Cutaneous Leishmaniasis in the Immunocompromised: Diagnostic and Therapeutic Insights from a Case Documented in Central Italy
Abstract
1. Introduction
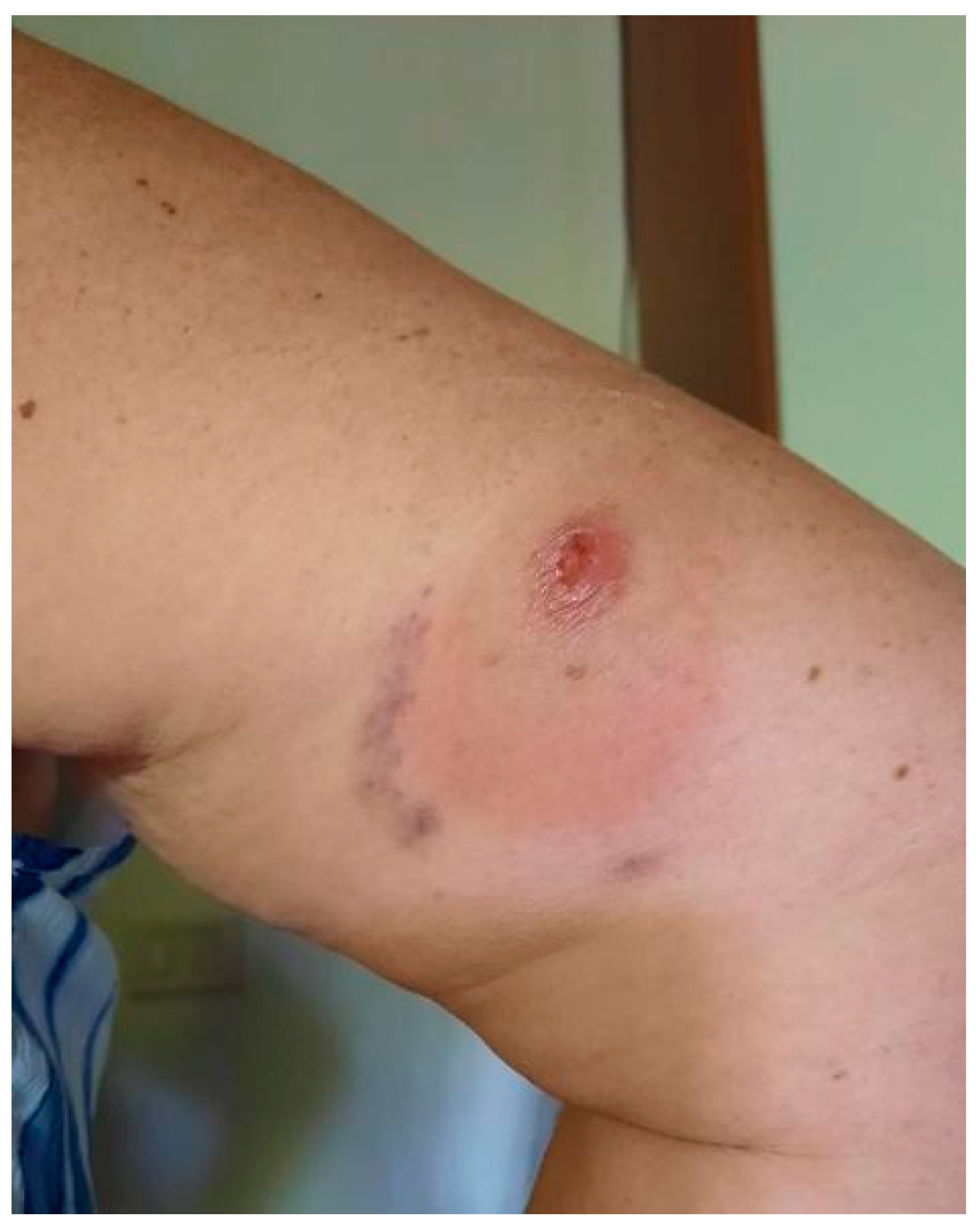
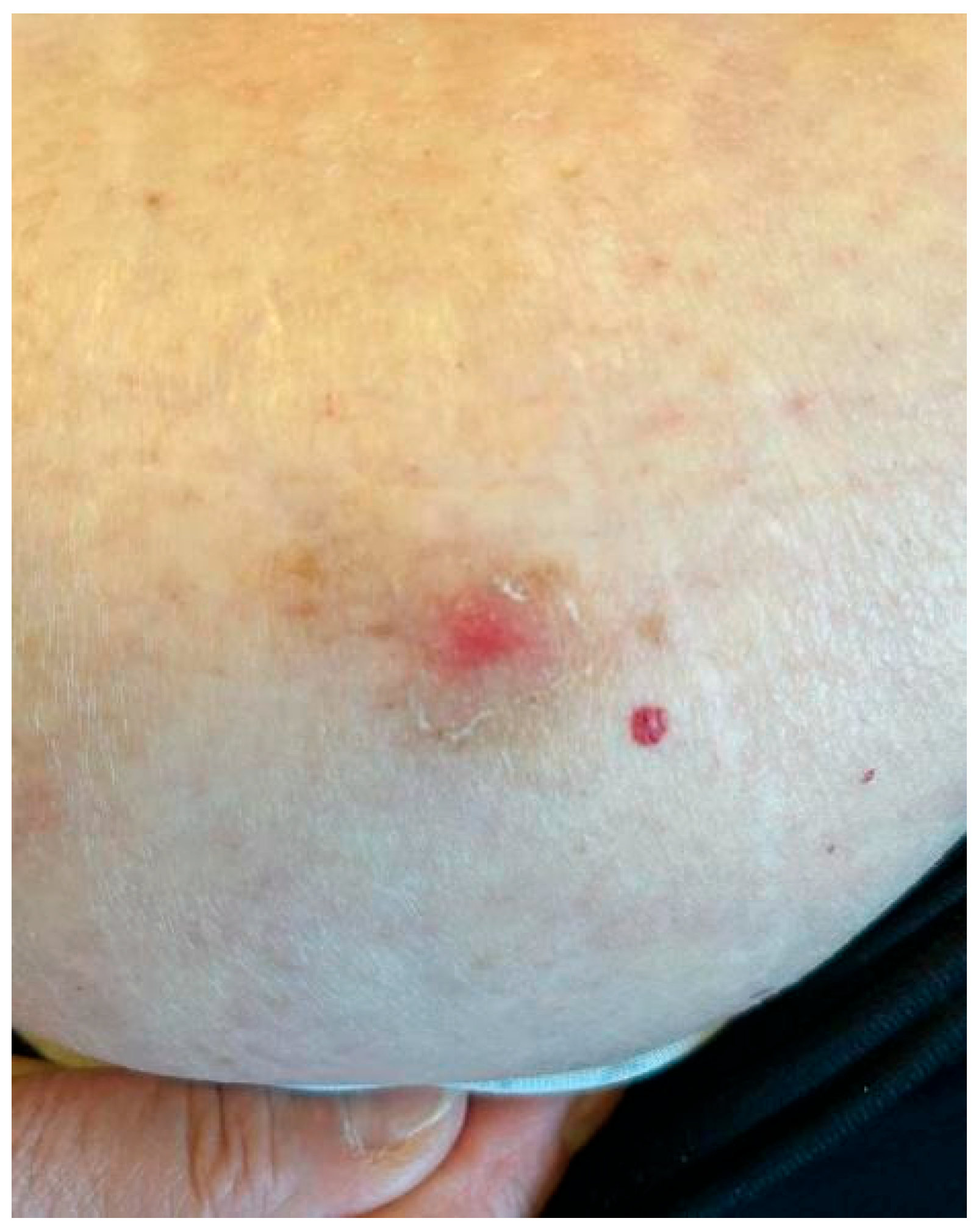
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guery, R.; Walker, S.L.; Harms, G.; Neumayr, A.; Van Thiel, P.; Gangneux, J.P.; Clerinx, J.; Söbirk, S.K.; Visser, L.; Lachaud, L.; et al. Clinical diversity and treatment results in Tegumentary Leishmaniasis: A European clinical report in 459 patients. PLoS Negl. Trop. Dis. 2021, 15, e0009863. [Google Scholar] [CrossRef]
- Maia, C.; Conceição, C.; Pereira, A.; Rocha, R.; Ortuño, M.; Muñoz, C.; Jumakanova, Z.; Pérez-Cutillas, P.; Özbel, Y.; Töz, S.; et al. The estimated distribution of autochthonous leishmaniasis by Leishmania infantum in Europe in 2005–2020. PLoS Negl. Trop. Dis. 2023, 17, e0011497. [Google Scholar] [CrossRef] [PubMed]
- Kassi, M.; Kassi, M.; Afghan, A.K.; Rehman, R.; Kasi, P.M. Marring leishmaniasis: The stigmatization and the impact of cutaneous leishmaniasis in Pakistan and Afghanistan. PLoS Negl. Trop. Dis. 2008, 2, e259. [Google Scholar] [CrossRef] [PubMed]
- Van Griensven, J.; Carrillo, E.; López-Vélez, R.; Lynen, L.; Moreno, J. Leishmaniasis in immunosuppressed individuals. Clin. Microbiol. Infect. 2014, 20, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Gramiccia, M.; Scalone, A.; Di Muccio, T.; Orsini, S.; Fiorentino, E.; Gradoni, L. The burden of visceral leishmaniasis in Italy from 1982 to 2012: A retrospective analysis of the multi-annual epidemic that occurred from 1989 to 2009. Eurosurveillance 2013, 18, 20535. [Google Scholar] [CrossRef]
- Gradoni, L.; Pozio, E.; Bettini, S.; Gramiccia, M. Leishmaniasis in Tuscany (Italy). (III) The prevalence of canine leishmaniasis in two foci of Grosseto Province. Trans. R. Soc. Trop. Med. Hyg. 1980, 74, 421–422. [Google Scholar] [CrossRef]
- Franceschini, E.; Puzzolante, C.; Menozzi, M.; Rossi, L.; Bedini, A.; Orlando, G.; Gennari, W.; Meacci, M.; Rugna, G.; Carra, E.; et al. Clinical and Microbiological Characteristics of Visceral Leishmaniasis Outbreak in a Northern Italian Nonendemic Area: A Retrospective Observational Study. BioMed Res. Int. 2016, 2016, 6481028. [Google Scholar] [CrossRef]
- Varani, S.; Cagarelli, R.; Melchionda, F.; Attard, L.; Salvadori, C.; Finarelli, A.C.; Gentilomi, G.A.; Tigani, R.; Rangoni, R.; Todeschini, R.; et al. Ongoing outbreak of visceral leishmaniasis in Bologna Province, Italy, November 2012 to May 2013. Eurosurveillance 2013, 18, 20530. [Google Scholar] [CrossRef]
- Barbiero, A.; Spinicci, M.; Aiello, A.; Maruotto, M.; Antonello, R.M.; Formica, G.; Piccica, M.; Isola, P.; Parisio, E.M.; Nardone, M.; et al. The Uprise of Human Leishmaniasis in Tuscany, Central Italy: Clinical and Epidemiological Data from a Multicenter Study. Microorganisms 2024, 12, 1963. [Google Scholar] [CrossRef]
- González, C.; Calderón, J.M.; López, A.M.; Bernardini, I.; Gradoni, L.; Pombi, M.; Bongiorno, G.; Gabrielli, S. Species-specific variation in predicted distribution and habitat suitability of phlebotomine sand flies in Italy under different climate change scenarios. Sci. Rep. 2025, 15, 13297. [Google Scholar] [CrossRef]
- Manual on Case Management and Surveillance of the Leishmaniases in the WHO European Region. Available online: https://www.who.int/publications/i/item/9789289052511 (accessed on 12 May 2025).
- Aronson, N.E.; Joya, C.A. Cutaneous Leishmaniasis: Updates in Diagnosis and Management. Infect. Dis. Clin. N. Am. 2019, 33, 101–117. [Google Scholar] [CrossRef]
- Boni, S.M.; Oyafuso, L.K.; Soler, R.D.C.; Lindoso, J.A.L. Efficiency of noninvasive sampling methods (swab) together with Polymerase Chain Reaction (PCR) for diagnosing American Tegumentary Leishmaniasis. Rev. Inst. De Med. Trop. São Paulo 2019, 59, e38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silgado, A.; Armas, M.; Sánchez-Montalvá, A.; Goterris, L.; Ubals, M.; Temprana-Salvador, J.; Aparicio, G.; Chicharro, C.; Serre-Delcor, N.; Ferrer, B.; et al. Changes in the microbiological diagnosis and epidemiology of cutaneous leishmaniasis in real-time PCR era: A six-year experience in a referral center in Barcelona. PLoS Negl. Trop. Dis. 2021, 15, e0009884. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.M.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 2016, 63, e202–e264. [Google Scholar] [CrossRef] [PubMed]
- Dinc, R. New developments in the treatment of cutaneous leishmaniasis. Asian Pac. J. Trop. Med. 2022, 15, 196–205. [Google Scholar] [CrossRef]
- Bosch-Nicolau, P.; Ubals, M.; Salvador, F.; Sánchez-Montalvá, A.; Aparicio, G.; Erra, A.; Martinez de Salazar, P.; Sulleiro, E.; Molina, I. Leishmaniasis and tumor necrosis factor alpha antagonists in the Mediterranean basin. A switch in clinical expression. PLoS Negl. Trop. Dis. 2019, 13, e0007708. [Google Scholar] [CrossRef]
- Galluzzi, L.; Ceccarelli, M.; Diotallevi, A.; Menotta, M.; Magnani, M. Real-time PCR applications for diagnosis of leishmaniasis. Parasites Vectors 2018, 11, 273. [Google Scholar] [CrossRef]
- Gomes, C.M.; Cesetti, M.V.; de Paula, N.A.; Vernal, S.; Gupta, G.; Sampaio, R.N.R.; Roselino, A.M. Field Validation of SYBR Green- and TaqMan-Based Real-Time PCR Using Biopsy and Swab Samples to Diagnose American Tegumentary Leishmaniasis in an Area Where Leishmania (Viannia) braziliensis Is Endemic. J. Clin. Microbiol. 2017, 55, 526–534. [Google Scholar] [CrossRef]
- Adams, E.R.; Gomez, M.A.; Scheske, L.; Rios, R.; Marquez, R.; Cossio, A.; Albertini, A.; Schallig, H.; Saravia, N.G. Sensitive diagnosis of cutaneous leishmaniasis by lesion swab sampling coupled to qPCR. Parasitology 2014, 141, 1891–1897. [Google Scholar] [CrossRef]
- Merino-Espinosa, G.; Rodríguez-Granger, J.; Morillas-Márquez, F.; Tercedor, J.; Corpas-López, V.; Chiheb, S.; Alcalde-Alonso, M.; Azaña-Defez, J.M.; Riyad, M.; Díaz-Sáez, V.; et al. Comparison of PCR-based methods for the diagnosis of cutaneous leishmaniasis in two different epidemiological scenarios: Spain and Morocco. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1999–2003. [Google Scholar] [CrossRef]
- Mimori, T.; Matsumoto, T.; Calvopiña, M.H.; Gomez, E.A.; Saya, H.; Katakura, K.; Nonaka, S.; Shamsuzzaman, S.M.; Hashiguchi, Y. Usefulness of sampling with cotton swab for PCR-diagnosis of cutaneous leishmaniasis in the New World. Acta Trop. 2002, 81, 197–202. [Google Scholar] [CrossRef]
- Bamorovat, M.; Sharifi, I.; Aflatoonian, M.R.; Sharifi, H.; Karamoozian, A.; Sharifi, F.; Khosravi, A.; Hassanzadeh, S. Risk factors for anthroponotic cutaneous leishmaniasis in unresponsive and responsive patients in a major focus, southeast of Iran. PLoS ONE 2018, 13, e0192236. [Google Scholar] [CrossRef]
- Mueller, M.C.; Fleischmann, E.; Grunke, M.; Schewe, S.; Bogner, J.R.; Löscher, T. Relapsing cutaneous leishmaniasis in a patient with ankylosing spondylitis treated with infliximab. Am. J. Trop. Med. Hyg. 2009, 81, 52–54. [Google Scholar] [CrossRef]
- Darcis, G.; Van der Auwera, G.; Giot, J.B.; Hayette, M.P.; Tassin, F.; Arrese Estrada, J.; Cnops, L.; Moutschen, M.; de Leval, L.; Leonard, P. Recurrence of visceral and muco-cutaneous leishmaniasis in a patient under immunosuppressive therapy. BMC Infect. Dis. 2017, 17, 478. [Google Scholar] [CrossRef]
- Eggers, Y.; Holtfreter, M.; Müller-Stoever, I.; Mischlinger, J.; Hammacher, A.; Hemmerlein, B.; Kreuter, A.; Oellig, F.; Tappe, D.; Luedde, T.; et al. A rare case of lingual mucosal leishmaniasis caused by reactivation of Leishmania infantum infection. BMC Infect. Dis. 2025, 25, 234. [Google Scholar] [CrossRef]
- Bailey, M.S.; Langman, G. Misdiagnosis of cutaneous leishmaniasis and recurrence after surgical excision. BMJ Mil. Health 2014, 160, 314–316. [Google Scholar] [CrossRef]
- Czechowicz, R.T.; Millard, T.P.; Smith, H.R.; Ashton, R.E.; Lucas, S.B.; Hay, R.J. Reactivation of cutaneous leishmaniasis after surgery. Br. J. Dermatol. 1999, 141, 1113–1116. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povolo, L.; Barbiero, A.; Spinicci, M.; Petrosillo, N.; Bartoloni, A.; Zammarchi, L. Cutaneous Leishmaniasis in the Immunocompromised: Diagnostic and Therapeutic Insights from a Case Documented in Central Italy. Infect. Dis. Rep. 2025, 17, 125. https://doi.org/10.3390/idr17050125
Povolo L, Barbiero A, Spinicci M, Petrosillo N, Bartoloni A, Zammarchi L. Cutaneous Leishmaniasis in the Immunocompromised: Diagnostic and Therapeutic Insights from a Case Documented in Central Italy. Infectious Disease Reports. 2025; 17(5):125. https://doi.org/10.3390/idr17050125
Chicago/Turabian StylePovolo, Laura, Anna Barbiero, Michele Spinicci, Nicola Petrosillo, Alessandro Bartoloni, and Lorenzo Zammarchi. 2025. "Cutaneous Leishmaniasis in the Immunocompromised: Diagnostic and Therapeutic Insights from a Case Documented in Central Italy" Infectious Disease Reports 17, no. 5: 125. https://doi.org/10.3390/idr17050125
APA StylePovolo, L., Barbiero, A., Spinicci, M., Petrosillo, N., Bartoloni, A., & Zammarchi, L. (2025). Cutaneous Leishmaniasis in the Immunocompromised: Diagnostic and Therapeutic Insights from a Case Documented in Central Italy. Infectious Disease Reports, 17(5), 125. https://doi.org/10.3390/idr17050125








