1. Introduction
Central venous catheters (CVCs) are essential in the management of pediatric patients, including low-birth-weight neonates, infants with short-gut syndrome, and children with cancer or chronic diseases [
1]. However, CVCs are frequently associated with bloodstream infections (BSIs), which may require catheter removal and can even result in mortality [
1]. The management of catheter-related BSIs (CRBSIs) remains challenging due to several factors, such as the rising prevalence of multidrug-resistant organisms, the choice of appropriate antimicrobial therapy, treatment duration, and the decision of whether to retain the catheter in situ [
1]. Because CVC insertion in pediatric patients requires general anesthesia or sedation, effective antimicrobial therapy is especially important to achieve catheter salvage. Blood culture (BC) remains the gold standard for the diagnosis and management of BSIs [
1,
2], but it is limited by low sensitivity and long turnaround times [
2]. Digital polymerase chain reaction (dPCR), a third-generation PCR technique, was developed in the 1990s and introduced into clinical practice more recently. dPCR is based on partitioning the DNA sample into thousands of microdroplets, each serving as an independent PCR reaction chamber, which allows absolute quantification of target DNA molecules. Compared with real-time quantitative PCR (RT-qPCR) and next-generation sequencing, dPCR offers higher sensitivity, shorter turnaround times, and lower costs. Quantitative measurement of bacterial DNA using dPCR has been shown to correlate with sepsis severity, disease progression, and treatment response [
3]. Although dPCR itself is not a new technology, its use in pediatric cases of suspected CRBSI remains limited. Importantly, dPCR requires only a small volume of blood and can provide rapid information on both causative pathogens and resistance genes, which may be particularly advantageous in children. In this case report, we evaluated the utility of dPCR for the quantitative detection of bacterial and drug-resistance DNA in managing a pediatric patient with suspected CRBSI.
3. Discussion
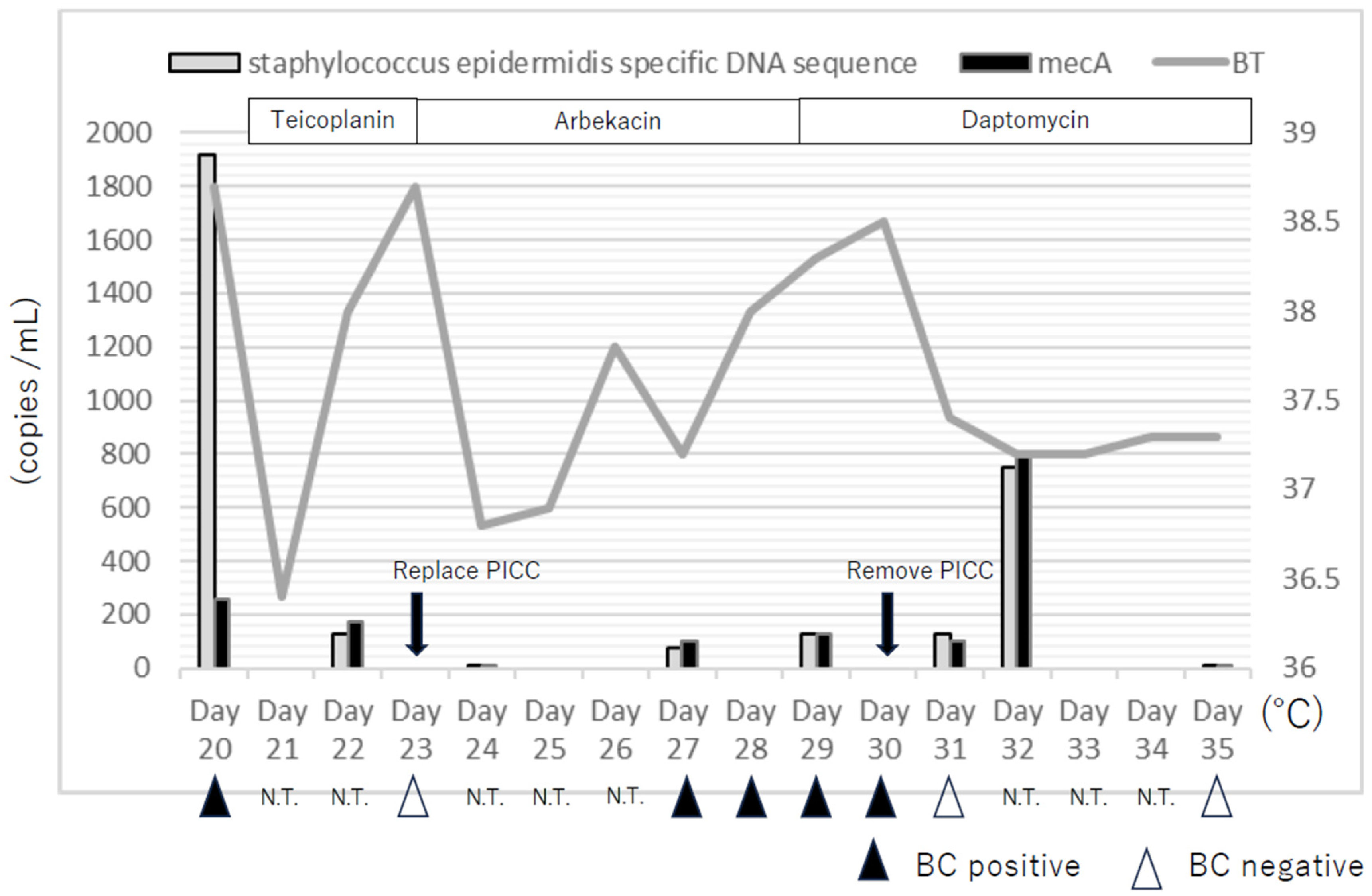
In this study, synchronous results between dPCR and BC were limited; however, when BC was positive, dPCR was also positive, and dPCR remained positive even when BC was negative. In particular, on the day after dPCR was positive, BT increased, and the BSI was more severe. The higher sensitivity of dPCR than BC was consistent with a previous study [
8]. However, it should also be emphasized that while blood culture requires viable bacteria to yield a positive result, dPCR can detect bacterial DNA fragments even after the organisms have been killed by antimicrobial therapy. This explains why dPCR may remain positive for longer periods even when blood culture has turned negative. The rapid increase in targeted DNA by dPCR on day 32 may represent transient release of DNA from non-viable bacteria into the bloodstream caused by thrombolysis of suppurative thrombophlebitis after PICC removal. We also emphasize that dPCR should not be regarded as a reliable tool for confirming bacterial eradication. Because dPCR can detect DNA fragments from non-viable bacteria or resistance genes that are not necessarily linked to active infection, its results may persist even when viable organisms have been cleared. From a clinical perspective, this means that dPCR cannot determine eradication, and over-interpretation could potentially lead to unnecessary or inappropriate antimicrobial treatment. Therefore, dPCR results must always be interpreted in conjunction with clinical findings and blood culture data, and should be considered complementary rather than definitive.
Because dPCR can detect minute amounts of DNA, false-positive results due to contamination or residual DNA are another concern. In this case, S. epidermidis was continuously detected using BC and dPCR, while other organisms were not detected during the course of the disease. Although there is little possibility of contamination, S. epidermidis exists ubiquitously on the skin, and a cautious interpretation of results is needed. Strict adherence to aseptic sampling, inclusion of negative controls, and the use of internal quality controls are necessary to minimize false-positive results. Re-testing of borderline positive samples can also help confirm reproducibility.
The turnaround time was significantly shorter with dPCR than with BC, which can facilitate rapid decision-making in the management of CRBSIs. This rapidity is particularly advantageous during the first febrile episode or early in suspected infection, when viable bacteria are more likely to be present and timely treatment decisions are critical. In this setting, dPCR may potentially contribute to therapeutic decisions in several ways: rapid detection of resistance genes, such as
mecA, could support the early choice of an appropriate antimicrobial agent; serial monitoring of bacterial DNA copy numbers might provide information on the duration of therapy, although such results must be interpreted with caution in accordance with existing guidelines for hospital-acquired infections, since DNA from non-viable or non-causative bacteria may persist; and persistently positive dPCR results despite adequate antimicrobial therapy might provide additional evidence to support catheter removal, particularly when consistent with clinical findings and blood culture data. However, these applications remain hypothetical and should be considered limitations of the present case report, as current evidence is insufficient to draw definitive conclusions. Nevertheless, the development of multiplex panels with species-specific primers and probes could further enhance the clinical utility of dPCR by enabling differentiation among clinically relevant organisms, thereby facilitating more targeted empirical therapy and potentially helping to limit the emergence of antibiotic resistance. In the present study, we employed primers and probes designed on the basis of BC results; however, preparing panels for other clinically important organisms, such as
Staphylococcus aureus, in advance would allow the application of dPCR independent of BC. Indeed, Liu et al. recently demonstrated the clinical utility of preset primer/probe panels for several viruses using multiplex digital PCR in the rapid diagnosis of suspected pediatric bloodstream infections [
9].
A definitive diagnosis of CRBSI is obtained mainly by comparative culture and differential time-to-positivity [
1]. CRBSI is diagnosed when BC via the CVC, becomes positive at least 2 h before BC, via the peripheral blood [
1]. In pediatrics, collecting adequate blood for BC is sometimes difficult. In our case, paired blood cultures were not obtained, and therefore the diagnosis of CRBSI could not be confirmed by strict laboratory criteria. Thus, our patient should be regarded as having a suspected rather than a laboratory-confirmed CRBSI, which represents an important limitation of this report. Nevertheless, dPCR required only a minimum of 400 µL of blood, and provided quantitative information that contributed to clinical management despite this limitation. Previous larger studies have already demonstrated the potential utility of dPCR in bloodstream infections [
10,
11,
12]. Our report adds a pediatric case perspective, but further multicenter studies are required to validate the role of dPCR compared with conventional BC in clinical practice.
In this case, the patient received chemotherapy and developed neutropenia and body temperature was the leading indicator of the effectiveness of the antimicrobial therapy, apart from the BC results. In patients with neutropenia, quantitatively measuring bacterial DNA using dPCR can help to monitor the severity of BSI, and the effectiveness of antimicrobial therapy. In this case, the copy numbers of the
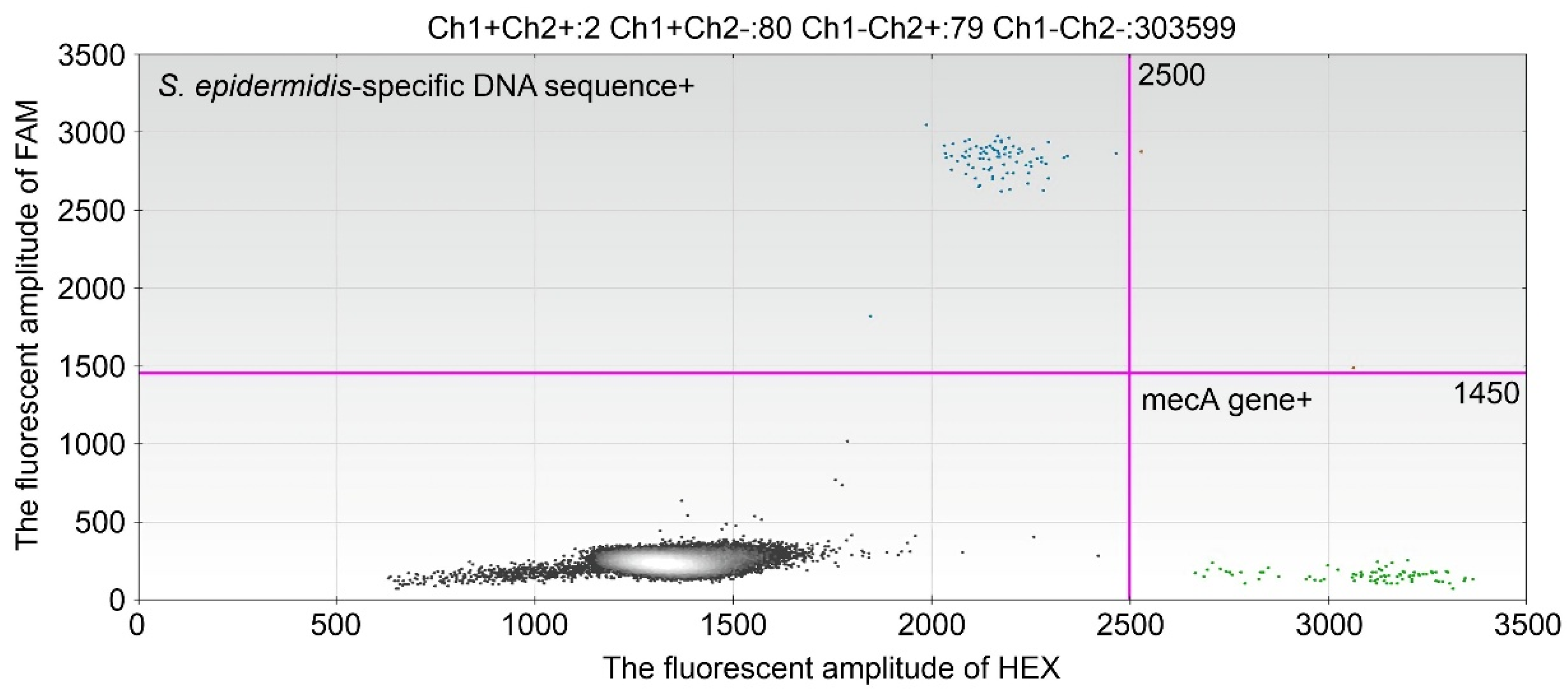
S. epidermidis specific sequence and
mecA gene in the blood were high even after the PICC was removed. Default DNA clearance is associated with treatment failure, and these results will help to determine the duration of antimicrobial therapy [
3].
Myeloablative chemotherapy in pediatric patients with malignancies frequently causes febrile neutropenia, which is managed using empirical broad-spectrum antibiotics. The use of empirical broad-spectrum antibiotics increases the number of multidrug-resistant organisms. Infections by multidrug-resistant organisms are associated with high mortality in immunocompromised pediatric patients with malignancies. In this case, we detected the methicillin-resistance gene
mecA in the blood using dPCR. This approach could also be applied to other drug-resistant genes, such as the carbapenem-resistance gene
blaKPC and the vancomycin-resistance gene
vanA [
13]. However, it is important to note that the mere presence of such genes does not necessarily indicate phenotypic resistance, as expression and resistance may be influenced by insertion sequences, mutations, or regulatory mechanisms. Therefore, dPCR findings of resistance genes should be interpreted cautiously and always in parallel with conventional phenotypic susceptibility testing, such as MIC determination by culture, as well as the patient’s clinical course. This combined approach would help ensure that the detection of resistance determinants by dPCR contributes meaningfully to appropriate antimicrobial decision-making in pediatric malignancies.
Although dPCR offers high sensitivity and rapid turnaround time, there are several limitations. First, dPCR is unable to provide information on antimicrobial susceptibility beyond the specific resistance genes targeted by primers. Therefore, in cases involving unexpected or novel resistance mechanisms, culture-based methods remain indispensable. Second, dPCR is not suitable when broad-range pathogen identification is required, such as in polymicrobial bloodstream infections or in patients with atypical clinical presentations. Unlike dPCR, blood culture currently allows comprehensive and unbiased detection of a wide range of organisms, including unexpected pathogens. This represents a major limitation of dPCR at present. However, with the development of multiplex primer/probe panels targeting multiple clinically relevant organisms and advances in broad-range digital PCR approaches, dPCR could eventually overcome this limitation and potentially serve as an alternative to blood culture in the future, offering rapid and highly sensitive detection across a broader spectrum of pathogens.
Another important limitation is cost. dPCR instruments and reagents remain relatively expensive, and their implementation in routine hospital practice may be challenging, particularly in low- and middle-income countries. Moreover, specialized technical expertise is required for assay setup and data interpretation. Large-scale health-economic evaluations are warranted to assess whether the rapid turnaround time and reduced empiric antibiotic use can offset these costs in routine pediatric care.
Finally, we emphasize that dPCR should be considered a complementary diagnostic tool rather than a replacement for blood culture. While dPCR can rapidly provide information on pathogen DNA and resistance genes, blood culture remains essential for isolating organisms, performing antimicrobial susceptibility testing, and supporting epidemiological surveillance. Future studies using animal models, such as S. epidermidis bacteremia in immunodeficient mice, also could help establish the translational utility of dPCR in bloodstream infections.
In conclusion, dPCR enables quantitative measurement of bacterial DNA and may facilitate rapid clinical decision-making in the management of CRBSI, as well as real-time monitoring of the therapeutic response. Its applicability in pediatrics is enhanced by the low blood volume requirement compared with BCs. Importantly, although dPCR can rapidly detect causative pathogens and resistance genes, it should be interpreted alongside conventional blood cultures, which remain indispensable for definitive diagnosis and antimicrobial susceptibility testing.