Clostridioides difficile Infection in the United States of America—A Comparative Event Risk Analysis of Patients Treated with Fidaxomicin vs. Vancomycin Across 67 Large Healthcare Providers
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Data Source
2.2. Definitions
2.3. Statistical Analysis
2.4. Ethical Considerations and Informed Consent
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feuerstadt, P.; Theriault, N.; Tillotson, G. The burden of CDI in the United States: A multifactorial challenge. BMC Infect. Dis. 2023, 23, 132. [Google Scholar] [CrossRef] [PubMed]
- U.S. Centers for Disease Control and Prevention (CDC)—2022 SPECIAL REPORT—COVID-19—U.S. IMPACT ON ANTIMICROBIAL RESISTANCE. Available online: https://www.cdc.gov/antimicrobial-resistance/media/pdfs/covid19-impact-report-508.pdf (accessed on 2 December 2022).
- Wingen-Heimann, S.M.; Davies, K.; Viprey, V.F.; Davis, G.; Wilcox, M.H.; Vehreschild, M.; Lurienne, L.; Bandinelli, P.A.; Cornely, O.A.; Vilken, T.; et al. Clostridioides difficile infection (CDI): A pan-European multi-center cost and resource utilization study, results from the Combatting Bacterial Resistance in Europe CDI (COMBACTE-CDI). Clin. Microbiol. Infect. 2023, 29, 651.e1–651.e8. [Google Scholar] [CrossRef] [PubMed]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.K. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef]
- Alsoubani, M.; Chow, J.K.; Rodday, A.M.; McDermott, L.A.; Walk, S.T.; Kent, D.M.; Snydman, D.R. The Clinical Effectiveness of Fidaxomicin Compared to Vancomycin in the Treatment of Clostridioides difficile Infection, A Single-Center Real-World Experience. J. Infect. Dis. 2024, 230, 1501–1509. [Google Scholar] [CrossRef]
- Cornely, O.A.; Crook, D.W.; Esposito, R.; Poirier, A.; Somero, M.S.; Weiss, K.; Sears, P.; Gorbach, S. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 2012, 12, 281–289. [Google Scholar] [CrossRef]
- Guery, B.; Menichetti, F.; Anttila, V.J.; Adomakoh, N.; Aguado, J.M.; Bisnauthsing, K.; Georgopali, A.; Goldenberg, S.D.; Karas, A.; Kazeem, G.; et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): A randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect. Dis. 2018, 18, 296–307. [Google Scholar] [CrossRef]
- Mikamo, H.; Tateda, K.; Yanagihara, K.; Kusachi, S.; Takesue, Y.; Miki, T.; Oizumi, Y.; Gamo, K.; Hashimoto, A.; Toyoshima, J.; et al. Efficacy and safety of fidaxomicin for the treatment of Clostridioides (Clostridium) difficile infection in a randomized, double-blind, comparative Phase III study in Japan. J. Infect. Chemother. 2018, 24, 744–752. [Google Scholar] [CrossRef]
- Pichenot, M.; Hequette-Ruz, R.; Le Guern, R.; Grandbastien, B.; Charlet, C.; Wallet, F.; Schiettecatte, S.; Loeuillet, F.; Guery, B.; Galperine, T. Fidaxomicin for treatment of Clostridium difficile infection in clinical practice: A prospective cohort study in a French University Hospital. Infection 2017, 45, 425–431. [Google Scholar] [CrossRef]
- Vargo, C.A.; Bauer, K.A.; Mangino, J.E.; Johnston, J.E.; Goff, D.A. An antimicrobial stewardship program’s real-world experience with fidaxomicin for treatment of Clostridium difficile infection: A case series. Pharmacotherapy 2014, 34, 901–909. [Google Scholar] [CrossRef]
- Wolf, J.; Kalocsai, K.; Fortuny, C.; Lazar, S.; Bosis, S.; Korczowski, B.; Petit, A.; Bradford, D.; Croos-Dabrera, R.; Incera, E. Safety and Efficacy of Fidaxomicin and Vancomycin in Children and Adolescents with Clostridioides (Clostridium) difficile Infection: A Phase 3, Multicenter, Randomized, Single-blind Clinical Trial (SUNSHINE). Clin. Infect. Dis. 2020, 71, 2581–2588. [Google Scholar] [CrossRef]
- TriNetX: Real-World Data for the Life Sciences and Healthcare. Available online: https://trinetx.com/real-world-resources/case-studies-publications/trinetx-publication-guidelines/ (accessed on 1 June 2025).
- United States Consensus Bureau—Census Regions and Divisions of the United States. Available online: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf (accessed on 2 July 2025).
- De-la-Rosa-Martinez, D.; Vilar-Compte, D.; Martinez-Rivera, N.; Ochoa-Hein, E.; Morfin-Otero, R.; Rangel-Ramirez, M.E.; Garciadiego-Fossas, P.; Mosqueda-Gomez, J.L.; Zulueta, A.P.R.; Medina-Pinon, I.; et al. Multicenter study on Clostridioides difficile infections in Mexico: Exploring the landscape. Infect. Control. Hosp. Epidemiol. 2024, 45, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Feuerstadt, P.; Boules, M.; Stong, L.; Dahdal, D.N.; Sacks, N.C.; Lang, K.; Nelson, W.W. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: A real-world data analysis. SAGE Open Med. 2021, 9, 2050312120986733. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Russo, A.; Iraci, F.; Carfagna, P.; Goldoni, P.; Vullo, V.; Venditti, M. Risk Factors and Outcomes for Bloodstream Infections Secondary to Clostridium difficile Infection. Antimicrob. Agents Chemother. 2016, 60, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Almendares, O.; Farley, M.M.; Reno, J.; Smith, Z.T.; Stein, B.; Magill, S.S.; Smith, R.M.; Cleveland, A.A.; Lessa, F.C. Epidemiology and factors associated with candidaemia following Clostridium difficile infection in adults within metropolitan Atlanta, 2009–2013. Epidemiol. Infect. 2016, 144, 1440–1444. [Google Scholar] [CrossRef]
- Chatila, W.; Manthous, C.A. Clostridium difficile causing sepsis and an acute abdomen in critically ill patients. Crit. Care Med. 1995, 23, 1146–1150. [Google Scholar] [CrossRef]
- Baggs, J.; Jernigan, J.A.; Halpin, A.L.; Epstein, L.; Hatfield, K.M.; McDonald, L.C. Risk of Subsequent Sepsis Within 90 Days After a Hospital Stay by Type of Antibiotic Exposure. Clin. Infect. Dis. 2018, 66, 1004–1012. [Google Scholar] [CrossRef]
- Louie, T.J.; Cannon, K.; Byrne, B.; Emery, J.; Ward, L.; Eyben, M.; Krulicki, W. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin. Infect. Dis. 2012, 55 (Suppl. 2), S132–S142. [Google Scholar] [CrossRef]
- Gonzales-Luna, A.J.; Carlson, T.J.; Garey, K.W. Gut microbiota changes associated with Clostridioides difficile infection and its various treatment strategies. Gut Microbes 2023, 15, 2223345. [Google Scholar] [CrossRef]
- Machado, S.; Kyriopoulos, I.; Orav, E.J.; Papanicolas, I. Association between Wealth and Mortality in the United States and Europe. N. Engl. J. Med. 2025, 392, 1310–1319. [Google Scholar] [CrossRef]
- Lubbert, C.; Zimmermann, L.; Borchert, J.; Horner, B.; Mutters, R.; Rodloff, A.C. Epidemiology and Recurrence Rates of Clostridium difficile Infections in Germany: A Secondary Data Analysis. Infect. Dis. Ther. 2016, 5, 545–554. [Google Scholar] [CrossRef]
| Cohort (Before PSM) | Characteristic | Mean ± SD | Patients (n) | % of Cohort | p-Value |
|---|---|---|---|---|---|
| VAN group | Age at day 0 | 59.8 ± 20.5 | 134,545 | 100% | <0.001 |
| FDX group | 57.5 ± 20.1 | 2170 | 100% | ||
| VAN group | Female gender | n.a. | 70,361 | 52.3% | <0.001 |
| FDX group | n.a. | 1353 | 62.4 | ||
| Cohort (after PSM) | |||||
| VAN group | Age at day 0 | 57.5 ± 20.1 | 2170 | 100% | 1 |
| FDX group | 57.5 ± 20.1 | 2170 | 100% | ||
| VAN group | Female gender | n.a. | 1353 | 62.4% | 1 |
| FDX group | n.a. | 1353 | 62.4% | ||
| Event | Event Occurred in FDX Group (Patients with Event/Overall Patients; n) | Event Occurred in VAN Group (Patients with Event/Overall Patients; n) | Risk Difference (95% CI) | Risk Difference p-Value | Risk Ratio (95% CI) | Odds Ratio (95% CI) |
|---|---|---|---|---|---|---|
| Candidiasis | 49/1963 | 105/1792 | 0.034 (0.021–0.047) | <0.001 | 2.347 (1.683–3.275) | 2.431 (1.721–3.434) |
| Cardiovascular disease | 76/715 | 64/379 | 0.063 (0.019–0.107) | 0.003 | 1.589 (1.167–2.162) | 1.708 (1.193–2.446) |
| CLABSI | 10/2161 | 18/2129 | 0.004 (−0.001–0.009) | 0.120 | 1.827 (0.845–3.949) | 1.834 (0.845–3.983) |
| Death | 244/2156 | 415/2144 | 0.080 (0.059–0.102) | <0.001 | 1.710 (1.477–1.980) | 1.881 (1.585–2.232) |
| IBD | 69/1646 | 67/1543 | 0.002 (−0.013–0.016) | 0.834 | 1.036 (0.745–1.439) | 1.037 (0.736–1.463) |
| Psychological disease | 109/1243 | 136/1146 | 0.031 (0.007–0.055) | 0.013 | 1.353 (1.066–1.718) | 1.401 (1.074–1.828) |
| Sepsis | 46/1826 | 113/1316 | 0.061 (0.044–0.077) | <0.001 | 3.409 (2.437–4.767) | 3.635 (2.560–5.161) |
| Surgical site infection | 15/2148 | 15/2129 | 0.000 (−0.005–0.005) | 0.981 | 1.009 (0.494–2.059) | 1.009 (0.492–2.069) |
| VAP | 10/2166 | 10/2138 | 0.000 (−0.004–0.004) | 0.977 | 1.013 (0.423–2.429) | 1.013 (0.421–2.439) |
| VRE infection | 75/1997 | 120/1891 | 0.026 (0.012–0.040) | <0.001 | 1.690 (1.275–2.239) | 1.736 (1.292–2.334) |
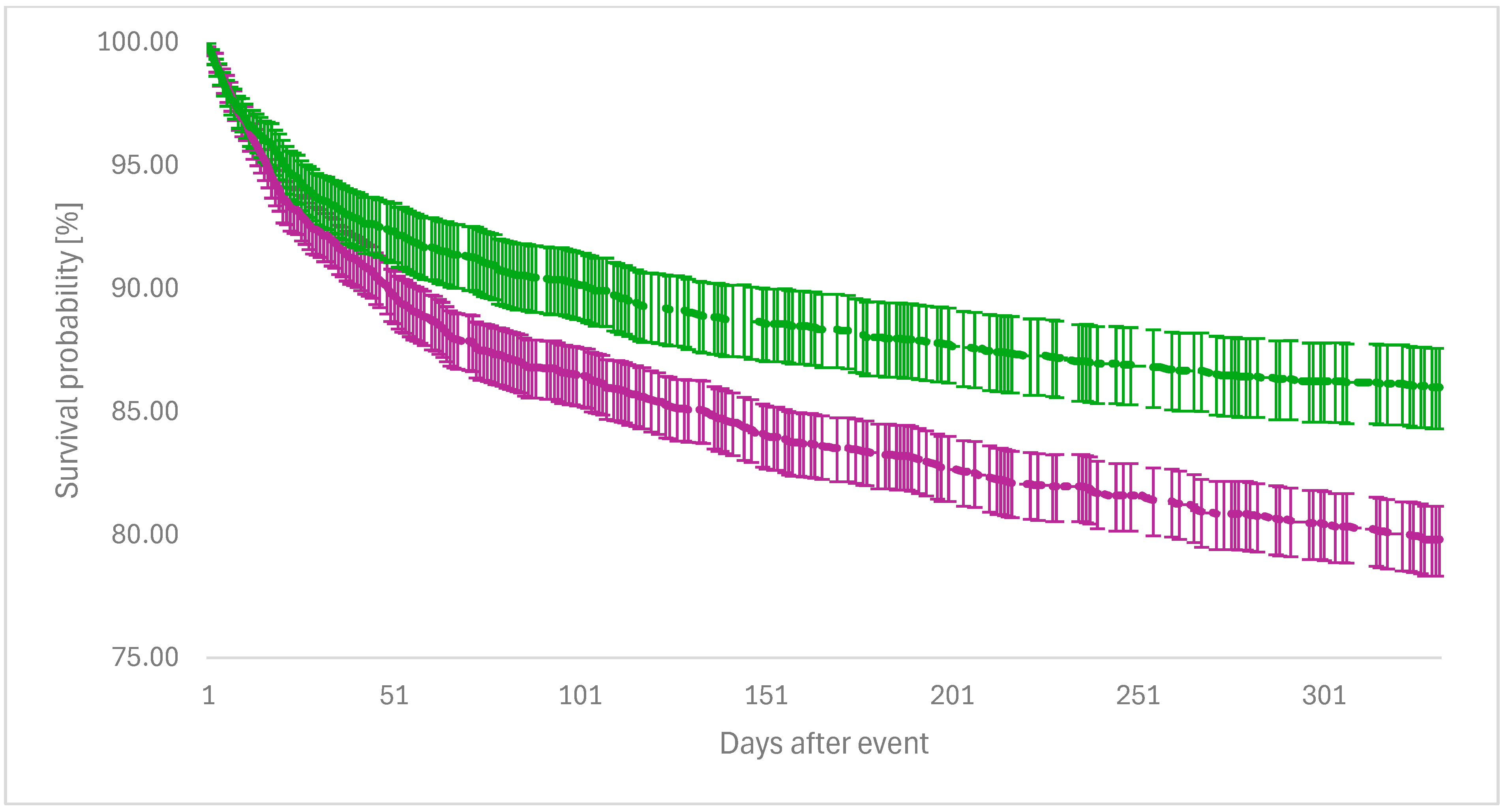
| Event | Survival Probability with Event in the FDX Group (%) | Survival Probability with Event in the VAN Group (%) | Log-Rank Test p-Value | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| Candidiasis | 96.82 | 92.74 | <0.001 | 2.350 (1.674–3.299) |
| Cardiovascular disease | 86.91 | 80.40 | 0.012 | 1.526 (1.094–2.128) |
| CLABSI | 99.87 | 98.96 | <0.001 | 9.105 (2.113–39.240) |
| IBD | 94.77 | 94.58 | 0.889 | 1.024 (0.732–1.433) |
| Psychological disease | 88.37 | 84.38 | 0.017 | 1.358 (1.055–1.747) |
| Sepsis | 96.93 | 89.95 | <0.001 | 3.399 (2.413–4.789) |
| Surgical site infection | 99.11 | 99.13 | 0.988 | 0.995 (0.486–2.034) |
| VAP | 99.76 | 99.51 | 0.252 | 1.991 (0.599–6.611) |
| VRE infection | 95.56 | 92.44 | <0.001 | 1.684 (1.262–2.247) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wingen-Heimann, S.M.; Lübbert, C.; Bavaro, D.F.; Hopff, S.M. Clostridioides difficile Infection in the United States of America—A Comparative Event Risk Analysis of Patients Treated with Fidaxomicin vs. Vancomycin Across 67 Large Healthcare Providers. Infect. Dis. Rep. 2025, 17, 87. https://doi.org/10.3390/idr17040087
Wingen-Heimann SM, Lübbert C, Bavaro DF, Hopff SM. Clostridioides difficile Infection in the United States of America—A Comparative Event Risk Analysis of Patients Treated with Fidaxomicin vs. Vancomycin Across 67 Large Healthcare Providers. Infectious Disease Reports. 2025; 17(4):87. https://doi.org/10.3390/idr17040087
Chicago/Turabian StyleWingen-Heimann, Sebastian M., Christoph Lübbert, Davide Fiore Bavaro, and Sina M. Hopff. 2025. "Clostridioides difficile Infection in the United States of America—A Comparative Event Risk Analysis of Patients Treated with Fidaxomicin vs. Vancomycin Across 67 Large Healthcare Providers" Infectious Disease Reports 17, no. 4: 87. https://doi.org/10.3390/idr17040087
APA StyleWingen-Heimann, S. M., Lübbert, C., Bavaro, D. F., & Hopff, S. M. (2025). Clostridioides difficile Infection in the United States of America—A Comparative Event Risk Analysis of Patients Treated with Fidaxomicin vs. Vancomycin Across 67 Large Healthcare Providers. Infectious Disease Reports, 17(4), 87. https://doi.org/10.3390/idr17040087








