; Yıldırım, F.; Özgen, Ö.; Erganiş, S.; Aydoğdu, M.; Dizbay, M.; Gürsel, G.; Kalkanci, A. Saccharomyces cerevisiae fungemia after probiotic treatment in an intensive care unit patient. J. Mycol. Med. 2018, 28, 218–221. [Google Scholar] [CrossRef]
- Chakravarty, S.; Parashar, A.; Acharyya, S. Saccharomyces cerevisiae Sepsis Following Probiotic Therapy in an Infant. Indian Pediatr. 2019, 56, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Landaburu, M.F.; Daneri, G.A.L.; Relloso, S.; Zarlenga, L.J.; Vinante, M.A.; Mujica, M.T. Fungemia following Saccharomyces cerevisiae var. boulardii probiotic treatment in an elderly patient. Rev. Argent. Microbiol. 2020, 52, 27–30. [Google Scholar] [CrossRef]
- Antony, S.J. Lactobacillemia: An emerging cause of infection in both the immunocompromised and the immunocompetent host. J. Natl. Med. Assoc. 2000, 92, 83. [Google Scholar] [PubMed]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Jarvinen, A. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef]
- Kunz, A.N.; Noel, J.M.; Fairchok, M.P. Two Cases of Lactobacillus Bacteremia during Probiotic Treatment of Short Gut Syndrome. J. Pediatr. Gastr. Nutr. 2004, 38, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Land, M.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005, 115, 178–181. [Google Scholar] [CrossRef] [PubMed]
- De Groote, M.A.; Frank, D.N.; Dowell, E.; Glode, M.P.; Pace, N.R. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr. Infect. Dis. J. 2005, 24, 278–280. [Google Scholar] [CrossRef]
- LeDoux, D.; LaBombardi, V.J.; Karter, D. Lactobacillus acidophilus bacteraemia after use of a probiotic in a patient with AIDS and Hodgkin’s disease. Int. J. STD AIDS 2006, 17, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Kochan, P.; Chmielarczyk, A.; Szymaniak, L.; Brykczynski, M.; Galant, K.; Zych, A.; Pakosz, K.; Giedrys-Kalemba, S.; Lenouvel, E.; Heczko, P.B. Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient—Is the time right to revise probiotic safety guidelines? Clin. Microbiol. Infect. 2011, 17, 1589–1592. [Google Scholar] [CrossRef]
- Vahabnezhad, E.; Mochon, A.B.; Wozniak, L.J.; Ziring, D.A. Lactobacillus Bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J. Clin. Gastroenterol. 2013, 47, 437–439. [Google Scholar] [CrossRef]
- Sadowska-Krawczenko, I.; Paprzycka, M.; Korbal, P.; Wiatrzyk, A.; Krysztopa-Grzybowska, K.; Polak, M.; Czajka, U.; Lutyńska, A. Lactobacillus rhamnosus GG suspected infection in a newborn with intrauterine growth restriction. Benef. Microb. 2014, 5, 397–402. [Google Scholar] [CrossRef]
- Coviello, C.C.; Corsini, I.; Arena, F.; Antonelli, A.; Rossolini, G.M.; Dani, C. Lactobacillus sepsis and probiotic therapy in newborns: Two new cases and literature review. AJP Rep. 2015, 6, e25–e29. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, L.; Crum-Cianflone, N.F. The potential risks of probiotics among HIV-infected persons: Bacteraemia due to Lactobacillus acidophilus and review of the literature. Int. J. STD AIDS 2016, 27, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Brecht, M.; Garg, A.; Longstaff, K.; Cooper, C.; Andersen, C. Lactobacillus Sepsis following a Laparotomy in a Preterm Infant: A Note of Caution. Neonatology 2016, 109, 186–189. [Google Scholar] [CrossRef]
- Cui, X.; Shi, Y.; Gao, S.; Xue, X.; Fu, J. Effects of Lactobacillus reuteri DSM 17938 in preterm infants: A double-blinded randomized controlled study. Ital. J. Pediatr. 2019, 45, 1–7. [Google Scholar] [CrossRef]
- Yelin, I.; Flett, K.B.; Merakou, C.; Mehrotra, P.; Stam, J.; Snesrud, E.; Hinkle, M.; Lesho, E.; McGann, P.; McAdam, A.J.; et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 2019, 25, 1728–1732. [Google Scholar] [CrossRef]
- Koyama, S.; the Yokohama Cooperative Study Group for Hematology (YACHT); Fujita, H.; Shimosato, T.; Kamijo, A.; Ishiyama, Y.; Yamamoto, E.; Ishii, Y.; Hattori, Y.; Hagihara, M.; et al. Septicemia from Lactobacillus rhamnosus GG, from a probiotic enriched yogurt, in a patient with autologous stem cell transplantation. Probiotics Antimicrob. Proteins 2019, 11, 295–298. [Google Scholar] [CrossRef]
- Ohishi, A.; Takahashi, S.; Ito, Y.; Ohishi, Y.; Tsukamoto, K.; Nanba, Y.; Ito, N.; Kakiuchi, S.; Saitoh, A.; Morotomi, M.; et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J. Pediatr. 2010, 156, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Jenke, A.; Ruf, E.-M.; Hoppe, T.; Heldmann, M.; Wirth, S. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Arch. Dis. 2012, 97, F217–F218. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Pillonel, T.; Torregrossa, A.; Prod’Hom, G.; Fischer, C.J.; Greub, G.; Giannoni, E. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clin. Infect. Dis. 2015, 60, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Esaiassen, E.; Cavanagh, P.; Hjerde, E.; Simonsen, G.S.; Støen, R.; Klingenberg, C. Bifidobacterium longum subspecies infantis bacteremia in 3 extremely preterm infants receiving probiotics. Emerg. Infect. Dis. 2016, 22, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Uchida, T.; Kuwana, S.; Sasaki, K.; Watanabe, T.; Saito, J.; Kawaji, T. Bacteremia induced by Bifidobacterium breve in a newborn with cloacal exstrophy. Pediatr. Int. 2016, 58, 1226–1228. [Google Scholar] [CrossRef]
- Pruccoli, G.; Silvestro, E.; Napoleone, C.P.; Aidala, E.; Garazzino, S.; Scolfaro, C. Are probiotics safe? Bifidobacterium bacteremia in a child with severe heart failure. Infez. Med. 2019, 27, 175–178. [Google Scholar] [PubMed]
- Guenther, K.; Straube, E.; Pfister, W.; Guenther, A.; Huebler, A. Sever sepsis after probiotic treatment with Escherichia coli NISSLE 1917. Pediatr. Infect. Dis. J. 2010, 29, 188–189. [Google Scholar] [CrossRef]
- Papanikolaou, M.N.; Balla, M.; Papavasilopoulou, T.; Kofinas, G.; Karatzas, S. Probiotics: An obedient ally or an insidious enemy? Crit. Care 2012, 16, 456. [Google Scholar] [CrossRef]
- Princess, I.; Natarajan, T.; Ghosh, S. When good bacteria behave badly: A case report of Bacillus clausii sepsis in an immunocompetant adult. Access Microbiol. 2020, 2, e000097. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.W.; Greer, F.R. American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics 2010, 126, 1217–1231. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.G.; Ganea, D.; Gamero, A.M. Cecal ligation puncture procedure. J. Vis. Exp. 2011, e2860. [Google Scholar] [CrossRef]
- Alverdy, J.C.; Keskey, R.; Thewissen, R. Can the Cecal Ligation and Puncture Model Be Repurposed to Better Inform Therapy in Human Sepsis? Infect. Immun. 2017, 88, e00942-19. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).