Distribution and Factors Associated with Neisseria gonorrhoeae Cases in Kampala, Uganda, 2016–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Study Population
2.3. Data Collection and Laboratory Procedures
2.4. Statistical Analysis
3. Results
3.1. Positivity Levels of N. gonorrhoeae in Kampala
3.2. Risk Factors for Testing Positive with N. gonorrhoeae
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NG | Neisseria gonorrhoeae |
| HIV | Human Immunodeficiency Virus |
| STD | Sexually Transmitted Disease |
| STI | Sexually Transmitted Infection |
References
- World Health Organization. Sexually Transmitted Infections (STIs) Geneva: World Health Organization. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 27 April 2024).
- Chidiac, O.; AlMukdad, S.; Harfouche, M.; Harding-Esch, E.; Abu-Raddad, L.J. Epidemiology of gonorrhoea: Systematic review, meta-analyses, and meta-regressions, World Health Organization European Region, 1949 to 2021. Eurosurveillance 2024, 29, 2300226. [Google Scholar] [CrossRef]
- Iwuji, C.; Pillay, D.; Shamu, P.; Murire, M.; Nzenze, S.; Cox, L.A.; Mullick, S. A systematic review of antimicrobial resistance in Neisseria gonorrhoeae and Mycoplasma genitalium in sub-Saharan Africa. J. Antimicrob. Chemother. 2022, 77, 2074–2093. [Google Scholar] [CrossRef]
- Martín-Sánchez, M.; Ong, J.J.; Fairley, C.K.; Chen, M.Y.; Williamson, D.A.; Maddaford, K.; Aung, E.T.; Carter, G.; Bradshaw, C.S.; Chow, E.P.F. Clinical presentation of asymptomatic and symptomatic heterosexual men who tested positive for urethral gonorrhoea at a sexual health clinic in Melbourne, Australia. BMC Infect. Dis. 2020, 20, 486. [Google Scholar] [CrossRef]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548. [Google Scholar] [CrossRef]
- Kassa, Z.Y.; Hussen, S.; Hadra, N.; Moges, Y.; Bonja, F. Prevalence of Neisseria gonorrhoeae infection among women of reproductive age in sub-Saharan Africa: A systematic review and meta-analysis. Eur. J. Contracept. Reprod. Health Care 2020, 25, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Longe, B.T.; Moses, O.; David, R.T.O. Neisseria gonorrhoaea Infections in Africa-Systemat-ic Review and Meta-Analysis. J. Microbiol. Antimicrob. Agents 2020, 5, 4–9. [Google Scholar]
- Kakooza, F.; Musinguzi, P.; Workneh, M.; Walwema, R.; Kyambadde, P.; Mande, E.; Lubega, C.; Nakasi, J.M.; Kiggundu, R.; Hamill, M.M. Implementation of a standardised and quality-assured enhanced gonococcal antimicrobial surveillance programme in accordance with WHO protocols in Kampala, Uganda. Sex. Transm. Infect. 2021, 97, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Hamill, M.M.; Onzia, A.; Wang, T.-H.; Kiragga, A.N.; Hsieh, Y.-H.; Parkes-Ratanshi, R.; Gough, E.; Kyambadde, P.; Melendez, J.H.; Manabe, Y.C. High burden of untreated syphilis, drug resistant Neisseria gonorrhoeae, and other sexually transmitted infections in men with urethral discharge syndrome in Kampala, Uganda. BMC Infect. Dis. 2022, 22, 440. [Google Scholar] [CrossRef] [PubMed]
- Nakku-Joloba, E.; Mboowa, G.; Ssengooba, W.; Kiyimba, A.; Kigozi, E.; Baluku, H.; Alinaitwe, L.; Nyote, R.; Kabahita, J.M.; Mutumba, P.; et al. Prevalence and antimicrobial resistance profiles of Neisseria gonorrhea and Chlamydia trachomatis isolated from individuals attending STD clinics in Kampala, Uganda. Afr. Health Sci. 2022, 22, 62–71. [Google Scholar] [CrossRef]
- Kakooza, F.; Kiggundu, R.; Mboowa, G.; Kateete, P.D.; Nsangi, O.T.; Kabahita, J.M.; Ssentalo Bagaya, B.; Golparian, D.; Unemo, M. Antimicrobial susceptibility surveillance and antimicrobial resistance in Neisseria gonorrhoeae in Africa from 2001 to 2020: A mini-review. Front. Microbiol. 2023, 14, 1148817. [Google Scholar] [CrossRef]
- Unemo, M.; Lahra, M.M.; Escher, M.; Eremin, S.; Cole, M.J.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.-A.R.; Galas, M. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017–18: A retrospective observational study. Lancet Microbe 2021, 2, e627–e636. [Google Scholar] [CrossRef]
- World Health Organization. Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP): General Protocol. Geneva: World Health Organization. 2021. Available online: https://www.who.int/publications/i/item/9789240021341 (accessed on 27 April 2024).
- World Health Organization. Global Health Sector Strategy on Sexually Transmitted Infections, 2016–2021 Geneva: World Health Organization. 2016. Available online: https://www.who.int/publications/i/item/WHO-RHR-16.09 (accessed on 27 April 2024).
- Francis, S.C.; Mthiyane, T.N.; Baisley, K.; McHunu, S.L.; Ferguson, J.B.; Smit, T.; Crucitti, T.; Gareta, D.; Dlamini, S.; Mutevedzi, T.; et al. Prevalence of sexually transmitted infections among young people in South Africa: A nested survey in a health and demographic surveillance site. PLoS Med. 2018, 15, e1002512. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030. Geneva: World Health Organization. 2022. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/strategies/global-health-sector-strategies (accessed on 27 April 2024).
- Workneh, M.; Hamill, M.M.; Kakooza, F.; Mande, E.; Wagner, J.; Mbabazi, O.; Mugasha, R.; Kajumbula, H.; Walwema, R.; Zenilman, J.; et al. Antimicrobial Resistance of Neisseria Gonorrhoeae in a Newly Implemented Surveillance Program in Uganda: Surveillance Report. JMIR Public Health Surveill. 2020, 6, e17009. [Google Scholar] [CrossRef] [PubMed]
- Kiyingi, J.; Nabunya, P.; Bahar, O.S.; Mayo-Wilson, L.J.; Tozan, Y.; Nabayinda, J.; Namuwonge, F.; Nsubuga, E.; Kizito, S.; Nattabi, J.; et al. Prevalence and predictors of HIV and sexually transmitted infections among vulnerable women engaged in sex work: Findings from the Kyaterekera Project in Southern Uganda. PLoS ONE 2022, 17, e0273238. [Google Scholar] [CrossRef] [PubMed]
- McHaro, R.D.; Kisinda, A.; Njovu, L.; McHaro, M.; Mbwilo, F.; Mihale, G.; Komba, B.; Andrew, E.; Mayaud, P.; Kroidl, A.; et al. Prevalence of and risk factors associated with HIV, Herpes Simplex Virus-type 2, Chlamydia trachomatis and Neisseria gonorrhoeae infections among 18–24 year old students attending Higher Learning Institutions in Mbeya-Tanzania. PLoS ONE 2022, 17, e0266596. [Google Scholar] [CrossRef] [PubMed]
- Siziba, A.; Nunu, W.N.; Mudonhi, N.; Ndlovu, V.; Munyai, O.; Ndlovu, B.; Sanganyado, E. Risk factors associated with a high incidence of sexually transmitted infections in Beitbridge, Zimbabwe. Curationis 2022, 45, e1–e5. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Tomy, M.; Chemaitelly, H.; Omori, R.; Buse, K.; Low, N.; Hawkes, S.; Abu-Raddad, L.J. Dynamics of Neisseria gonorrhoeae transmission among female sex workers and clients: A mathematical modeling study. Epidemics 2024, 48, 100785. [Google Scholar] [CrossRef]
- World Health Organization. Gonorrhoea (Neisseria Gonorrhoeae Infection) 10 August 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/gonorrhoea-(neisseria-gonorrhoeae-infection)#:~:text=Most%20cases%20of%20gonorrhoea%20can%20be%20prevented%20with%20consistent%20and,the%20spread%20of%20the%20disease (accessed on 10 August 2024).
- Peace, N.; Elliot, A.; Lawrence, N.; Felix, T.; Apecu, R.O. Prevalence and antimicrobial susceptibility patterns of Neisseria gonorrhea among the symptomatic patients attending outpatient department in Lyatonde District Hospital Southwestern Uganda. Int. J. Sci. Rep. 2019, 5. [Google Scholar] [CrossRef]
- Mabonga, E.; Manabe, Y.C.; Elbireer, A.; Mbazira, J.K.; Nabaggala, M.S.; Kiragga, A.; Kisakye, J.; Gaydos, C.A.; Taylor, C.; Parkes-Ratanshi, R. Prevalence and predictors of asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae in a Ugandan population most at risk of HIV transmission. Int. J. STD AIDS 2021, 32, 510–516. [Google Scholar] [CrossRef]
- Sahile, A.; Teshager, L.; Fekadie, M.; Gashaw, M. Prevalence and Antimicrobial Susceptibility Patterns of Neisseria gonorrhoeae among Suspected Patients Attending Private Clinics in Jimma, Ethiopia. Int. J. Microbiol. 2020, 2020, 7672024. [Google Scholar]
- Ssekamatte, T.; Nalugya, A.; Mugambe, R.K.; Wagaba, B.; Nakalembe, D.; Mutebi, A.; Bagonza, R.A.; Tigaiza, A.; Kansiime, W.K.; Ssempala, R.; et al. Prevalence and predictors of sex under the influence of psychoactive substances among young people in informal settlements in Kampala, Uganda. BMC Public Health 2023, 23, 801. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Positive for N. gonorrhoeae (Gram Staining) n = 1218 | Positive for N. gonorrhoeae (Gram Staining and Culture) n = 923 |
|---|---|---|
| HIV Status | ||
| Negative | 874 (71.8%) * | 658 (71.2%) |
| Positive | 78 (6.4%) | 55 (5.9%) |
| Unknown | 266 (21.8%) | 210 (22.7%) |
| Age group | ||
| Median (IQR) age | 24 (21, 30) ** | |
| ≤24 years | 610 (50.0%) | 476 (51.6%) |
| Greater than 24 years | 580 (47.6%) | 425 (46.0%) |
| Unknown | 28 (2.3%) | 22 (2.4%) |
| Number of Sex partners in the last six months | ||
| Median (IQR) no. of sexual partners | 2.00 (1.00, 2.00) | |
| ≤1 Partner | 553 (45.4%) | 412 (44.6%) |
| >1 Partner | 595 (48.8%) | 469 (50.8%) |
| Unknown | 70 (5.7%) | 42 (4.6%) |
| Condom Use | ||
| Always | 10 (0.8%) | 8 (0.8%) |
| Never | 463 (38.0%) | 336 (36.4%) |
| Sometimes | 722 (59.3%) | 565 (61.2%) |
| Unknown | 23 (1.9%) | 14 (1.5%) |
| Facility Name | ||
| IDI Clinic | 11 (0.9%) | 9 (0.9%) |
| Kampala Remand Prison HC III | 43 (3.5%) | 26 (2.8%) |
| Kawaala HC III | 189 (15.5%) | 147 (15.9%) |
| Kirruddu GH | 150 (12.3%) | 115 (12.5%) |
| Kisenyi HC IV | 332 (27.3%) | 282 (30.6%) |
| Luzira Prison HC IV | 79 (6.5%) | 48 (5.2%) |
| Luzira Upper Prison HC III | 20 (1.6%) | 3 (0.3%) |
| MARPI Mulago | 90 (7.4%) | 77 (8.3%) |
| Murchison Bay hospital | 37 (3.0%) | 13 (1.4%) |
| Naguru hospital | 267 (21.9%) | 203 (22.0%) |
| History of Sex exchange for Money | 167 (13.7%) | 128 (13.9%) |
| Urethral discharge | 1185 (97.2%) | 916 (99.2%) |
| Dysuria | 1082 (88.8%) | 822 (89.1%) |
| Characteristic | Overall n = 1663 (95% CI 1) | Central n = 113 (95% CI) | Kawempe n = 218 (95% CI) | Makindye n = 340 (95% CI) | Nakawa n = 579 (95% CI) | Rubaga n = 413 (95% CI) |
|---|---|---|---|---|---|---|
| Positivity (Gram stain) | 1218 (73%) (71%, 75%) 1 | 91 (7.5%) (6.1%, 9.1%) | 155 (12.7%) (11%, 15%) | 244 (20%) (18%, 22%) | 409 (33.5%) (31%, 36%) | 319 (26.4%) (24%, 29%) |
| Positivity (Gram stain & Culture) | 923 (56%) (53%, 58%) | 75 (8.1%) (6.5%, 10%) | 113 (12.2%) (10%, 15%) | 185 (20.0%) (18%, 623%) | 291 (31.5%) (29%, 35%) | 259 (28.1%) (25%, 31%) |
| Characteristic | Bivariable Analysis (cPR 1 (95% CI 2)) | p-Value | Multivariable Analysis (aPR 3 (95%CI)) | p-Value |
|---|---|---|---|---|
| Patient Age group | ||||
| ≤24 years | 1 | 1 | ||
| Greater than 24 years | 0.93 (0.88, 0.99) 2 | 0.02 * | 0.93 (0.87, 0.99) | 0.017 ** |
| HIV Status | ||||
| Negative | 1 | |||
| Positive | 0.98 (0.87, 0.75) | 0.79 | ||
| Unknown | 1.04 (0.96, 1.14) | 0.27 | ||
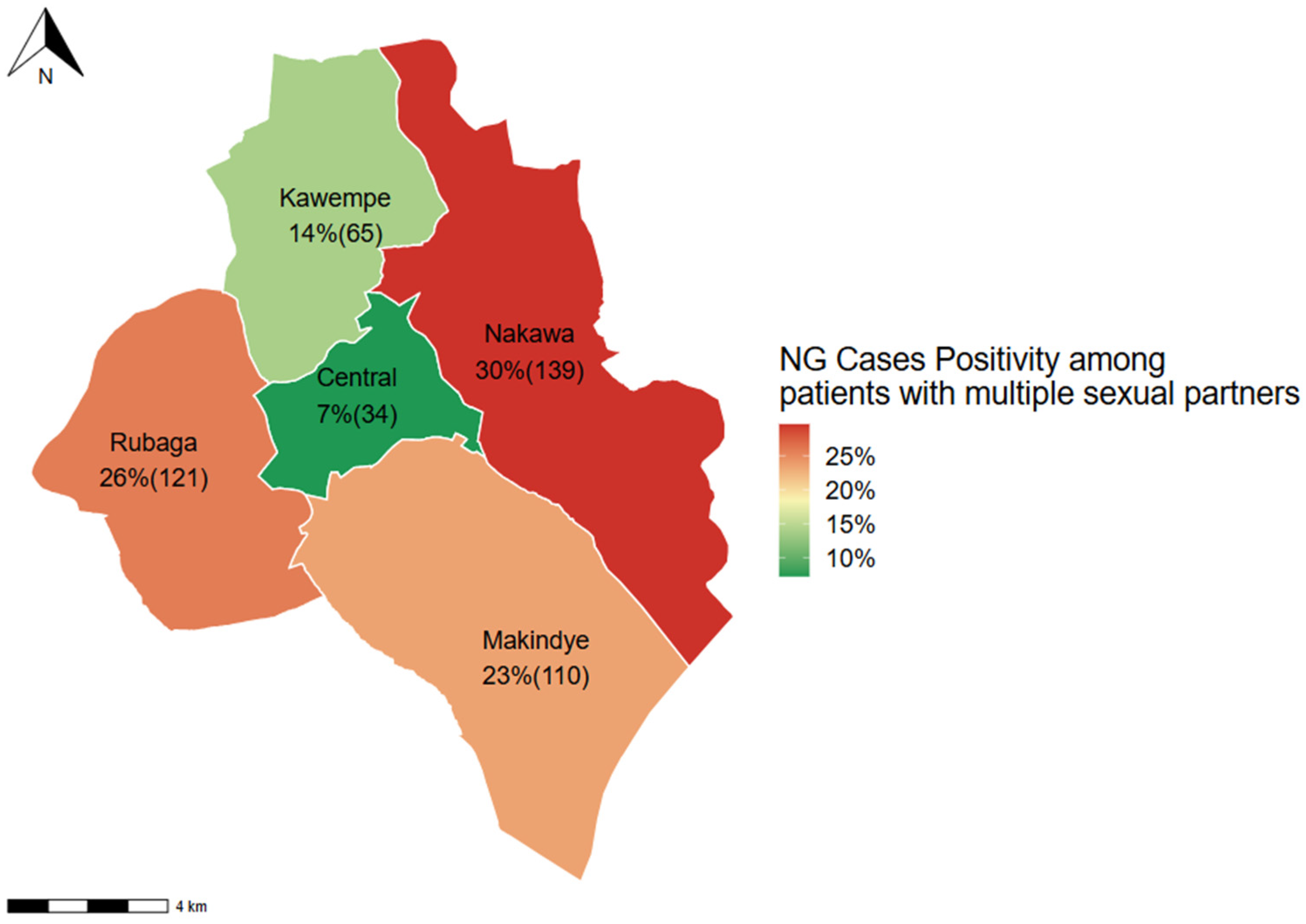
| Number of sex partners in the last 6 months | ||||
| ≤1 Partner | 1 | |||
| >1 Partner | 0.99 (0.93, 1.05) | 0.82 | ||
| Condom Use | ||||
| Always | 1 | 1 | ||
| Never | 1.44 (0.90, 2.29) | 0.12 * | 1.43 (0.89, 2.30) | 0.141 |
| Sometimes | 1.48 (0.93, 2.36) | 0.09 * | 1.47 (0.93, 2.36) | 0.115 |
| History of Sex Money Exchange | ||||
| No | 1 | |||
| Yes | 0.96 (0.88, 1.06) | 0.48 | ||
| Division | ||||
| Central | 1 | 1 | ||
| Kawempe | 0.88 (0.77, 1.01) | 0.08 * | 0.90 (0.79, 1.03) | 0.145 |
| Makindye | 0.90 (0.80, 1.02) | 0.13 * | 0.91 (0.81, 1.03) | 0.169 |
| Nakawa | 0.89 (1.00, 0.07) | 0.07 * | 0.90 (0.80, 1.01) | 0.07 |
| Rubaga | 0.98 (0.88, 1.11) | 0.84 | 0.99 (0.88, 1.11) | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lwigale, F.; Tumwine, C.; Kiggundu, R.; Elungat, P.; Mackline, H.; Byonanebye, D.M.; Kambugu, A.; Kakooza, F. Distribution and Factors Associated with Neisseria gonorrhoeae Cases in Kampala, Uganda, 2016–2020. Infect. Dis. Rep. 2025, 17, 132. https://doi.org/10.3390/idr17050132
Lwigale F, Tumwine C, Kiggundu R, Elungat P, Mackline H, Byonanebye DM, Kambugu A, Kakooza F. Distribution and Factors Associated with Neisseria gonorrhoeae Cases in Kampala, Uganda, 2016–2020. Infectious Disease Reports. 2025; 17(5):132. https://doi.org/10.3390/idr17050132
Chicago/Turabian StyleLwigale, Fahad, Conrad Tumwine, Reuben Kiggundu, Patrick Elungat, Hope Mackline, Dathan M. Byonanebye, Andrew Kambugu, and Francis Kakooza. 2025. "Distribution and Factors Associated with Neisseria gonorrhoeae Cases in Kampala, Uganda, 2016–2020" Infectious Disease Reports 17, no. 5: 132. https://doi.org/10.3390/idr17050132
APA StyleLwigale, F., Tumwine, C., Kiggundu, R., Elungat, P., Mackline, H., Byonanebye, D. M., Kambugu, A., & Kakooza, F. (2025). Distribution and Factors Associated with Neisseria gonorrhoeae Cases in Kampala, Uganda, 2016–2020. Infectious Disease Reports, 17(5), 132. https://doi.org/10.3390/idr17050132







