Public Health Impact of the MVA-BN Vaccine During the 2022 Mpox Outbreak: A Systematic Review
Abstract
1. Introduction
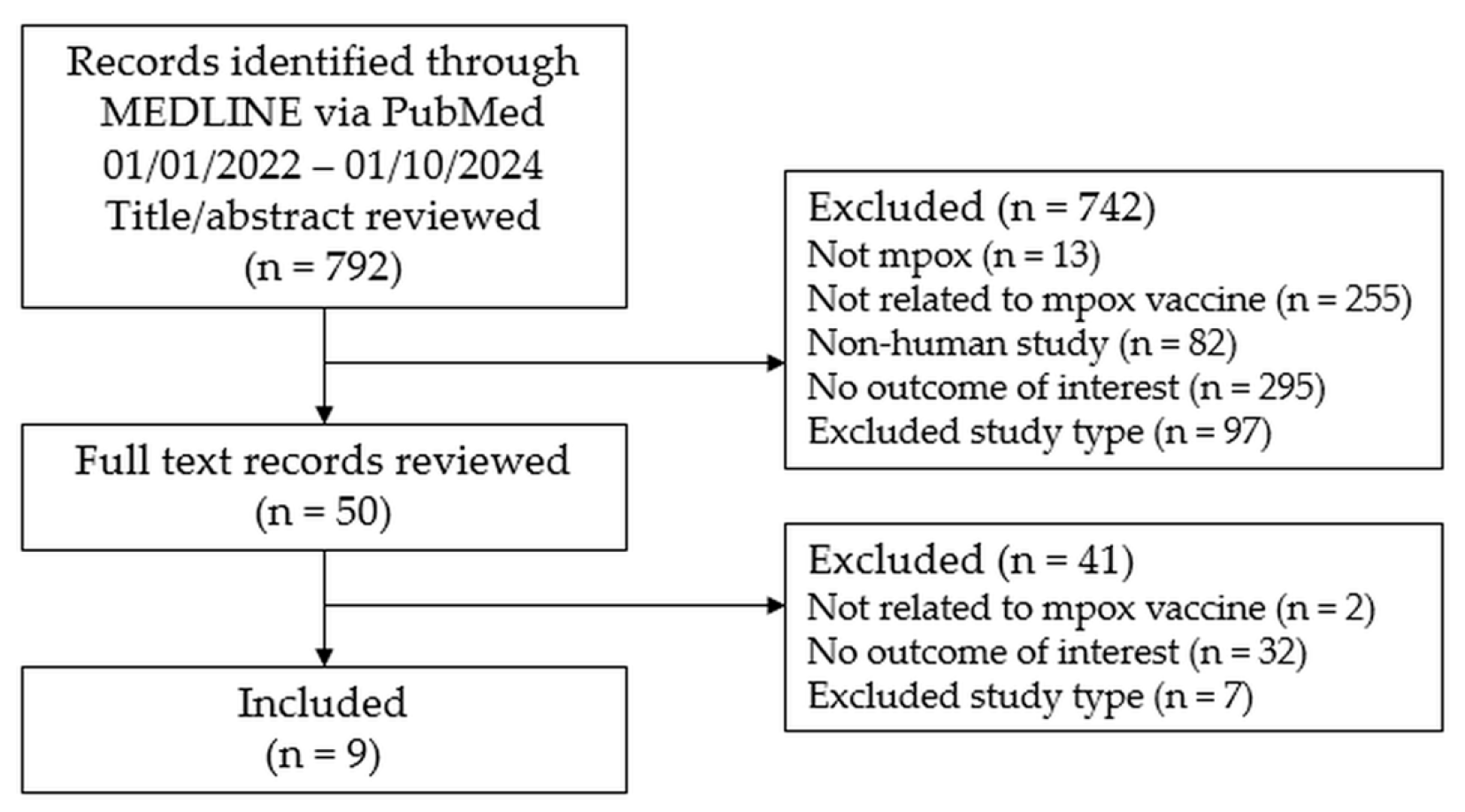
2. Materials and Methods
3. Results
3.1. SLR Findings
3.2. Studies That Measured the Impact of the Vaccine During the 2022 Outbreak
3.2.1. Lin, 2024 [17]
3.2.2. Zhang X, 2024 [18]
3.2.3. Brand, 2023 [19]
3.2.4. Clay, 2024 [20]
3.3. Studies That Measured the Impact of the Vaccine on a Future Outbreak
Shamier, 2024 [21]
3.4. Studies That Measured the Impact of a Hypothetical Vaccination Program or Strategies
3.4.1. Zheng, 2022 [22]
3.4.2. Knight, 2022 [23]
3.4.3. Gan, 2023 [24]
3.4.4. Zhang L, 2024 [25]
3.5. Estimate of Cases Averted in the US
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| CI | Confidence Interval |
| COVID-19 | Coronavirus Disease 2019 |
| DRC | Democratic Republic of the Congo |
| EUA | Emergency Use Authorization |
| EU | European Union |
| EUL | Emergency Use Listing |
| FDA | Food and Drug Administration |
| GBMSM | Gay and Bisexual Men who have Sex with Men |
| IQR | Interquartile Range |
| MPXV | Monkeypox Virus |
| MVA-BN | Modified Vaccinia Ankara-Bavarian Nordic |
| PHEIC | Public Health Emergency of International Concern |
| PI | Prediction Interval |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| R0 | Basic reproductive number |
| SEIR | Susceptible, Exposed, Infected, Recovered |
| SLR | Systematic Literature Review |
| UK | United Kingdom |
| US | United States |
| VE | Vaccine Efficacy |
| WHO | World Health Organization |
References
- World Health Organization. Smallpox and Mpox (Orthopoxviruses): WHO Position Paper, August 2024. Published 23 August 2024. Available online: https://www.who.int/publications/i/item/who-wer-9934-429-456 (accessed on 14 October 2024).
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mpox (Monkeypox). Updated 26 August 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/mpox (accessed on 14 November 2023).
- Centers for Disease Control and Prevention. 2022 Mpox Outbreak Global Map. 6 August 2024. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html (accessed on 29 August 2024).
- World Health Organization. Global Mpox Trends. Updated 19 September 2025. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 21 July 2025).
- Centers for Disease Control and Prevention. Mpox Vaccination Administration in the US. Published 9 January 2024. Available online: https://stacks.cdc.gov/view/cdc/145636/cdc_145636_DS1.pdf (accessed on 16 August 2024).
- Centers for Disease Control and Prevention. U.S. Mpox Case Trends Reported to CDC. Updated 1 September 2025. Available online: https://www.cdc.gov/monkeypox/data-research/cases/index.html (accessed on 30 August 2024).
- Bavarian Nordic. Bavarian Nordic’s Mpox Vaccine Receives Recommendation from U.S. CDC Advisory Committee for Routine Use in Adults at Risk. Published 25 October 2023. Available online: https://www.bavarian-nordic.com/investor/news/news.aspx?news=6840 (accessed on 14 November 2023).
- EMA. EMA Recommends Extending Indication of Mpox Vaccine to Adolescents. 19 September 2024. Available online: https://www.ema.europa.eu/en/news/ema-recommends-extending-indication-mpox-vaccine-adolescents (accessed on 22 September 2025).
- UK Health Security Agency. Mpox Vaccination: Information for Healthcare Practitioners. 7 April 2025. Available online: https://www.gov.uk/government/publications/vaccination-against-mpox-information-for-healthcare-practitioners/mpox-vaccination-information-for-healthcare-practitioners (accessed on 22 September 2025).
- NACI. Interim Guidance on the Use of Imvamune® in the Context of a Routine Immunization Program. 24 May 2024. Available online: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-interim-guidance-imvamune-routine-immunization-program.html (accessed on 22 September 2025).
- WHO. WHO Prequalifies the First Vaccine Against Mpox. 13 September 2024. Available online: https://www.who.int/news/item/13-09-2024-who-prequalifies-the-first-vaccine-against-mpox (accessed on 22 September 2025).
- WHO. Multi-Country Outbreak of Mpox, External Situation Report #46—28 January 2025. 28 January 2025. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report--46---28-january-2025 (accessed on 22 September 2025).
- European Commission. Mpox: HERA to Donate Over 215,000 Vaccine Doses to Africa CDC Amid Urgent Outbreak. 14 August 2024. Available online: https://health.ec.europa.eu/latest-updates/mpox-hera-donate-over-215000-vaccine-doses-africa-cdc-amid-urgent-outbreak-2024-08-14_en (accessed on 22 September 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Kallmes, K.M.; Kallmes, K.R.; Holub, K. Nested Knowledge Version 1.78.1. St. Paul, MN. Available online: https://nested-knowledge.com/ (accessed on 22 September 2025).
- Lin, Y.C.; Wen, T.H.; Shih, W.L.; Vermund, S.H.; Fang, C.T. Impact of vaccination and high-risk group awareness on the mpox epidemic in the United States, 2022–2023: A modelling study. EClinicalMedicine 2024, 68, 102407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Mandal, S.; Mohammed, H.; Turner, C.; Florence, I.; Walker, J.; Niyomsri, S.; Amirthalingam, G.; Ramsay, M.; Charlett, A.; et al. Transmission dynamics and effect of control measures on the 2022 outbreak of mpox among gay, bisexual, and other men who have sex with men in England: A mathematical modelling study. Lancet Infect. Dis. 2024, 24, 65–74. [Google Scholar] [CrossRef]
- Brand, S.P.C.; Cavallaro, M.; Cumming, F.; Turner, C.; Florence, I.; Blomquist, P.; Hilton, J.; Guzman-Rincon, L.M.; House, T.; Nokes, D.J.; et al. The role of vaccination and public awareness in forecasts of Mpox incidence in the United Kingdom. Nat. Commun. 2023, 14, 4100. [Google Scholar] [CrossRef]
- Clay, P.A.; Asher, J.M.; Carnes, N.; Copen, C.E.; Delaney, K.P.; Payne, D.C.; Pollock, E.D.; Mermin, J.; Nakazawa, Y.; Still, W.; et al. Modelling the impact of vaccination and sexual behaviour adaptations on mpox cases in the USA during the 2022 outbreak. Sex. Transm. Infect. 2024, 100, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Shamier, M.C.; Zaeck, L.M.; Götz, H.M.; Vieyra, B.; Verstrepen, B.E.; Wijnans, K.; Welkers, M.R.; Hoornenborg, E.; van Cleef, B.A.; van Royen, M.E.; et al. Scenarios of future mpox outbreaks among men who have sex with men: A modelling study based on cross-sectional seroprevalence data from the Netherlands, 2022. Eurosurveillance 2024, 29, 2300532. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, C.; Li, P.; de Vries, A.; Giordano, G.; Pan, Q. Projecting the impact of testing and vaccination on the transmission dynamics of the 2022 monkeypox outbreak in the USA. J. Travel Med. 2022, 29, taac101. [Google Scholar] [CrossRef]
- Knight, J.; Tan, D.; Mishra, S. Maximizing the impact of limited vaccine supply under different early epidemic conditions: A 2-city modelling analysis of monkeypox virus transmission among men who have sex with men. CMAJ 2022, 194, E1560–E1567. [Google Scholar] [CrossRef]
- Gan, G.; Janhavi, A.; Tong, G.; Lim, J.; Dickens, B. The need for pre-emptive control strategies for mpox in Asia and Oceania. Infect. Dis. Model. 2023, 9, 214–223. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Yan, W.; Zhao, Y.; Wang, D.; Chen, B. Global prediction for mpox epidemic. Env. Res. 2024, 243, 117748. [Google Scholar] [CrossRef] [PubMed]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; Van Royen, M.E.; Götz, H.; Shamier, M.C.; Van Leeuwen, L.P.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e9. [Google Scholar] [CrossRef]
- Criscuolo, E.; Giuliani, B.; Ferrarese, R.; Ferrari, D.; Locatelli, M.; Clementi, M.; Mancini, N.; Clementi, N. Smallpox vaccination-elicited antibodies cross-neutralize 2022-Monkeypox virus Clade II. J. Med. Virol. 2023, 95, e28643. [Google Scholar] [CrossRef] [PubMed]
- Hubert, M.; Guivel-Benhassine, F.; Bruel, T.; Porrot, F.; Planas, D.; Vanhomwegen, J.; Wiedemann, A.; Burrel, S.; Marot, S.; Palich, R.; et al. Complement-dependent mpox-virus neutralizing antibodies in infected and vaccinated individuals. Cell Host Microbe 2023, 31, 937–948.e4. [Google Scholar] [CrossRef]
- Kottkamp, A.C.; Samanovic, M.I.; Duerr, R.; Oom, A.L.; Belli, H.M.; Zucker, J.R.; Rosen, J.B.; Mulligan, M.J. Antibody Titers against Mpox Virus after Vaccination. N. Engl. J. Med. 2023, 389, 2299–2301. [Google Scholar] [CrossRef]
- Oom, A.L.; Wilson, K.K.; Yonatan, M.; Rettig, S.; Youn, H.A.; Tuen, M.; Shah, Y.; DuMont, A.L.; Belli, H.M.; Zucker, J.R.; et al. The two-dose MVA-BN mpox vaccine induces a nondurable and low avidity MPXV-specific antibody response. J. Virol. 2025, 99, e00253-25. [Google Scholar] [CrossRef]
- Mason, L.M.K.; Betancur, E.; Riera-Montes, M.; Lienert, F.; Scheele, S. MVA-BN vaccine effectiveness: A systematic review of real-world evidence in outbreak settings. Vaccine 2024, 42, 126409. [Google Scholar] [CrossRef]
- WHO. Disease Outbreak News: Multi-Country Monkeypox Outbreak Situation Update. 4 June 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390 (accessed on 30 September 2024).
- Savinkina, A.; Kindrachuk, J.; Bogoch, I.I.; Rimoin, A.W.; Hoff, N.A.; Shaw, S.Y.; Pitzer, V.E.; Mbala-Kingebeni, P.; Gonsalves, G.S. Modelling vaccination approaches for mpox containment and mitigation in the Democratic Republic of the Congo. Lancet Glob. Health 2024, 12, e1936–e1944. [Google Scholar] [CrossRef]
- Jin, S.; Asakura, T.R.; Murayama, H.; Niyukuri, D.; Saila-Ngita, D.; Lim, J.T.; Endo, A.; Dickens, B.L. Vaccination strategies to achieve outbreak control for MPXV Clade I with a one-time mass campaign in sub-Saharan Africa: A scenario-based modelling study. PLoS Med. 2025, 22, e1004726. [Google Scholar] [CrossRef]
- Vakaniaki, E.H.; Kacita, C.; Kinganda-Lusamaki, E.; O’toole, Á.; Wawina-Bokalanga, T.; Mukadi-Bamuleka, D.; Amuri-Aziza, A.; Malyamungu-Bubala, N.; Mweshi-Kumbana, F.; Mutimbwa-Mambo, L.; et al. Sustained human outbreak of a new MPXV clade I lineage in eastern Democratic Republic of the Congo. Nat. Med. 2024, 30, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Nizigiyimana, A.; Ndikumwenayo, F.; Houben, S.; Manirakiza, M.; van Lettow, M.; Liesenborghs, L.; Mbala-Kingebeni, P.; Rimoin, A.W.; Bogoch, I.I.; Kindrachuk, J. Epidemiological analysis of confirmed mpox cases, Burundi, 3 July to 9 September 2024. Eurosurveillance 2024, 29, 2400647. [Google Scholar] [CrossRef] [PubMed]
- Kinganda-Lusamaki, E.; Amuri-Aziza, A.; Fernandez-Nuñez, N.; Makangara-Cigolo, J.C.; Pratt, C.; Vakaniaki, E.H.; Hoff, N.A.; Luakanda-Ndelemo, G.; Akil-Bandali, P.; Nundu, S.S.; et al. Clade I mpox virus genomic diversity in the Democratic Republic of the Congo, 2018–2024: Predominance of zoonotic transmission. Cell 2025, 188, 4–14.e6. [Google Scholar] [CrossRef] [PubMed]
| Study | Objective | Methods | Results | Key Parameters | Setting |
|---|---|---|---|---|---|
| Impact of the Vaccine During the 2022 Outbreak | |||||
| Lin, 2024 [17] | Proportion of cases averted by vaccination and risk behavior modification | Deterministic transmission compartmental model | Vaccines (2-dose campaign) could prevent 21.2% of cases (approximately 8096 cases); Behavior change: 15.4%; Both: 64.0% (approximately 53,499 cases) | R0 = 3.88 (high-risk) R0 = 0.39 (low-risk) | US |
| Zhang X, 2024 [18] | Estimate the effect of vaccination on the outbreak | Structured dynamic compartmental transmission model | Vaccination marginally increased the number of infections prevented (approx. 185 cases) but minimized a resurgence in cases from Jan 2023; could have averted 4x more if initiated earlier | R0 (homogenous) = 1.41–2.17 R0 (structured) = 1.94 (high-risk) and 0.67 (low-risk) | England |
| Brand, 2023 [19] | Make projections of future mpox incidence over a medium-term time horizon (26 weeks) | Bayesian, compartmental transmission model | Vaccination did not cause mpox incidence to turn over; however, a rebound in cases due to behavior reversion was prevented by high-risk group-targeted vaccination | R0 (GBMSM pop) = 5.16 (2.96–9.24) R0 (Overall) = 5.16 (2.96–9.24) | UK |
| Clay, 2024 [20] | Estimate the relative effects of behavioral adaptation and vaccination on the 2022 outbreak, and the theoretical impact if vaccines had been distributed earlier | Dynamic network transmission model incorporating both vaccine administration data and sexual partner acquisition | Initial declines in cases were likely caused by behavioral adaptations, but vaccination alone averted 79% (IQR: 64–88%) of cases, compared with behavioral adaptation alone (25% (IQR: 10–42%)) | VE (first dose) = 75.2% VE (second) = 85.9% | US |
| Impact of the Vaccine on a Future Outbreak | |||||
| Shamier, 2024 [21] | Future outbreak scenarios based on seroprevalence data | Stochastic transmission compartmental model | Marginal decrease in cases due to vaccine: 1427 vs. 1321 | Reduction in infection risk = 85% for historically vaccinated individuals (i.e., smallpox); 78% for recently vaccinated | Netherlands |
| Impact of a Hypothetical Vaccination Program or Strategies | |||||
| Zheng, 2022 [22] | Estimate the impact of diagnostic testing interventions and ring vaccination on cumulative cases | Epidemic dynamical model | Ring vaccination of 20% of exposed contacts reduces cumulative cases by 61.1% by end of 2022 If 40%, then 78.3% reduction; if 60%, then 81.8% reduction | Smallpox vaccine VE = 85% | US |
| Knight, 2022 [23] | Determine the optimal vaccination allocation strategy to minimize cumulative cases | Deterministic compartmental mpox virus transmission model | A limited mpox vaccine supply would avert more early infections when prioritized to larger networks with more initial infections or had a higher R0 | Mpox vaccine VE = 85% | Canada |
| Gan, 2023 [24] | Simulate outbreaks and demonstrate value of pre-emptive vaccination before arrival of the virus | Individual-based SEIR compartmental model | Mass vaccination can reduce total cases by 22.3% to 96.1%. Targeted vaccination: cases can be reduced by 8.4% to 66.9% For mass vaccination the average number of cases averted per vaccine dose: 0.82 Singapore, 0.96 Hong Kong, and 0.85 Sydney | VE: 85% (Sensitivity analysis VE = 66%) | Singapore, Hong Kong, Sydney |
| Zhang L, 2024 [25] | Simulate global mpox transmission and countermeasure scenarios for the 2022 outbreak | Modified SEIR model | A 20% vaccination rate by the end of 2022 could have reduced mpox infection rates by 16%; a 30% rate could have reduced it by 29% | Mpox vaccine VE = 78% | Various |
| Proportion of Mpox Cases Averted | |||||
|---|---|---|---|---|---|
| Objective | Study | Country or City | Due to Vaccine Alone | Due to Behavioral Changes Alone | Due to Behavioral Changes and Vaccination |
| Impact of the Vaccine During the 2022 Outbreak | Lin, 2024 [17] | US | 21.2% | 15.4% | 64.0% |
| Zhang X, 2024 [18] | England | 9.8% | 98% | 98.1% | |
| Brand, 2023 [19] | UK | 45–53% * | - | - | |
| Clay, 2024 [20] | Washington DC | 79% | 25% | 84% | |
| Impact on a Future Outbreak | Shamier, 2024 [21] | Netherlands | 74% | - | - |
| Impact of a Hypothetical Vaccination Program or Strategies | Zheng, 2022 [22] | US | 61.1–81.8% † | - | - |
| Knight, 2022 [23] | Toronto-like city Ontario-like city | Displayed in figure only | - | - | |
| Gan, 2023 [24] | Singapore Hong Kong Sydney | 25–78% ** 29–96% ** 22–71% ** | - | - | |
| Zhang L, 2024 [25] | Various | 16–29% ‡ | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsandres, S.C.; Scheele, S.K.; Kiener, T.; Bloudek, L. Public Health Impact of the MVA-BN Vaccine During the 2022 Mpox Outbreak: A Systematic Review. Infect. Dis. Rep. 2025, 17, 124. https://doi.org/10.3390/idr17050124
Katsandres SC, Scheele SK, Kiener T, Bloudek L. Public Health Impact of the MVA-BN Vaccine During the 2022 Mpox Outbreak: A Systematic Review. Infectious Disease Reports. 2025; 17(5):124. https://doi.org/10.3390/idr17050124
Chicago/Turabian StyleKatsandres, Sarah C., Suzanne K. Scheele, Takako Kiener, and Lisa Bloudek. 2025. "Public Health Impact of the MVA-BN Vaccine During the 2022 Mpox Outbreak: A Systematic Review" Infectious Disease Reports 17, no. 5: 124. https://doi.org/10.3390/idr17050124
APA StyleKatsandres, S. C., Scheele, S. K., Kiener, T., & Bloudek, L. (2025). Public Health Impact of the MVA-BN Vaccine During the 2022 Mpox Outbreak: A Systematic Review. Infectious Disease Reports, 17(5), 124. https://doi.org/10.3390/idr17050124







