Abstract
Quorum sensing (QS) is a form of bacterial communication involved in the production of virulence factors in many species. As a result, inhibition of quorum sensing may be of use in mitigating pathogenesis. The signaling molecule indole is currently being investigated as a target for quorum sensing inhibition (QSI) and the indole derivative indole-3-carboxaldehyde (ICA) has been shown to inhibit quorum sensing-mediated behaviors in Escherichia coli. In this study, we investigate bromination as a method of increasing the QSI capabilities of indole carboxaldehydes. The IC50 values of three monobrominated indole carboxaldehydes (5-bromoindole-3-carboxaldehyde, 6-bromoindole-3-carboxaldehyde, and 7-bromoindole-3-carboxaldehyde) were determined and compared to the IC50 value of ICA. The bromination of these indole carboxaldehydes reduced the IC50 values between 2- and 13-fold, indicating that bromination significantly increases the potency of these indole carboxaldehydes.
1. Introduction
Quorum sensing (QS) is a type of intercellular communication used by many bacterial species wherein bacteria produce and secrete signaling molecules to affect changes in a bacterial population. As the population of bacteria grows, the concentration of signaling molecules secreted into the environment increases. Once the signaling molecules reach a critical threshold concentration, the molecules saturate their respective receptors on, or in, the bacteria, altering gene expression [1]. Quorum sensing mediates a wide range of bacterial behaviors. These behaviors include biofilm formation, biosynthesis of virulence factors, and antibiotic resistance. Since QS is frequently involved in the activation of pathogenesis, inhibition of quorum sensing provides a potential treatment mechanism for bacterial infections [2]. Inhibition of these QS systems may be accomplished in a variety of ways. These methods include inhibition of signal molecule synthesis, impairment of signal function, or interference with signal receptors [3].
One of the more common quorum sensing systems in bacteria is the N-acyl homoserine lactone (AHL) class of signaling molecules. In the AHL system, the signaling molecules are produced by LuxI-type synthases and bind to the receptor LuxR. LuxR then acts as a transcription factor to modify gene expression in the cell [2]. In Vibrio fischeri, a bioluminescent species known for its symbiosis with the Hawaiian bobtail squid, LuxR activates the operon coding for the enzyme luciferase, which catalyzes a reaction that produces the bacterium’s signature bioluminescence [4]. The opportunistic pathogen Pseudomonas aeruginosa also utilizes the AHL system as it interacts with other quorum sensing systems to regulate the production of virulence factors and biofilms [1]. Although Escherichia coli cells are Gram-negative, like the preceding examples, they do not produce AHLs as signaling molecules. However, they are able to utilize the LuxR homologue SdiA to respond to AHLs produced by other bacteria. Activation of SdiA, through indole, or homoserine lactones, has been shown to inhibit virulence, chemotaxis, and motility in E. coli [5].
Indoles, products of tryptophan metabolism, are intercellular and intracellular signaling molecules utilized by E. coli, other species of bacteria, and many eukaryotic organisms [6]. Further, indole and indole byproducts are produced by Lactobacilli spp. and other human commensal bacteria. These indole byproducts help influence the activity of the human immune system by regulating T cells, lymphocytes, and inflammatory processes. This enables commensal bacteria to outcompete pathogenic bacteria in the human gut [7]. In E. coli, the effects of indole vary widely depending on environmental conditions [8]. In one study, high concentrations of indole and indole-3-carboxaldehyde reduced the virulence of enteropathogenic E. coli (EPEC) through the inhibition of the enterocyte effacement (LEE) genes involved in pathogenicity [6]. It was found that a carboxaldehyde functional group was vital to the ability of indoles to inhibit pathogenicity in this example. Indole-3-carboxaldehyde also reduced mortality and morbidity in mice infected with Citrobacter rodentium [6]. Based on these properties, indole and its derivatives, especially indole carboxaldehydes, may be useful in treating E. coli infections by interfering with normal indole signaling.
Functional alteration of indole carboxaldehydes may increase their effectiveness as quorum sensing inhibitors (QSIs). Previous work has shown that bromination of biologically active compounds, such as pharmaceuticals, can increase their effectiveness [9]. This increase in activity may occur through a variety of mechanisms such as helping a compound to penetrate the cell membrane more effectively, reducing the degradation of the compound, or increasing the affinity of the compound for a receptor’s binding pocket [9]. Due to these potential effects, bromination may increase the potency of indole carboxaldehydes as QSIs.
In order to examine if this was the case, the effects of three differently brominated indole carboxaldehydes on QS were evaluated in this study. The compounds used were 5-bromoindole-3-carboxaldehyde, 6-bromoindole-3-carboxaldehyde, and 7-bromoindole-3-carboxaldehyde. None of these compounds have previously been reported as QSIs. Although indole carboxaldehydes as a group have been shown to affect QS-mediated behaviors, such as biofilm formation and production of virulence factors [8], the effect of bromination on quorum sensing inhibition has not been studied. This study examines different bromination patterns of indole carboxaldehydes to determine how bromination affects quorum sensing inhibition in Chromobacterium violaceum, an organism previously used as a reporter for AHL-based QS.
2. Materials and Methods
2.1. Test Compounds
To determine the effect of bromine substitution on QS inhibition of indole carboxaldehydes, 5-bromoindole-3-carboxaldehyde, 6-bromoindole-3-carboxaldehyde, and 7-bromoindole-3-carboxaldehyde were tested for quorum sensing inhibition activity. Indole-3-carboxaldehyde was used as a control (Figure 1). All compounds were purchased from Sigma-Aldrich (St. Lewis, MO, USA).
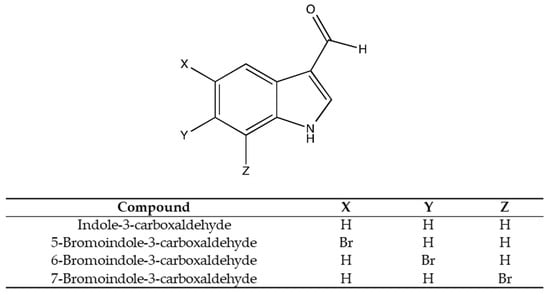
Figure 1.
Test compounds and structures.
2.2. Model Organism
Chromobacterium violaceum is a Gram-negative species that uses the AHL quorum sensing system to regulate the production of the purple pigment violacein. When QS is active, the bacteria produce violacein, giving the colonies their distinctive purple hue. When QS is inhibited, the bacteria stop producing violacein and the colonies turn white [10].
2.3. Quorum Sensing Inhibition Screening
Preliminary screening was conducted using a disk diffusion assay and sought to determine whether the selected brominated indole carboxaldehydes inhibited quorum sensing in C. violaceum without inhibiting growth [11]. Briefly, 1 mg of each compound was dissolved in ethanol and applied to blank disks. The ethanol was then allowed to evaporate. Following evaporation, these discs were placed on Lysogeny Broth (LB) agar plates and overlaid with 5 mL of 0.5% LB agar containing 5 µL of overnight C. violaceum culture. One plate was prepared for each compound during initial screening. Plates were incubated overnight at 30 °C and were then examined for zones of quorum sensing inhibition and growth inhibition. A positive result was indicated by the presence of quorum sensing inhibition without visible growth inhibition. Positive results were re-evaluated two more times by the above method while negative results were not pursued at this time.
2.4. Quantification of Quorum Sensing Inhibition
The Skogman et al. (2016) protocol [12] was used to determine the concentration necessary to inhibit 50% of QS (IC50) for compounds with a positive result in the preliminary disk assay. Briefly, inhibition of QS was determined by the reduction in violacein production. A 10−3 dilution was made of an overnight culture of C. violaceum with the original optical density adjusted to 0.7 OD595 before said dilution. Compounds were dissolved in ethanol to achieve concentrations of 40 mM, 20 mM, 10 mM, and 5 mM. A 96-well plate was prepared with 198 µl of diluted C. violaceum and 2 µL of the appropriate compound concentration to reach the final compound concentrations of 400 µM, 200 µM, 100 µM, and 50 µM. Four replicates were performed for each concentration. For the control, 2 µl of ethanol was added to C. violaceum cultures. Plates were incubated overnight at 30 °C. After incubation, plates were centrifuged at 3000 rpm for 10 min, the supernatant was removed from each well, and 200 µL of EtOH was added. The sides of the wells were scraped to dislodge the biofilms and to enhance violacein extraction. After scraping, the wells were allowed to sit for 15 min to extract the pigment. The plate was then centrifuged at 3000 rpm for 10 min to pellet the cells. The supernatant (100 µL) containing the dissolved pigment was transferred to a clean microplate and the absorbance was measured at 595 nm [12].
2.5. Statistical Analysis
Absorbance was averaged for each concentration, accounting for the background absorbance of ethanol. Average absorbances were then plotted against concentration and exponential regressions were used to calculate the IC50 value for each compound. Three plates were prepared for each compound and the IC50 value was calculated for each plate. The individual IC50 values were used to calculate the average IC50 value for each compound. The percent reduction from the control was calculated for each test compound. A one-way ANOVA with a post hoc Tukey HSD test was performed to compare the potencies of the compounds.
3. Results
The effects of monobrominated indole carboxaldehydes 5-bromoindole-3-carboxaldehyde, 6-bromoindole-3-carboxaldehyde, and 7-bromoindole-3-carboxaldehyde on QS in C. violaceum were tested using a disk diffusion assay. Although these compounds have not been previously reported as QSIs, 5-bromoindole-3-carboxaldehyde has been shown to inhibit bioluminescence in Vibrio fischeri. This inhibition was attributed to the toxicity of the compound by the authors [11]. However, bioluminescence in V. fischeri is mediated by an AHL system [4]. Therefore, it is possible that the bioluminescence inhibition was due to quorum sensing inhibition instead of growth inhibition alone. Our results today seem to indicate that inhibition of QS being the cause, rather than toxicity, is supportable.
In our work, all three monobrominated indole carboxaldehyde compounds and the control exhibited QS inhibition, as indicated by the presence of white colonies surrounding the applied compounds with no growth inhibition observed (Figure S1). Of the compounds, 7-bromoindole-3-carboxaldehyde exhibited the largest zone of quorum sensing inhibition, followed by 5-bromoindole-3-carboxaldehyde, and then 6-bromoindole-3-carboxaldehyde (Table 1). This preliminary assay indicated, but could not confirm, the relative potential of each compound as a QSI, as the diffusion of the compound through the agar may be affected by many factors. Consequently, the diameter of the zone of inhibition cannot necessarily be correlated with compound potency. Rather, potency was determined by the IC50 value.

Table 1.
Average diameter of zone of quorum sensing inhibition. 7-bromoindole-3-carboxaldehyde exhibited the greatest diameter. (A) Indole-3-carboxaldehyde, (B) 5-bromoindole-3-carboxaldehyde, (C) 6-bromoindole-3-carboxaldehyde, (D) 7-bromoindole-3-carboxaldehyde.
For indole-3-carboxaldehyde, the average IC50 value was 171 µM. For 5-bromo-3-carboxaldehyde, the average IC50 value was 13 µM, which represents a 13-fold reduction over the control. For 6-bromo-3-carboxaldehyde, the average IC50 value was 19 µM and was 9-fold reduced compared to the control. For 7-bromoindole-3-carboxaldehyde, the average IC50 value was 72 µM and was a 2-fold reduction when compared to the control (Figure 2, Table 2). One-way ANOVA tests indicated that the amount of brominated indoles necessary to inhibit 50% of quorum sensing was significantly decreased from the control (F = 39.6285, P = 3.80 × 10−5). Post hoc Tukey HSD analysis indicated that the IC50 values of all brominated indoles were significantly decreased from indole-3-carboxaldehyde (QAB = 13.6296, PAB = 0.001; QAC = 13.0513, PAC = 0.001; QAD = 8.5804, PAD = 0.001). The IC50 of 5-bromoindole-3-carboxaldehyde was significantly decreased from the IC50 of 7-bromoindole-3-carboxaldehyde, indicating an effect of bromine position on IC50.
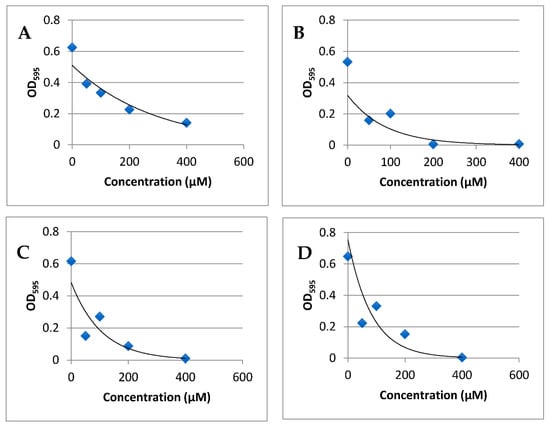
Figure 2.
Absorbance at OD595 for varying concentrations of brominated indole carboxaldehydes. For all compounds, absorbance decreased with increasing concentration. (A) Indole-3-carboxaldehyde, (B) 5-Bromoindole-3-carboxaldehyde, (C) 6-Bromoindole-3-carboxaldehyde, (D) 7-Bromoindole-3-carboxaldehyde.

Table 2.
Average IC50 values of brominated indole carboxaldehydes and fold reduction in IC50 from the control. The control, indole-3-carboxaldehyde, had the highest IC50 value. Test compound 5-bromindole-3-carboxaldehyde had the lowest IC50 value and the greatest reduction from the control. Test compound 7-bromoindole-3-carboxaldehyde had the highest IC50 value and the smallest reduction from the control.
4. Discussion
Since bromination increased QS inhibition in the indole carboxaldehydes in our model system, it may also increase the ability of indole carboxaldehydes to inhibit other indole-mediated processes such as biofilm formation in E. coli (and related Gram-negative bacteria). The mechanism by which bromine can increase the effectiveness of indole’s QS inhibition is still somewhat ambiguous. It may be due to bromine’s ability to increase the permeability across bacterial cell membranes, or change the membrane potential [8], thereby allowing more indole molecules to cross into the cell. There are previous reports of indole augmenting the gene expression of some bacterial species [9], but it is clear from these results that the added bromine molecule is at least non-hindering and, more likely, helpful in the QSI action of indole.
The fact that different bromine positions correspond with differing increases in QS inhibition may indicate that the indole molecule is directly affecting the interaction of indole with the AHL receptors. Indoles have previously been shown to be direct agonists for some transcription factor receptors [7]. Therefore, the brominated derivatives may also be interacting with the AHL receptor. Further, at high concentrations, indole can inhibit the detection of AHL by some receptors [13], making it possible that by adding bromine, which has the ability to form x-bonds [14], the molecules were better able to interact with a portion of the AHL receptor active site. Additionally, as bromine is quite electronegative, it may be augmenting a bond that already forms within an AHL receptor, making said bond stronger due to the stabilizing ability of highly electronegative species.
5. Conclusions
From this study, we conclude that bromination of indole carboxaldehydes can increase the QSI action of these compounds. Further, the position of such bromination has shown varying degrees of increase in this activity, indicating that position and not just bromination is important in the QSI of these compounds. More studies to understand how the molecule indole, as well as its brominated derivatives, disrupts normal QS functions will need to be conducted. Additionally, as the effects of these molecules in inhibiting QS have been demonstrated in our model system, we anticipate furthering this work in potentially pathogenic organisms.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microbiolres12020025/s1, Figure S1: Inhibition of QS-mediated violacein production in C. violaceum tested in disk diffusion assay.
Author Contributions
Conceptualization, C.A.K., D.B., and P.J.T.J.; methodology, D.B. and D.K.M.; software, P.J.T.J.; validation, C.A.K. and D.K.M.; formal analysis, C.A.K. and P.J.T.J.; investigation, C.A.K. and D.K.M.; data curation, P.J.T.J.; writing—original draft preparation, C.A.K., D.K.M.; writing—review and editing, D.B. and P.J.T.J.; supervision, D.B. and P.J.T.J.; project administration, D.B. and P.J.T.J.; funding acquisition, D.B. and P.J.T.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of North Georgia Center for Undergraduate Research and Creative Activities (CURCA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to funding source limitations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galloway, W.R.; Hodgkinson, J.T.; Bowden, S.; Welch, M.; Spring, D.R. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends Microbiol. 2012, 20, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Rampioni, G.; Leoni, L.; Williams, P. The art of antibacterial warfare: Deception through interference with quorum sensing–mediated communication. Bioorg. Chem. 2014, 55, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miyashiro, T. Quorum sensing in the squid-vibrio symbiosis. Int. J. Mol. Sci. 2013, 14, 16386–16401. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jayaraman, A.; Wood, T.K. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 2007, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, B.; Anyanful, A.; Izrayelit, Y.; Bhatt, S.; Cartwright, E.; Wang, W.; Swimm, A.I.; Benian, G.M.; Schroeder, F.C.; Kalman, D. A Family of indoles regulate virulence and shiga toxin production in pathogenic E. coli. PLoS ONE 2013, 8, e54456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Minvielle, M.J.; Melander, C. Controlling bacterial behavior with indole-containing natural products and derivatives. Tetrahedron 2014, 70, 6363–6372. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; El-Alfy, A.; Ezel, K.; Radwan, M.; Shilabin, A.; Kochanowska-Karamyan, A.; Abd-Alla, H.; Otsuka, M.; Hamann, M. Marine inspired 2-(5-Halo-1H-indol-3-yl)-N,N-dimethylethanamines as modulators of serotonin receptors: An example illustrating the power of bromine as Part of the uniquely marine chemical space. Mar. Drugs 2017, 15, 248. [Google Scholar] [CrossRef] [PubMed]
- Mclean, R.J.; Pierson, L.S.; Fuqua, C. A simple screening protocol for the identification of quorum signal antagonists. J. Microbiol. Methods 2004, 58, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Reineke, N.; Biselli, S.; Franke, S.; Francke, W.; Heinzel, N.; Hühnerfuss, H.; Iznaguen, H.; Kammann, U.; Theobald, N.; Vobach, M. Brominated indoles and phenols in marine sediment and water extracts from the North and Baltic Seas–Concentrations and effects. Arch. Environ. Contam. Toxicol. 2006, 51, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Skogman, M.; Kanerva, S.; Manner, S.; Vuorela, P.; Fallarero, A. Flavones as quorum sensing inhibitors identified by a newly optimized screening platform using Chromobacterium violaceum as reporter bacteria. Molecules 2016, 21, 1211. [Google Scholar] [CrossRef] [PubMed]
- Sabag-Daigle, A.; Soares, J.A.; Smith, J.N.; Elmasry, M.E.; Ahmer, B.M.M. The acyl homoserine lactone receptor, SdiA, of Escherichia coli and Salmonella enterica serovar typhimurium does not respond to indole. Appl. Environ. Microbiol. 2012, 78, 5424–5431. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.; Cui, D.; Yao, Z.; Gao, B.; Lin, J.; Wei, D. The important role of halogen bond in substrate selectivity of enzymatic catalysis. Sci. Rep. 2016, 6, 34750. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).