Implementation and Adherence of a Custom Mobile Application for Anonymous Bidirectional Communication Among Nearly 4000 Participants: Insights from the Longitudinal RisCoin Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Concept and Architecture of the RisCoin Study App on CentraXX App
2.2. Technical, Ethical, and Data Protection Considerations of CentraXX App
2.3. Administration of the RisCoin Study Participant Profile
2.4. User Journey and Messaging Functions
2.4.1. Mass Messaging
2.4.2. Hotline
2.4.3. Short Questionnaire
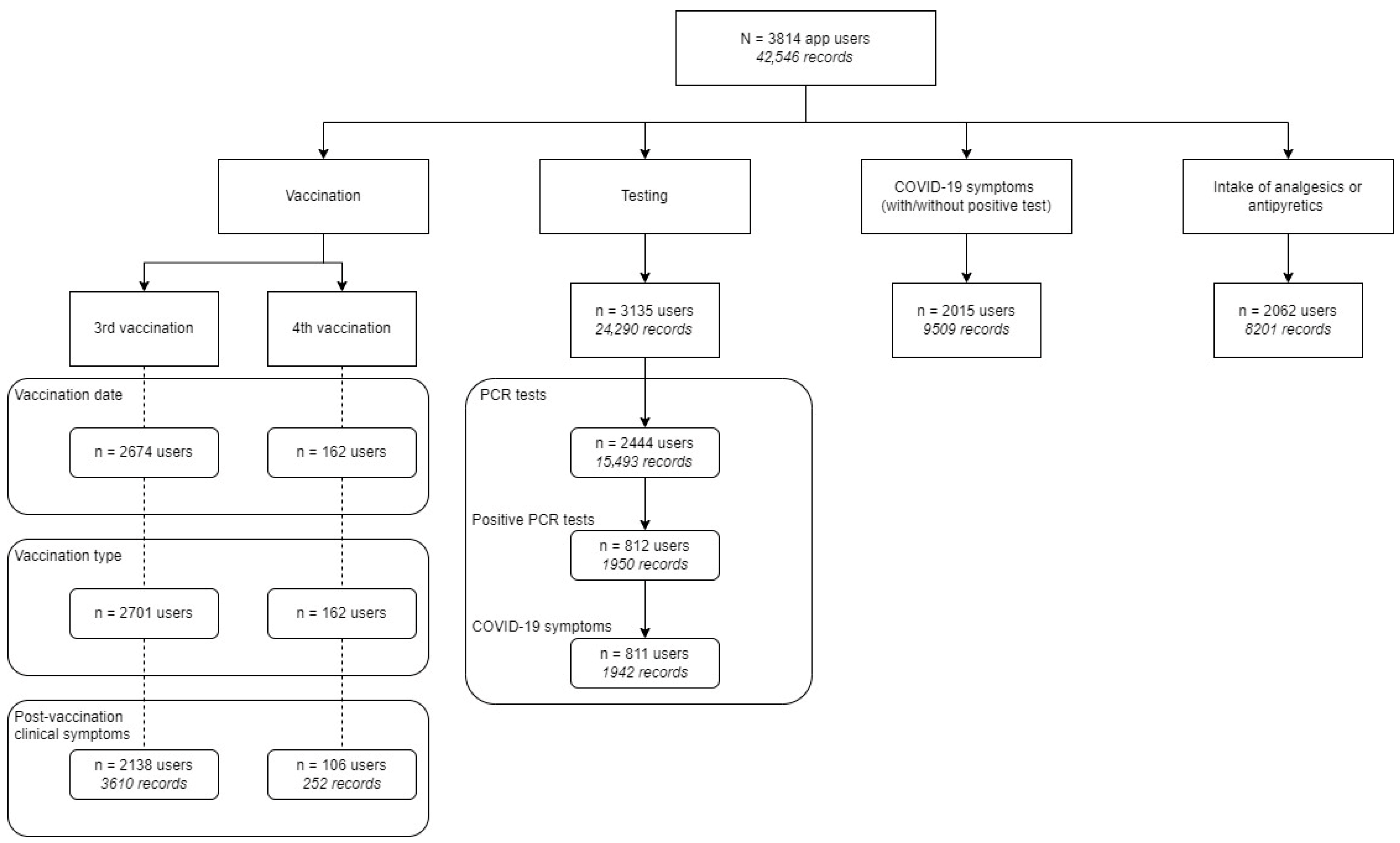
2.5. Data Extraction and Analysis
3. Results
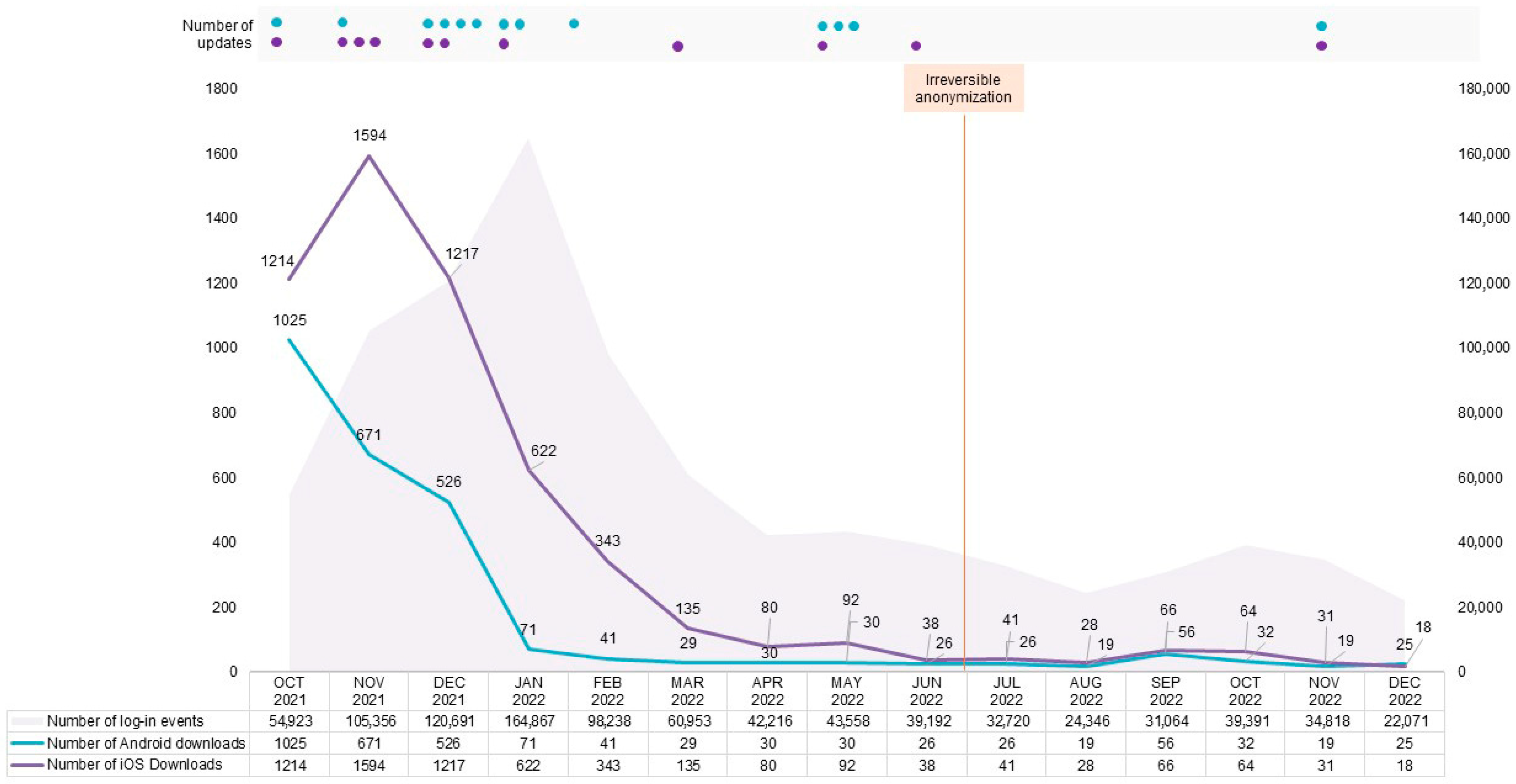
3.1. App Usage
3.2. Hotline
3.3. User Statistics Through Short Questionnaire and Hotline Module
3.4. Active User Rate, Adherence, and Associated Factors
4. Discussion
4.1. Pitfalls and Frequent Issues of an App-Based Study Design
4.1.1. Usability Issues
4.1.2. Validity and Reliability of the Data
4.2. Technical Issues Unrelated to Study Design
4.2.1. Older Phones Incapable of Supporting the App
4.2.2. Differences Between Operational Systems
4.3. Benefits of an App-Based Study Design
4.3.1. Agility and Flexibility
4.3.2. Facilitating Data Collection and Overview
4.4. Strengths and Limitations of Our Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrecht, U.-V.; von Jan, U.; Pramann, O.; Fangerau, H. Kapitel 7—Gesundheits-Apps im Forschungskontext. Chancen Und Risiken Von Gesundh. Apps 2016, 14–47. [Google Scholar] [CrossRef]
- LoPresti, M.A.; Abraham, M.E.; Appelboom, G.; Bruyère, O.; Slomian, J.; Reginster, J.-Y.; Connolly, E.S. Clinical Trials Using Mobile Health Applications. Pharm Med. 2015, 29, 17–25. [Google Scholar] [CrossRef]
- Lane, S.J.; Heddle, N.M.; Arnold, E.; Walker, I. A review of randomized controlled trials comparing the effectiveness of hand held computers with paper methods for data collection. BMC Med. Inform. Decis. Mak. 2006, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Nowojewski, A.; Bark, E.; Shih, V.H.; Dearden, R. Patient adherence and response time in electronic patient-reported outcomes: Insights from three longitudinal clinical trials. Qual. Life Res. 2024, 33, 1691–1706. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Kleen, S. Possibilities, Problems, and Perspectives of Data Collection by Mobile Apps in Longitudinal Epidemiological Studies: Scoping Review. J. Med. Internet Res. 2021, 23, e17691. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Le Thi, T.G.; Zhelyazkova, A.; Osterman, A.; Wichert, S.P.; Breiteneicher, S.; Koletzko, L.; Schwerd, T.; Völk, S.; Jebrini, T.; et al. A prospective longitudinal cohort study on risk factors for COVID-19 vaccination failure (RisCoin): Methods, procedures and characterization of the cohort. Clin. Exp. Med. 2023, 23, 4901–4917. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.; Campbell, A.N.C.; Miele, G.M.; Brunner, M.; Winstanley, E.L. Using e-technologies in clinical trials. Contemp. Clin. Trials 2015, 45, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Deutsches Institut für Normung. Medizinprodukte—Qualitätsmanagementsysteme—Anforderungen für Regulatorische Zwecke (ISO 13485:2016); Deutsche Fassung EN ISO 13485:2016 + AC:2018 + A11:2021: Medical devices—Quality Management systems—Requirements for Regulatory Purposes (ISO 13485:2016); German Version EN ISO 13485:2016 + AC:2018 + A11:2021, 03.100.70, 11.040.01. Available online: https://www.dinmedia.de/de/norm/din-en-iso-13485/332674603 (accessed on 20 October 2024).
- International Society for Pharmaceutical Engineering. Good (Automated) Manufacturing Process, 2008. Available online: https://guidance-docs.ispe.org/doi/book/10.1002/9781946964571?utm_source=google&utm_medium=paidsearch&utm_campaign=gamp5guide_wiley&gad_source=1&gad_campaignid=21032404309&gbraid=0AAAAAqlypk1EYqG6GqPeS9CxYSjR4f6z8&gclid=CjwKCAjw4efDBhATEiwAaDBpboUahI9w885qpsbVAXuD4KahvrKLpu--yFBeVd3faxacOyB5nHf8RhoCGv8QAvD_BwE (accessed on 19 October 2024).
- Marcano Belisario, J.S.; Jamsek, J.; Huckvale, K.; O’Donoghue, J.; Morrison, C.P.; Car, J. Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database Syst. Rev. 2015, 2015, MR000042. [Google Scholar] [CrossRef] [PubMed]
- Apple Developer. Human Interface Guidelines (HIG). Available online: https://developer.apple.com/design/human-interface-guidelines/ (accessed on 19 July 2024).
- Android Developer. Design for Mobile. Available online: https://developer.android.com/design/ui/mobile (accessed on 19 July 2024).
- Fliege, H.; Rose, M.; Arck, P.; Levenstein, S.; Klapp, B.F. Validierung des “Perceived Stress Questionnaire” (PSQ) an einer deutschen Stichprobe. Diagnostica 2001, 47, 142–152. [Google Scholar] [CrossRef]
- Fliege, H.; Rose, M.; Arck, P.; Walter, O.B.; Kocalevent, R.-D.; Weber, C.; Klapp, B.F. The Perceived Stress Questionnaire (PSQ) reconsidered: Validation and reference values from different clinical and healthy adult samples. Psychosom. Med. 2005, 67, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, L.; Klucker, E.; Le Thi, T.G.; Breiteneicher, S.; Rubio-Acero, R.; Neuhaus, L.; Stark, R.G.; Standl, M.; Wieser, A.; Török, H.; et al. Following Pediatric and Adult IBD Patients through the COVID-19 Pandemic: Changes in Psychosocial Burden and Perception of Infection Risk and Harm over Time. J. Clin. Med. 2021, 10, 4124. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity-Preventing and Managing the Global Epidemic: Report on a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; ISBN 9789241208949. [Google Scholar]
- Scott Kruse, C.; Karem, P.; Shifflett, K.; Vegi, L.; Ravi, K.; Brooks, M. Evaluating barriers to adopting telemedicine worldwide: A systematic review. J. Telemed. Telecare 2018, 24, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Becker, R. Gender and Survey Participation An Event History Analysis of the Gender Effects of Survey Participation in a Probability-based Multi-wave Panel Study with a Sequential Mixed-mode Design. Methods Data Anal. 2017, 16, 29. [Google Scholar] [CrossRef]
- Jackson, L.A.; Ervin, K.S.; Gardner, P.D.; Schmitt, N. Gender and the Internet: Women Communicating and Men Searching. Sex Roles 2001, 44, 363–379. [Google Scholar] [CrossRef]
- Slauson-Blevins, K.; Johnson, K.M. Doing Gender, Doing Surveys? Women’s Gatekeeping and Men’s Non-Participation in Multi-Actor Reproductive Surveys. Sociol. Inq. 2016, 86, 427–449. [Google Scholar] [CrossRef]
- Smith, W.G. Does Gender Influence Online Survey Participation? A Record-Linkage Analysis of University Faculty Online Survey Response Behavior; San Jose State University: San Jose, CA, USA, 2008. [Google Scholar]
- Kelso, M.; Feagins, L.A. Can Smartphones Help Deliver Smarter Care for Patients with Inflammatory Bowel Disease? Inflamm. Bowel Dis. 2018, 24, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Olivero, E.; Bert, F.; Thomas, R.; Scarmozzino, A.; Raciti, I.M.; Gualano, M.R.; Siliquini, R. E-tools for the hospital management: An overview of smartphone applications for health professionals. Int. J. Med. Inform. 2019, 124, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Csollarova, K.; Koletzko, L.; Le Thi, T.G.; Wratil, P.R.; Zhelyazkova, A.; Breiteneicher, S.; Stern, M.; Lupoli, G.; Schwerd, T.; Choukér, A.; et al. Factors Associated with Impaired Humoral Immune Response to mRNA Vaccines in Patients with Inflammatory Bowel Disease: A Matched-Cohort Analysis from the RisCoin Study. Vaccines 2025, 13, 673. [Google Scholar] [CrossRef]
- Grimm, M.; Link, E.; Albrecht, M.; Czerwinski, F.; Baumann, E.; Suhr, R. Exploring Functions and Predictors of Digital Health Engagement Among German Internet Users: Survey Study. J. Med. Internet Res. 2023, 25, e44024. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Neto, E.C.; Snyder, P.; Stepnowsky, C.; Elhadad, N.; Grant, D.; Mohebbi, M.H.; Mooney, S.; Suver, C.; Wilbanks, J.; et al. Indicators of retention in remote digital health studies: A cross-study evaluation of 100,000 participants. NPJ Digit. Med. 2020, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Corace, K.M.; Srigley, J.A.; Hargadon, D.P.; Yu, D.; MacDonald, T.K.; Fabrigar, L.R.; Garber, G.E. Using behavior change frameworks to improve healthcare worker influenza vaccination rates: A systematic review. Vaccine 2016, 34, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Prematunge, C.; Corace, K.; McCarthy, A.; Nair, R.C.; Pugsley, R.; Garber, G. Factors influencing pandemic influenza vaccination of healthcare workers—A systematic review. Vaccine 2012, 30, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Leitmeyer, K.; Buchholz, U.; Kramer, M.; Schenkel, K.; Stahlhut, H.; Köllstadt, M.; Haas, W.; Meyer, C. Influenza vaccination in German health care workers: Effects and findings after two rounds of a nationwide awareness campaign. Vaccine 2006, 24, 7003–7008. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; White, T.M.; Wyka, K.; Ratzan, S.C.; Rabin, K.; Larson, H.J.; Martinon-Torres, F.; Kuchar, E.; Abdool Karim, S.S.; Giles-Vernick, T.; et al. Influence of COVID-19 on trust in routine immunization, health information sources and pandemic preparedness in 23 countries in 2023. Nat. Med. 2024, 30, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Le Thi, T.G.; Osterman, A.; Badell, I.; Huber, M.; Zhelyazkova, A.; Wichert, S.P.; Litwin, A.; Hörmansdorfer, S.; Strobl, F.; et al. Dietary habits, traveling and the living situation potentially influence the susceptibility to SARS-CoV-2 infection: Results from healthcare workers participating in the RisCoin Study. Infection 2024, 52, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Nilan, K.; McKeever, T.M.; McNeill, A.; Raw, M.; Murray, R.L. Prevalence of tobacco use in healthcare workers: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0220168. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Chen, J.L.; Li, G.; Baláž, V. Risk, uncertainty and ambiguity amid Covid-19: A multi-national analysis of international travel intentions. Ann. Tour. Res. 2022, 92, 103346. [Google Scholar] [CrossRef] [PubMed]
- Zhelyazkova, A.; Adorjan, K.; Kim, S.; Klein, M.; Prueckner, S.; Kressirer, P.; Choukér, A.; Coenen, M.; Horster, S. Are We Prepared for the Next Pandemic? Management, Systematic Evaluation and Lessons Learned from an In-Hospital COVID-19 Vaccination Centre for Healthcare Workers. Int. J. Environ. Res. Public Health 2022, 19, 16326. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, Z.; Ji, Y.; Ma, L.; Liu, F.; Chi, M.; Deng, N.; An, J. Using Goal-Directed Design to Create a Mobile Health App to Improve Patient Compliance with Hypertension Self-Management: Development and Deployment. JMIR Mhealth Uhealth 2020, 8, e14466. [Google Scholar] [CrossRef] [PubMed]
- Menvielle, L.; Ertz, M.; François, J.; Audrain-Pontevia, A.-F. User-Involved Design of Digital Health Products. In Revolutions in Product Design for Healthcare: Advances in Product Design and Design Methods for Healthcare, 1st ed.; Subburaj, K., Sandhu, K., Ćuković, S., Eds.; Springer Singapore; Imprint Springer: Singapore, 2022; pp. 1–19. ISBN 978-981-16-9454-7. [Google Scholar]
- Bandarian-Balooch, S.; Martin, P.R.; McNally, B.; Brunelli, A.; Mackenzie, S. Electronic-Diary for Recording Headaches, Triggers, and Medication Use: Development and Evaluation. Headache 2017, 57, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Kristman, V.; Manno, M.; Côté, P. Loss to follow-up in cohort studies: How much is too much? Eur. J. Epidemiol. 2004, 19, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Vernet, P.; Miro, J. The Mobile App Development and Assessment Guide (MAG): Delphi-Based Validity Study. JMIR Mhealth Uhealth 2020, 8, e17760. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Li, K.; Feng, C.; Asim, S.M.; Yousif, A.; Ge, S. An Empirical Study of Investigating Mobile Applications Development Challenges. IEEE Access 2018, 6, 17711–17728. [Google Scholar] [CrossRef]
- Gustavell, T.; Sundberg, K.; Langius-Eklof, A. Using an Interactive App for Symptom Reporting and Management Following Pancreatic Cancer Surgery to Facilitate Person-Centered Care: Descriptive Study. JMIR Mhealth Uhealth 2020, 8, e17855. [Google Scholar] [CrossRef] [PubMed]
- Ali Sherazi, B.; Laeer, S.; Krutisch, S.; Dabidian, A.; Schlottau, S.; Obarcanin, E. Functions of mHealth Diabetes Apps That Enable the Provision of Pharmaceutical Care: Criteria Development and Evaluation of Popular Apps. Int. J. Environ. Res. Public Health 2023, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawashdeh, M.; Keikhosrokiani, P.; Belaton, B.; Alawida, M.; Zwiri, A. IoT Adoption and Application for Smart Healthcare: A Systematic Review. Sensors 2022, 22, 5377. [Google Scholar] [CrossRef] [PubMed]
- Vielhauer, J.; Mahajan, U.M.; Adorjan, K.; Benesch, C.; Oehrle, B.; Beyer, G.; Sirtl, S.; Johlke, A.-L.; Allgeier, J.; Pernpruner, A.; et al. Electronic data capture in resource-limited settings using the lightweight clinical data acquisition and recording system. Sci. Rep. 2024, 14, 19056. [Google Scholar] [CrossRef] [PubMed]
- Babre, D. Electronic data capture—Narrowing the gap between clinical and data management. Perspect. Clin. Res. 2011, 2, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.A.; Shiffman, S.; Schwartz, J.E.; Broderick, J.E.; Hufford, M.R. Patient compliance with paper and electronic diaries. Control Clin. Trials 2003, 24, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Marceau, L.D.; Link, C.; Jamison, R.N.; Carolan, S. Electronic diaries as a tool to improve pain management: Is there any evidence? Pain Med. 2007, 8, S101–S109. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Perry, H.B.; Long, L.A.; Labrique, A.B. Evidence on feasibility and effective use of mHealth strategies by frontline health workers in developing countries: Systematic review. Trop. Med. Int. Health 2015, 20, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Bot, B.M.; Suver, C.; Neto, E.C.; Kellen, M.; Klein, A.; Bare, C.; Doerr, M.; Pratap, A.; Wilbanks, J.; Dorsey, E.R.; et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci. Data 2016, 3, 160011. [Google Scholar] [CrossRef] [PubMed]
- Cooray, C.; Matusevicius, M.; Wahlgren, N.; Ahmed, N. Mobile Phone-Based Questionnaire for Assessing 3 Months Modified Rankin Score After Acute Stroke: A Pilot Study. Circ. Cardiovasc. Qual. Outcomes 2015, 8, S125–S130. [Google Scholar] [CrossRef] [PubMed]

| RisCoin Sub-Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|
| HCW | Patients with IBD | |||||||
| Non-Adherent Users N = 2521 (73%) | Adherent Users N = 958 (27%) | Total N = 3479 | p-Value | Non-Adherent Users N = 85 (60%) | Adherent Users N = 58 (40%) | Total N = 143 | p-Value | |
| RisCoin HCWs health status | <0.001 1 | N/A | N/A | |||||
| HCW-plus 2 | 1125 (68%) | 539 (32%) | 1664 (48%) | N/A | N/A | |||
| HCW-healthy 3 | 1396 (77%) | 419 (23%) | 1815 (52%) | N/A | N/A | |||
| Gender | <0.001 1 | 0.960 1 | ||||||
| Males | 706 (80%) | 173 (20%) | 879 (25%) | 48 (59%) | 33 (41%) | 81 (57%) | ||
| Females | 1808 (70%) | 784 (30%) | 2592 (75%) | 37 (60%) | 25 (40%) | 62 (43%) | ||
| Age groups | <0.001 1 | 0.029 1 | ||||||
| 18–30 | 873 (86%) | 137 (14%) | 1010 (29%) | 22 (85%) | 4 (15%) | 26 (18%) | ||
| 31–40 | 678 (78%) | 191 (22%) | 869 (25%) | 22 (61%) | 14 (39%) | 36 (25%) | ||
| 41–50 | 424 (66%) | 218 (34%) | 642 (18%) | 16 (59%) | 11 (41%) | 27 (19%) | ||
| 51–60 | 417 (59%) | 292 (41%) | 709 (20%) | 16 (46%) | 19 (54%) | 35 (24%) | ||
| >60 | 129 (52%) | 120 (48%) | 249 (7%) | 9 (47%) | 10 (53%) | 19 (13%) | ||
| BMI in 4 categories 4 | 0.004 1 | 0.188 1 | ||||||
| Underweight | 72 (71%) | 29 (29%) | 101 (3%) | 3 (75%) | 1 (25%) | 4 (3%) | ||
| Normal weight | 1602 (74%) | 565 (26%) | 2167 (62%) | 45 (63%) | 26 (37%) | 71 (50%) | ||
| Pre-obesity | 610 (72%) | 235 (28%) | 845 (24%) | 24 (48%) | 26 (52%) | 50 (35%) | ||
| Obesity all classes | 236 (65%) | 129 (35%) | 365 (10%) | 13 (72%) | 5 (28%) | 18 (13%) | ||
| Part-time employment | <0.001 1 | 0.876 1 | ||||||
| Yes | 737 (67%) | 363 (33%) | 1100 (32%) | 20 (61%) | 13 (39%) | 33 (23%) | ||
| No | 1782 (75%) | 594 (25%) | 2376 (68%) | 65 (59%) | 45 (41%) | 110 (77%) | ||
| Education | <0.001 1 | 0.326 1 | ||||||
| Middle school diploma | 217 (63%) | 128 (37%) | 345 (10%) | 19 (66%) | 10 (34%) | 29 (21%) | ||
| High school diploma | 628 (74%) | 220 (26%) | 848 (25%) | 15 (71%) | 6 (29%) | 21 (15%) | ||
| Completed apprenticeship | 481 (67%) | 239 (33%) | 720 (21%) | 18 (56%) | 14 (44%) | 32 (23%) | ||
| University degree | 1170 (77%) | 353 (23%) | 1523 (44%) | 29 (51%) | 28 (49%) | 57 (41%) | ||
| Healthcare occupation | <0.001 1 | 0.207 1 | ||||||
| Nurses | 592 (71%) | 246 (29%) | 838 (24%) | 4 (80%) | 1 (20%) | 5 (3%) | ||
| Physicians | 482 (83%) | 100 (17%) | 582 (17%) | 2 (67%) | 1 (33%) | 3 (2%) | ||
| Administration | 452 (65%) | 245 (35%) | 697 (20%) | 0 (0%) | 2 (100%) | 2 (1%) | ||
| Others including TA, service staff | 783 (73%) | 288 (27%) | 1071 (31%) | 1 (25%) | 3 (75%) | 4 (3%) | ||
| Clinical scientists not working in patient care | 199 (73%) | 74 (27%) | 273 (8%) | 78 (60%) | 51 (40%) | 129 (90%) | ||
| Avoiding special foods i.e., due to allergy or intolerance | 0.003 1 | 0.804 1 | ||||||
| Yes | 148 (64%) | 83 (36%) | 231 (7%) | 19 (58%) | 14 (42%) | 33 (23%) | ||
| No | 2373 (73%) | 875 (27%) | 3248 (93%) | 66 (60%) | 44 (40%) | 110 (77%) | ||
| Participants with direct patient contact | <0.001 1 | 0.487 1 | ||||||
| Yes | 1631 (75%) | 535 (25%) | 2166 (62%) | 6 (50%) | 6 (50%) | 12 (8%) | ||
| No | 887 (68%) | 422 (32%) | 1309 (38%) | 79 (60%) | 52 (40%) | 131 (92%) | ||
| COVID-19-related questions | ||||||||
| Contact with confirmed SARS-CoV-2 infected person | 0.001 1 | 0.049 1 | ||||||
| Yes | 1232 (75%) | 410 (25%) | 1642 (47%) | 11 (42%) | 15 (58%) | 26 (18%) | ||
| No or Unknown | 1276 (70%) | 544 (30%) | 1820 (53%) | 74 (63%) | 43 (37%) | 117 (82%) | ||
| Intensity of clinical symptoms after the 2nd COVID-19 vaccination | 0.094 1 | 0.074 1 | ||||||
| None | 1052 (73%) | 395 (27%) | 1447 (43%) | 56 (65%) | 30 (35%) | 86 (61%) | ||
| Mild or moderate complaints | 1144 (71%) | 462 (29%) | 1606 (47%) | 23 (46%) | 27 (54%) | 50 (36%) | ||
| Severe complaints | 258 (77%) | 77 (23%) | 335 (10%) | 3 (75%) | 1 (25%) | 4 (3%) | ||
| Vaccinated against influenza during the last flu season | <0.001 1 | 0.040 1 | ||||||
| Yes | 1231 (68%) | 571 (32%) | 1802 (53%) | 43 (51%) | 41 (49%) | 84 (63%) | ||
| No | 1224 (77%) | 375 (23%) | 1599 (47%) | 34 (69%) | 15 (31%) | 49 (37%) | ||
| Pollen allergy | <0.001 1 | 0.908 1 | ||||||
| Yes | 735 (69%) | 337 (31%) | 1072 (31%) | 25 (58%) | 18 (42%) | 43 (30%) | ||
| No | 1776 (74%) | 621 (26%) | 2397 (69%) | 58 (59%) | 40 (41%) | 98 (70%) | ||
| Smoking status (consumption of tobacco products e-cigarettes hookah pipe) | <0.001 1 | 0.209 1 | ||||||
| Current smoker | 475 (79%) | 128 (21%) | 603 (18%) | 18 (69%) | 8 (31%) | 26 (19%) | ||
| Non-smoker/Previous smoker | 2004 (71%) | 822 (29%) | 2826 (82%) | 63 (56%) | 50 (44%) | 113 (81%) | ||
| Self-perceived stress, PSQ > 33 5 | 0.108 1 | 0.992 1 | ||||||
| Yes | 1401 (74%) | 505 (26%) | 1906 (55%) | 43 (59%) | 30 (41%) | 73 (52%) | ||
| No | 1107 (71%) | 451 (29%) | 1558 (45%) | 40 (59%) | 28 (41%) | 68 (48%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhelyazkova, A.; Koletzko, S.; Adorjan, K.; Schrimf, A.; Völk, S.; Koletzko, L.; Fabry-Said, A.; Osterman, A.; Badell, I.; Eden, M.; et al. Implementation and Adherence of a Custom Mobile Application for Anonymous Bidirectional Communication Among Nearly 4000 Participants: Insights from the Longitudinal RisCoin Study. Infect. Dis. Rep. 2025, 17, 88. https://doi.org/10.3390/idr17040088
Zhelyazkova A, Koletzko S, Adorjan K, Schrimf A, Völk S, Koletzko L, Fabry-Said A, Osterman A, Badell I, Eden M, et al. Implementation and Adherence of a Custom Mobile Application for Anonymous Bidirectional Communication Among Nearly 4000 Participants: Insights from the Longitudinal RisCoin Study. Infectious Disease Reports. 2025; 17(4):88. https://doi.org/10.3390/idr17040088
Chicago/Turabian StyleZhelyazkova, Ana, Sibylle Koletzko, Kristina Adorjan, Anna Schrimf, Stefanie Völk, Leandra Koletzko, Alexandra Fabry-Said, Andreas Osterman, Irina Badell, Marc Eden, and et al. 2025. "Implementation and Adherence of a Custom Mobile Application for Anonymous Bidirectional Communication Among Nearly 4000 Participants: Insights from the Longitudinal RisCoin Study" Infectious Disease Reports 17, no. 4: 88. https://doi.org/10.3390/idr17040088
APA StyleZhelyazkova, A., Koletzko, S., Adorjan, K., Schrimf, A., Völk, S., Koletzko, L., Fabry-Said, A., Osterman, A., Badell, I., Eden, M., Choukér, A., Tuschen, M., Koletzko, B., Hao, Y., Tu, L., Török, H. P., Wichert, S. P., & Le Thi, T. G., on behalf of Members of RisCoin Study Group. (2025). Implementation and Adherence of a Custom Mobile Application for Anonymous Bidirectional Communication Among Nearly 4000 Participants: Insights from the Longitudinal RisCoin Study. Infectious Disease Reports, 17(4), 88. https://doi.org/10.3390/idr17040088






