Abstract
Background: Methicillin-resistant Staphylococcus aureus (MRSA) colonization is a risk factor for potential staphylococcal infection and outbreaks. Although it is recommended to obtain a swab culture to detect nasal colonization its necessity in low-prevalence countries is debated. The aim of this study was to determine the prevalence of MRSA nasal colonization, the rate of invasive infection development, and the risk factors for invasive infections in patients admitted to the intensive care unit. Materials and Methods: This retrospective study included patients who were followed up in one of the adult intensive care units at Kayseri City Training and Research Hospital between 1 January 2019 and 31 December 2024 (6 years) and from whom a culture was taken at the time of hospital admission to detect MRSA colonization in the nose. MRSA carriers were examined for the development of any invasive infection caused by MRSA within 28 days of their relevant admission. Results: Over a total period of six years, nasal swab samples were collected from 22,913 patients, and MRSA colonization was detected in 939 (4.0%). Of the patients with MRSA colonization, 32 (3.4%) were excluded from the analysis because they already had invasive MRSA infection. Additionally, 431 patients (45.8%) were excluded from the analysis because they were discharged or died within the first seven days of their admission. Consequently, invasive MRSA infection developed within 28 days in 29 of the 476 patients with MRSA colonization (6.0%). Patients who developed invasive infection had a higher rate of chronic renal failure (p < 0.001), hemodialysis (p < 0.001), central venous catheter (p = 0.028), staying in nursing home (p = 0.001), and a history of hospitalization within the last 90 days (p = 0.015). In the multivariable regression analysis, routine hemodialysis (OR: 5.216, p = 0.015), nursing home stay (OR: 3.668, p = 0.014), and a history of hospitalization within the last 90 days (OR: 2.458, p = 0.028) were found to be risk factors for developing invasive infection. The most common invasive infections were ventilator-associated pneumonia (n = 9), surgical site infection (n = 7), and catheter-related bloodstream infection (n = 6). All 29 strains were susceptible to vancomycin, linezolid, and daptomycin, while one strain was resistant to teicoplanin (3.5%). Conclusions: MRSA colonization has been detected in 4% of patients admitted to the intensive care unit. Screening should be performed because MRSA colonization may be a risk factor for invasive infections; however, screening all patients would be prohibitively expensive and labor-intensive. Instead, it may be more appropriate to identify risk factors and then screen select patients.
1. Introduction
Staphylococcus aureus is one of the most common causative agents of healthcare-associated infections recently [1]. Hospital infections caused by S. aureus have become an increasingly significant problem in recent years due to their multidrug resistance to methicillin and many other antibiotics [2]. Methicillin-Resistant S. aureus (MRSA) strains have become a serious global problem due to limited treatment options and high treatment costs [3]. In Türkiye, 12% of staphylococci isolated from an intensive care unit were MRSA [4]. However, according to the World Health Organization (WHO) report, MRSA rate in bloodstream infections in 2021 was over 30 percent [5]. Also, according to the Antimicrobial resistance surveillance in Europe report, the proportion of MRSA strains exceeded 30% in our country [6].
Colonization with S. aureus is the first step in a potential staphylococcal infection. In nasal carrier patients, these bacteria can colonize the skin and mucous membranes and spread to the patient from vascular access sites, causing infection [7]. In particular, it has been found that the incidence of S. aureus colonization and infection is significantly increased in patients with S. aureus nasal colonization in intensive care units. It has been reported that failure to take the necessary infection control measures after admitting a patient colonized with MRSA to the intensive care unit can lead to an outbreak [8].
To prevent possible outbreaks and invasive infections, it is recommended that swab cultures be taken to detect nasal colonization during hospital admission and that isolation be applied to patients carrying MRSA. In regions with low prevalence, this screening is not recommended as it is not cost-effective [9,10,11]. Although there are facilities in our country that perform MRSA screening, the prevalence rate among hospitalised patients, the rate of invasive infections caused by MRSA in patients defined as carriers, and the frequency of infection development from colonisation are unknown.
The aim of this study is to determine the prevalence of nasal MRSA colonization among patients admitted to the adult intensive care units of our hospital and to identify risk factors for the development of infection from MRSA colonization.
2. Materials and Methods
2.1. Study Design and Patients
This retrospective study was conducted in a tertiary care hospital with 175 adult intensive care beds and a total bed capacity of 1600.
Patients treated in the adult intensive care unit of Kayseri City Hospital between 1 January 2019 and 31 December 2024 (6 years) and tested for MRSA colonization with a nasal culture on admission were included.
Patient data were obtained from the records of the Infection Control Committee. Among these patients, those identified as MRSA carriers were evaluated for the development of any invasive MRSA-related infection within 28 days following the corresponding hospitalization. Those who developed MRSA infection were classified as “group 1,” while patients who did not develop invasive infection were classified as “group 2.” Invasive infections include all hospital-acquired infections, such as catheter-related or primary bloodstream infections, pneumonia or ventilator-associated pneumonia, and upper urinary tract infections.
In order to identify risk factors for the development of invasive infections in patients with nasal MRSA colonisation, a comparison was made between those with invasive infections and those without. Variables such as age, gender, comorbidities, concomitant infections and invasive procedures were analysed to determine their relationship with the development of infection.
Patients with invasive MRSA infection on admission to the intensive care unit, suspicion COVID-19, patients under the age of 18, and pregnant women were excluded from the study. Patients who were colonized with MRSA but died/discharged within the first 7 days of hospitalization were excluded from the study because analysis could not be performed.
2.2. Infection Control Precautions
In accordance with our hospital infection prevention protocol, swabs are taken from patients admitted to the intensive care unit during admission for MRSA screening. Patients who are confirmed to be MRSA carriers are placed in contact isolation. Contact isolation requires hand hygiene, wearing a gown and gloves.
2.3. Ethical Approval
This study was approved by local ethical committee (No:298 Date: 28 January 2025).
2.4. Microbiological Analysis
The swab samples taken from the patients were inoculated on blood agar (Salubris, İstanbul, Türkiye) medium and incubated at 35.5 °C to 37 °C for 24 to 48 h. The VITEK 2 system (Compact, bioMérieux, Marcy l'Etoile, France) was used to identify colonies growing on the medium. Antibiotic susceptibility testing was performed using the VITEK 2 system and Kirby–Bauer disk diffusion. The results were evaluated according to the recommendations of the European Committee on Antimicrobial Susceptibility Testing.
Weekly internal quality control strains are used for VITEK 2 Systems. Quality control tests for S. aureus are performed using the automated and standard Kirby–Bauer disk diffusion susceptibility test and the ETEST method (Bioanalyse, Ankara, Türkiye) with the ATCC 2913 Methicillin-Sensitive S. aureus strain.
2.5. Statistical Analysis
SPSS 22.0 (IBM Corp., Armonk, NY, USA) package program was used for statistical analysis. Categorical variables were expressed as numbers and percentages, and Chi-square was used for comparisons. For nonparametric data, Mann–Whitney U test was used between groups. Data that are not normally distributed will be displayed as median (min-max). In all analyses, p-value (two-tailed) < 0.05 will be considered statistically significant. Values with p < 0.05 were taken in binary logistic regression analysis.
3. Results
A total of 22,913 patients were tested with nasal swabs, and MRSA was isolated in 939 (4.0%) of them. Thirty-two of these patients had invasive MRSA infection and concurrent clinical culture growth on admission to intensive care unit. In addition, 431 patients who were discharged/exitus within the first seven days of hospitalization were excluded from the analysis. Of the 476 patients analysed, 29 (6.0%) developed invasive infections (Group 1), while 447 (94.0%) did not develop invasive infections (Group 2) within 28 days (Figure 1).
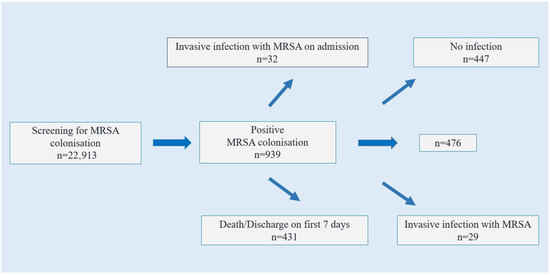
Figure 1.
Flowchart of patients included in the study.
The demographic data and invasive procedures of the patients are presented in Table 1. According to this, there was no statistically significant difference between group 1 and 2 in terms of age and gender. Chronic kidney disease was present in 41.4% (n = 12) of group 1 and 14.3% (n = 64) of group 2 (p < 0.001). The proportion of patients undergoing routine hemodialysis was 20.7% (n = 6) in group 1 and 2.2% (n = 10) in group 2 (p < 0.001).

Table 1.
Features and risk factors of patients.
Central venous catheters were used in 96.6% (n = 28) of group 1 and 80.1% (n = 358) of group 2. The use of intubation, urinary catheterization, surgery, tracheostomy and percutaneous endoscopic gastrostomy was statistically similar between the groups (p > 0.05).
The rate of staying in a nursing home was 31.0% (n = 9) in group 1 and 6.5% (n = 29) in group 2 (p < 0.001). The rate of hospitalization in the last 90 days was 48.3% (n = 14) in group 1 and 27.3% (n = 122) in group 2 (p = 0.015).
According to multivariable regression analysis, routine hemodialysis (OR: 5.216, p = 0.015), staying in a nursing home (OR: 3.668, p = 0.014), and a history of hospitalization in the last 90 days (OR: 2.458, p = 0.028) were found to be risk factors for the development of invasive infection in MRSA carriers.
The most common invasive infections were ventilator-associated pneumonia (n = 9), surgical site infection (n = 7), and catheter-related bloodstream infection (n = 6) (Figure 2). All 29 strains were susceptible to vancomycin, linezolid, and daptomycin, while one strain was resistant to teicoplanin (3.5%). Resistance to quinolones was detected in 11 strains (37.9%), to clindamycin in 8 strains (27.5%), and to aminoglycosides in 6 strains (20.6%).
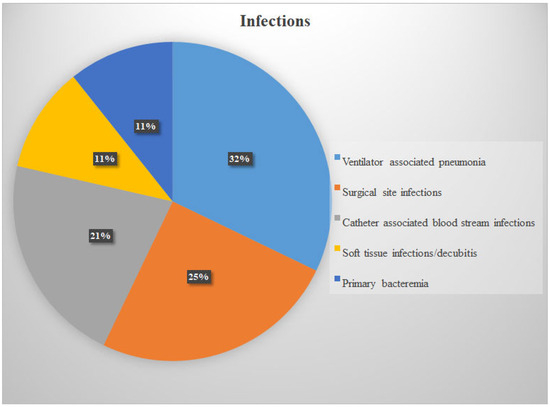
Figure 2.
Infection types.
4. Discussion
In this retrospective study, the prevalence of nasal MRSA colonization among patients admitted to our adult intensive care unit was found to be 4.0%. Among patients with nasal MRSA colonization who met the follow-up criteria, 6.0% developed an invasive MRSA infection during their hospital stay. Multivariable analysis demonstrated that regular hemodialysis, a history of nursing home residence, and a history of hospitalization within the last 90 days were independent risk factors for the development of invasive infection in patients with MRSA colonization.
Although there are a large number of studies on MRSA prevalence, the target groups vary, including healthy peoples, healthcare workers, or patients [12,13,14,15]. MRSA prevalence in our region was reported as 1.2% in a study conducted approximately 10 years ago in the pulmonary diseases department [16]. A recent study found a prevalence of 11.3% among healthy students in Türkiye [17]. However, a meta-analysis identified a carrier rate of 10–20% in our region [18]. In a study from Türkiye, 900 patients were screened and MRSA was isolated in 11 (1.2%) patients. Multivariate analysis showed that insulin use was the only significant risk factor for MRSA colonization [19]. In our study, nasal MRSA colonization screening was performed in patients admitted to the intensive care unit to evaluate the prevalence of progression to invasive infection and risk factors. Our study has the largest patient population and the longest screening period conducted in our region and country to date. In our study, a prevalence of 4% was detected, and invasive infection was identified in 6% of colonized cases. Considering these relatively low rates, screening only patients with risk factors may be an approach for developing countries, considering staff, workload, and testing costs. Surveillance with screening cultures should be evaluated for cost-effectiveness for every patient admitted to the intensive care unit or hospital in developing countries. Conducting studies that include cost analyses on this matter will enable us to provide clearer explanations in the future.
In patients colonized with MRSA, the most important risk factor for developing invasive infection was routine hemodialysis in this study. The literature reports that MRSA colonization is significantly higher in hemodialysis patients compared to the general population. In a prospective study monitoring S. aureus colonization in hemodialysis patients, the S. aureus colonization rate varied cross-sectionally between 33% and 46% depending on the month and was found to be higher than in the general population [20]. In a study conducted by Sathish and colleagues, MRSA nasal colonization was found to be an independent risk factor for the development of MRSA bloodstream infections [21]. In a meta-analysis, the likelihood of progressing to an MRSA infection was 19% in colonized hemodialysis patients over the long term (6–20 months), compared to only 2% in non-colonized patients [22]. These data support the view that colonization and invasive infection are more common in this group. However, we were unable to obtain data showing how many patients had HD catheters or how many patients received HD treatment via a fistula. This data should be recorded in future studies.
The rate of nasal MRSA colonization of elderly patients in a nursing home in Brazil was 26% [23]. In Slovakia, it was found that staying in a geriatric care center nursing home and older age could be risk factors for the development of high-risk MRSA strains [24]. Recent hospitalization is also a risk factor for MRSA colonization [15,25]. In our study, both a history of nursing home stay and a history of hospitalization within the last 90 days were risk factors for the development of invasive MRSA infection from colonization. Since retrospective screening could not be performed prior to admission for these patients, the duration of colonization is unknown. Due to the retrospective nature of the study, no geriatric assessment or frailty scale was applied, and the pre-admission colonization period is unknown. Factors such as the immune system and colonization duration may have facilitated progression to invasive infection.
Intubation, urinary catheterization, and surgery did not show a statistically significant difference between the two groups. This may be due to the fact that these invasive procedures are performed on most patients in the intensive care unit. Studies conducted on a non-ICU, more general patient population may reach different conclusions on this matter.
5. Limitations
This study has some limitations. First, since this study is retrospective and single-center, the data may be limited. Second, MRSA colonization was detected only through nasal swabs; other colonization sites such as the perineum or throat were not screened, which may have led to an underestimation of the true prevalence. Another limitation is that, since only 29 patients developed invasive infections, it could lead to overfitting, leading to errors in the multivariable statistical model. Nearly half of MRSA-positive patients were excluded due to discharge or death within 7 days. On the other hand, infections that may have progressed to infection after discharge could not be followed up. Infections that developed after discharge may have been missed.
6. Conclusions
Our study has revealed the prevalence of MRSA colonization in patients admitted to the intensive care unit and the risk factors for progression to invasive infection. Based on our results, it should be discussed whether screening should be performed on selected patients rather than on all patients admitted to the ICU. This discussion must also include economic data. Hospitalization within the last 90 days, nursing home stay, and routine hemodialysis treatment are risk factors. Particularly in developing countries, rather than admitting all patients to the intensive care unit, considering the workload and costs, screening these three patient groups and implementing decolonization should be considered.
Author Contributions
E.E.E.: Methodology; Formal analysis; Investigation; Writing—original draft. N.K.: Conceptualisation; Methodology; Formal analysis; Investigation. E.S.: Conceptualisation; Methodology; Formal analysis; Investigation. İ.Ç.: Conceptualisation; Methodology; Formal analysis; Investigation. E.A.M.: Conceptualisation, Investigation, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Kayseri City Hospital Clinical Research Ethics Committe (protocol code: 268 and date: 28 January 2025).
Informed Consent Statement
Patient consent was waived due to retrospectively study. The relevant ethics committee has decided that participation in the study does not require consent.
Data Availability Statement
The data supporting the findings of this study are not publicly available due to sensitivity and are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thanks Kayseri City Training and Research Hospital Infection Control Committee for their contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, A.; Garrett, S.; Hong, W.; Zhang, J. Staphylococcus aureus Infections and Human Intestinal Microbiota. Pathogens 2024, 13, 276. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, L.; Wei, J.; Xu, B. Progress in the Prevalence, Classification and Drug Resistance Mechanisms of Methicillin-Resistant Staphylococcus aureus. Infect. Drug Resist. 2023, 16, 3271–3292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pannewick, B.; Baier, C.; Schwab, F.; Vonberg, R.P. Infection control measures in nosocomial MRSA outbreaks-Results of a systematic analysis. PLoS ONE 2021, 16, e0249837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orhan, Z.; Kayış, A.; Küçük, B.; Doğaner, A.; Aral, M. Antimicrobial Resistance in Staphylococci Isolated from Various Clinical Specimens of Inpatients in Intensive Care Units: A 4-year Evaluation. Turk. J. Intensive Care 2024, 22, 24–30. [Google Scholar] [CrossRef]
- World Health Organization. Proportion of Bloodstream Infection Due to Methicillin-Resistant Staphylococcus Aureus (MRSA). Available online: https://data.who.int/indicators/i/918081E/5DD9606 (accessed on 9 October 2025).
- Central Asian and European Surveillance of Antimicrobial Resistance (CAESAR) Reports. Antimicrobial Resistance Surveillance in Europe 2023–2021 Data. Available online: https://www.who.int/europe/publications/i/item/9789289058537 (accessed on 9 October 2025).
- Qiao, F.; Huang, W.; Cai, L.; Zong, Z.; Yin, W. Methicillin-resistant Staphylococcus aureus nasal colonization and infection in an intensive care unit of a university hospital in China. J. Int. Med. Res. 2018, 46, 3698–3708. [Google Scholar] [CrossRef] [PubMed]
- Touaitia, R.; Mairi, A.; Ibrahim, N.A.; Basher, N.S.; Idres, T.; Touati, A. Staphylococcus aureus: A Review of the Pathogenesis and Virulence Mechanisms. Antibiotics 2025, 14, 470. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Transmission Precautions. Transmission-Based Precautions Basics Infection Control. Available online: https://www.cdc.gov/infection-control/hcp/isolation-precautions/appendix-a-type-duration.html (accessed on 9 October 2025).
- Suratwala, S.; Kommareddy, D.; Duvvuri, P.; Woltmann, J.; Segal, A.; Krauss, E. Cost-effectiveness and clinical utility of universal pre-admission MRSA screening in total joint arthroplasty patients. J. Hosp. Infect. 2023, 138, 27–33. [Google Scholar] [CrossRef] [PubMed]
- McKinnell, J.A.; Bartsch, S.M.; Lee, B.Y.; Huang, S.S.; Miller, L.G. Cost-benefit analysis from the hospital perspective of universal active screening followed by contact precautions for methicillin-resistant Staphylococcus aureus carriers. Infect. Control Hosp. Epidemiol. 2015, 36, 2–13. [Google Scholar] [CrossRef]
- Locke, T.E.; Keeley, A.J.; Laundy, N.; Keil, C.; Hamilton, J.; Pandor, A.; de Silva, T.I.; Darton, T.C. Prevalence and risk factors for Staphylococcus aureus colonisation among healthy individuals in low- and middle-income countries: A systematic review and meta-analysis. J. Infect. 2025, 90, 106462. [Google Scholar] [CrossRef]
- Giri, S.; Ghimire, A.; Mishra, A.; Acharya, K.; Kuikel, S.; Tiwari, A.; Mishra, S.K. Prevalence of methicillin-resistant Staphylococcus aureus carriage among healthcare workers in South Asia in non-outbreak settings: A systematic review and meta-analysis. Am. J. Infect. Control 2023, 51, 184–193. [Google Scholar] [CrossRef]
- Mekuriya, E.; Manilal, A.; Aklilu, A.; Woldemariam, M.; Hailu, T.; Wasihun, B. Methicillin-resistant Staphylococcus aureus colonization among medicine and health science students, Arba Minch University, Ethiopia. Sci. Rep. 2022, 12, 10161. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.; Khaled, H.; Fayed, H.M.; Mansour, Y.M.; Eldalil, M.; Elshennawy, E.; Salem, H.; Elkatan, H.A. Prevalence, antibiogram, and risk factors of methicillin-resistant Staphylococcus aureus (MRSA) asymptomatic carriage in Africa: A systematic review and meta-analysis. BMC Infect. Dis. 2025, 25, 505. [Google Scholar] [CrossRef] [PubMed]
- Oguzkaya-Artan, M.; Artan, C.; Baykan, Z. Prevalence and risk factors for Staphylococcus aureus and methicillin-resistant Staphylococcus aureus nasal carriage inpatients in a tertiary care hospital’s chest clinic in Turkey. Niger. J. Clin. Pract. 2016, 19, 313–317. [Google Scholar] [CrossRef]
- Şanlıtürk, G.; Ahmadkhanpour, B.; Al-Numan, S.; Betar, S.; Bozkurt, İ.; Hatami, K. Nasal Methicillin-resistant Staphylococcus aureus Carriage and Related Risk Factors in a University Student Population. Mediterr. J. Infect. Microb. Antimicrob. 2020, 9, 2. [Google Scholar] [CrossRef]
- Akpınar, O.; Yigit, A.; Güzel, M.; Akdogan, D. The investigation of nasal carriage rate of methicillin-resistant Staphylococcus aureus among Turkish healthcare workers, 1990-2019: Meta-analysis. Turk. Hij. Den. Biyol. Derg. 2020, 77, 217–226. [Google Scholar] [CrossRef]
- Baykam, N.; Esener, H.; Ergonul, O.; Kosker, P.Z.; Cirkin, T.; Celikbas, A.; Eren, S.; Dokuzoguz, B. Methicillin-resistant Staphylococcus aureus on hospital admission in Turkey. Am. J. Infect. Control. 2009, 37, 247–249. [Google Scholar] [CrossRef]
- Scheuch, M.; Freiin von Rheinbaben, S.; Kabisch, A.; Engeßer, J.; Ahrendt, S.; Dabers, T.; Kohler, C.; Holtfreter, S.; Bröker, B.M.; Stracke, S. Staphylococcus aureus colonization in hemodialysis patients: A prospective 25 months observational study. BMC Nephrol. 2019, 20, 153. [Google Scholar] [CrossRef]
- Sathish, J.V.; Malleshappa, P.; Yashaswini, M.K.; Shariff, S. Influence of methicillin-resistant Staphylococcus aureus (MRSA) nasal carriage on MRSA bloodstream infections among patients on chronic hemodialysis. Dial. Transplant. 2014, 35, 143–147. [Google Scholar] [CrossRef]
- Zacharioudakis, I.M.; Zervou, F.N.; Ziakas, P.D.; Mylonakis, E. Meta-analysis of methicillin-resistant Staphylococcus aureus colonization and risk of infection in dialysis patients. J. Am. Soc. Nephrol. 2014, 25, 2131–2141. [Google Scholar] [CrossRef]
- Martins, D.M.; Cardoso, E.M.; Capellari, L.; Botelho, L.A.B.; Ferreira, F.A. Detection of Staphylococcus aureus from nares of elderly living in a Brazilian nursing home. Diagn. Microbiol. Infect. Dis. 2024, 108, 116089. [Google Scholar] [CrossRef]
- Kaiglová, A.; Melnikov, K.; Bárdyová, Z.; Kucharíková, S. The prevalence of methicillin-resistant Staphylococcus aureus among nursing home residents for the elderly in Slovakia. Epidemiol. Mikrobiol. Imunol. 2023, 72, 195–198. [Google Scholar] [PubMed]
- Al-Iede, M.; Ayyad, D.M.; Etoom, R.A.; Aldameiry, R.H.; Toubasi, A.A. The prevalence and risk factors of methicillin-resistant Staphylococcus aureus among pediatric populations: A systematic review and meta-analysis. Eur. J. Pediatr. 2024, 183, 3679–3687. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).