Molecular Epidemiology of Hepatitis C Virus Genotypes in Northern Thailand: A Retrospective Study from 2016 to 2024
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. RNA Extraction, HCV Viral Load, and Genotyping
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Distribution of HCV Genotypes and Subtypes
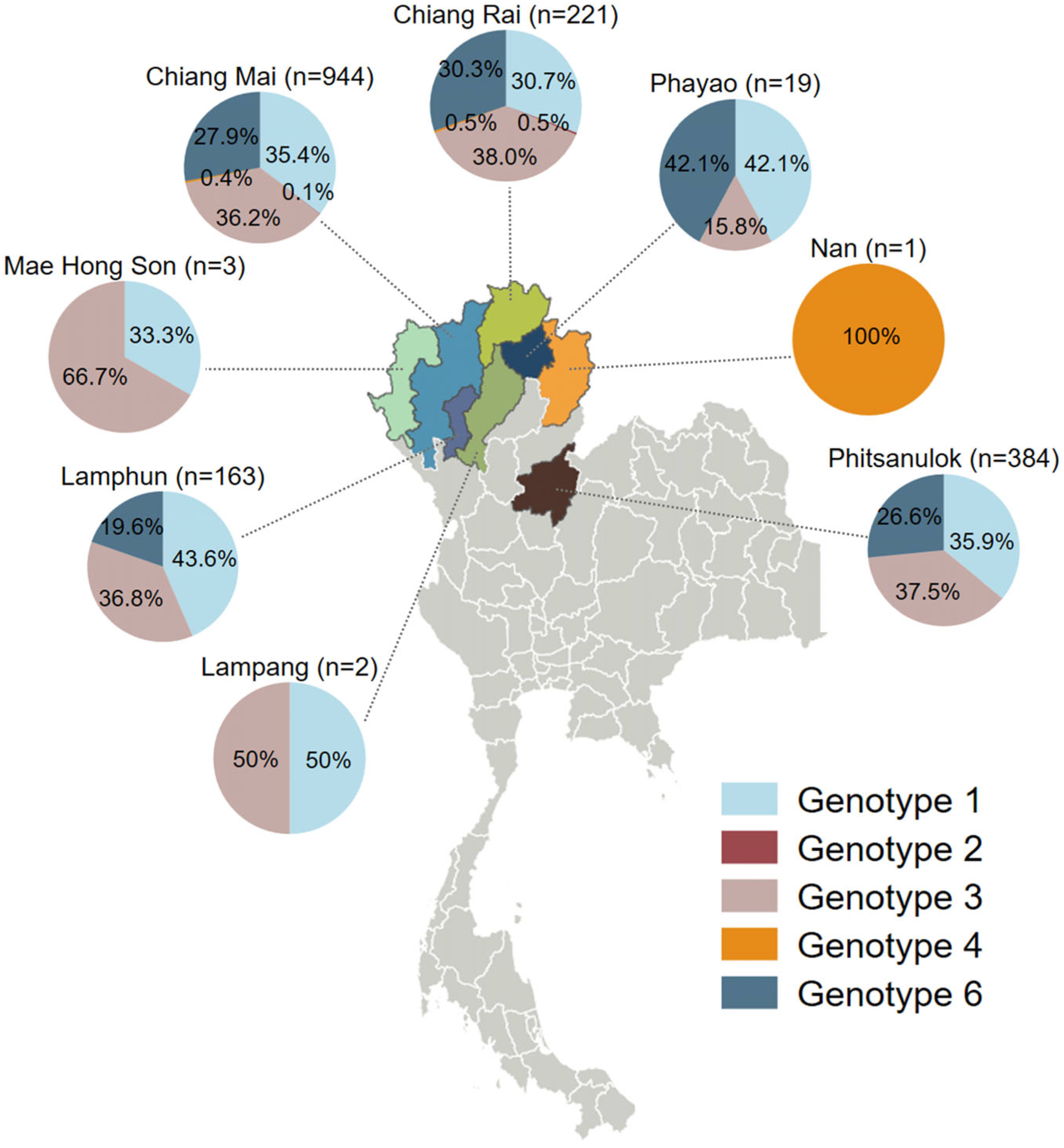
3.3. Geographic Distribution of HCV Genotypes in Northern Thailand
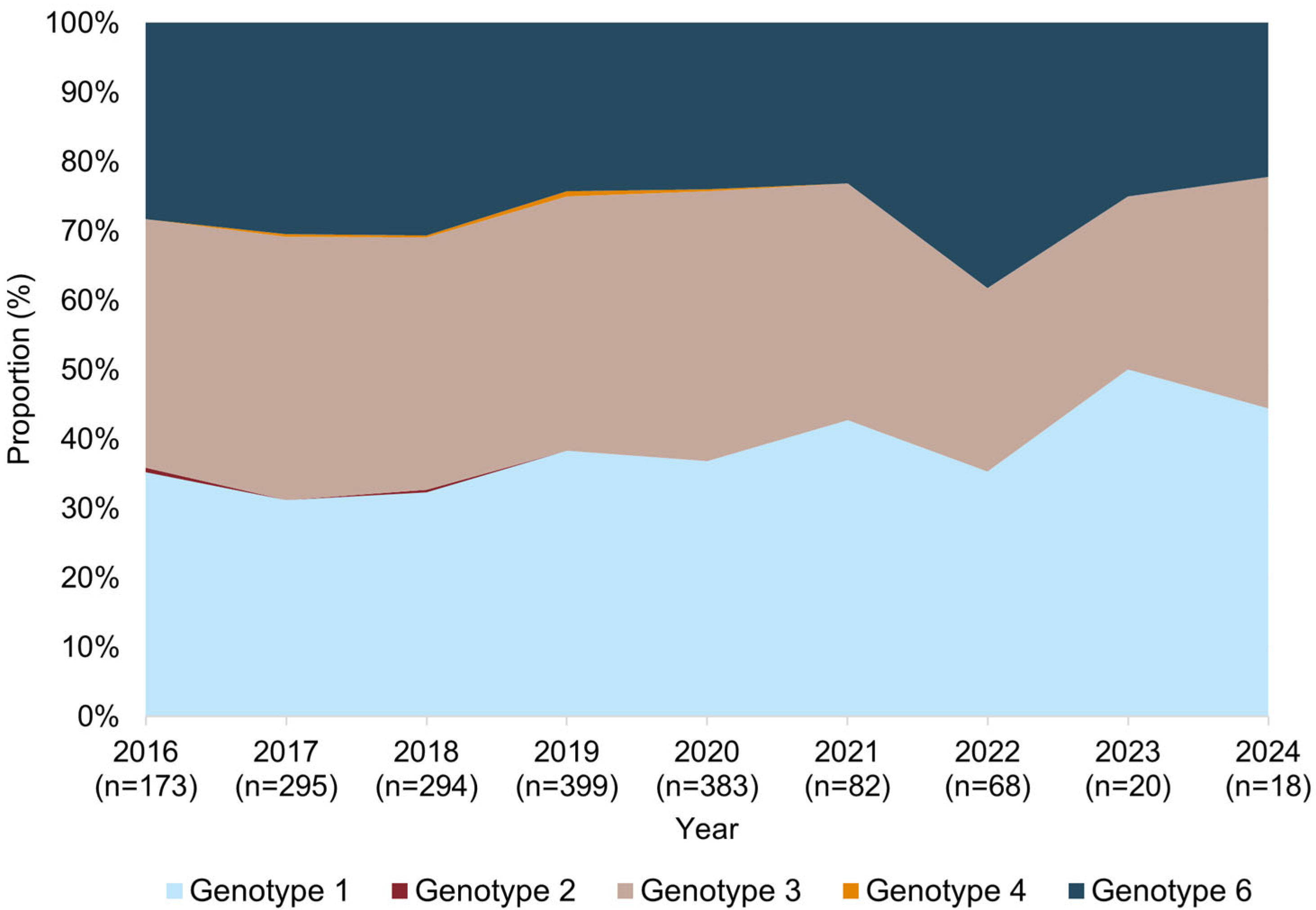
3.4. Temporal Trends in HCV Genotype Distribution
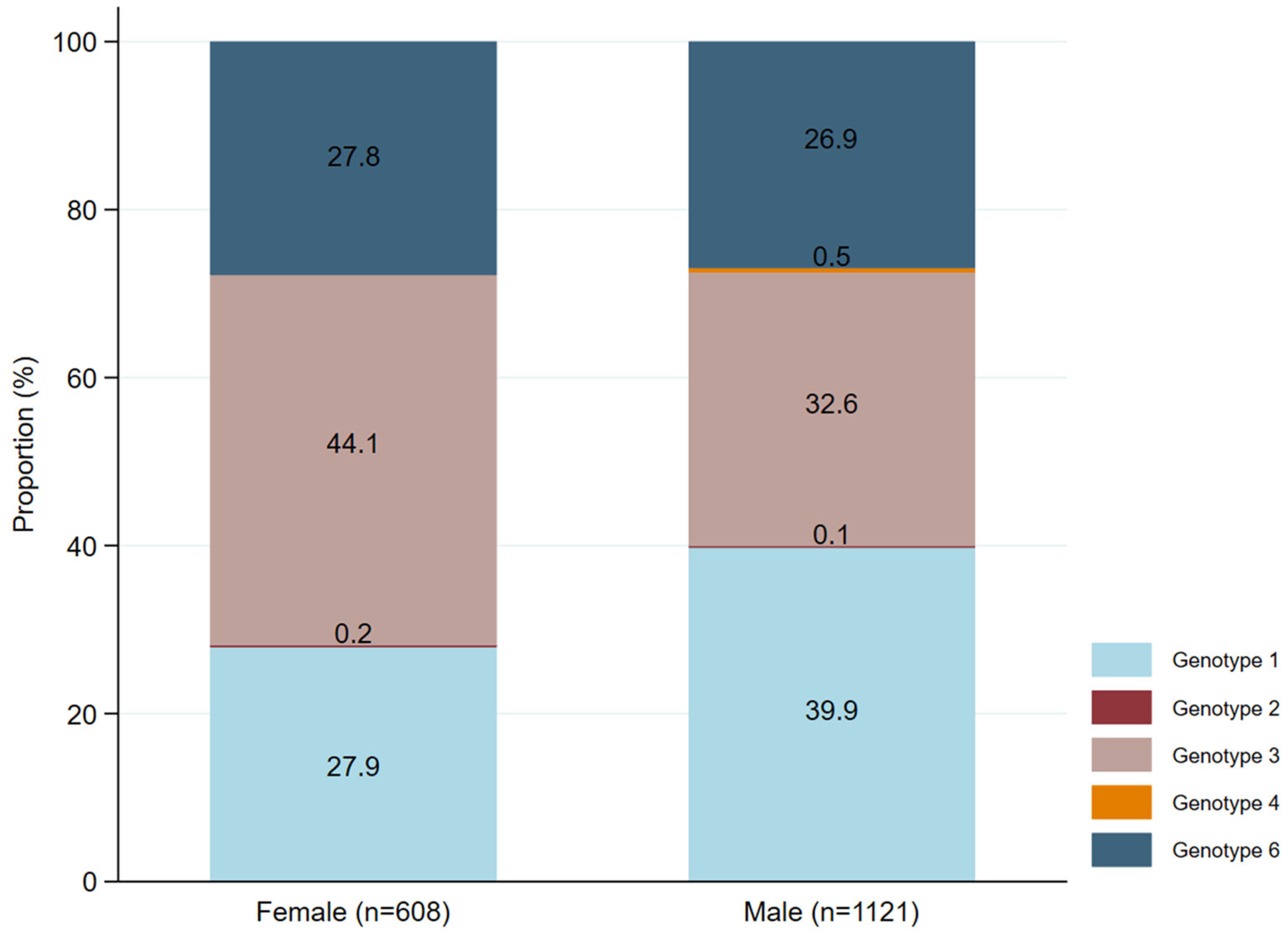
3.5. Distribution of HCV Genotypes by Sex
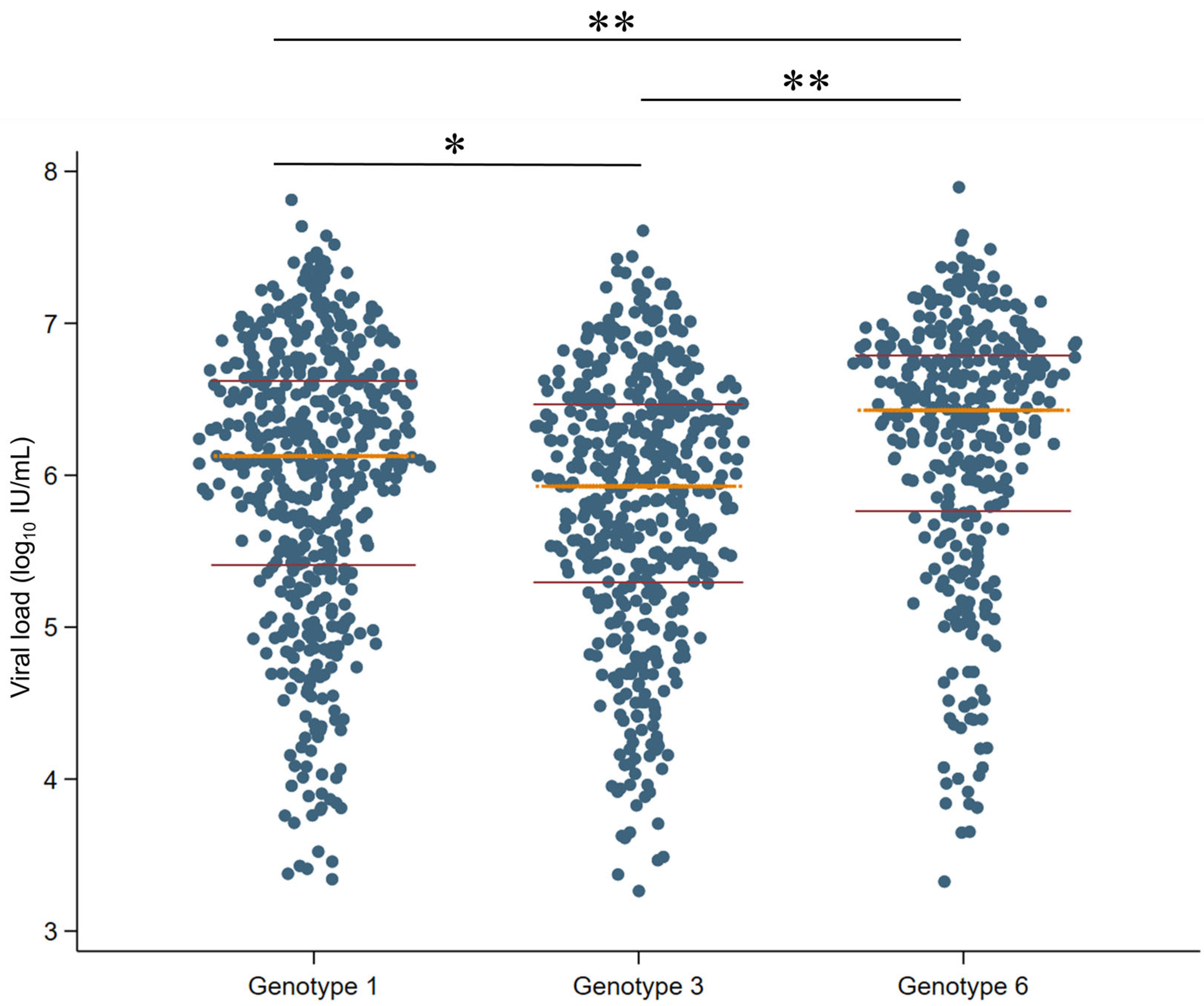
3.6. Association Between Different HCV Genotypes and Viral Load
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 26 November 2024).
- Nakamura, F.; Takeda, H.; Ueda, Y.; Takai, A.; Takahashi, K.; Eso, Y.; Arasawa, S.; Iguchi, E.; Shimizu, T.; Mishima, M.; et al. Mutational spectrum of hepatitis C virus in patients with chronic hepatitis C determined by single molecule real-time sequencing. Sci. Rep. 2022, 12, 7083. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.M.; González-Candelas, F.; Moya, A.; Sanjuán, R. Effect of ribavirin on the mutation rate and spectrum of hepatitis C virus in vivo. J. Virol. 2009, 83, 5760–5764. [Google Scholar] [CrossRef] [PubMed]
- Geller, R.; Estada, Ú.; Peris, J.B.; Andreu, I.; Bou, J.V.; Garijo, R.; Cuevas, J.M.; Sabariegos, R.; Mas, A.; Sanjuán, R. Highly heterogeneous mutation rates in the hepatitis C virus genome. Nat. Microbiol. 2016, 1, 16045. [Google Scholar] [CrossRef] [PubMed]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses: ICTV. Flaviviridae: Hepacivirus C Classification. Available online: https://ictv.global/sg_wiki/flaviviridae/hepacivirus (accessed on 5 February 2025).
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Fahmy, A.M.; Hammad, M.S.; Mabrouk, M.S.; Al-Atabany, W.I. On leveraging self-supervised learning for accurate HCV genotyping. Sci. Rep. 2024, 14, 15463. [Google Scholar] [CrossRef]
- Keikha, M.; Eslami, M.; Yousefi, B.; Ali-Hassanzadeh, M.; Kamali, A.; Yousefi, M.; Karbalaei, M. HCV genotypes and their determinative role in hepatitis C treatment. Virusdisease 2020, 31, 235–240. [Google Scholar] [CrossRef]
- Cunningham, E.B.; Applegate, T.L.; Lloyd, A.R.; Dore, G.J.; Grebely, J. Mixed HCV infection and reinfection in people who inject drugs—Impact on therapy. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 218–230. [Google Scholar] [CrossRef]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. European Association for the Study of the Liver (EASL). EASL recommendations on treatment of hepatitis C: Final update of the series (☆). J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Sarrazin, C. Treatment failure with DAA therapy: Importance of resistance. J. Hepatol. 2021, 74, 1472–1482. [Google Scholar] [CrossRef]
- Pawlotsky, J.M. Hepatitis C Virus Resistance to Direct-Acting Antiviral Drugs in Interferon-Free Regimens. Gastroenterology 2016, 151, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Ilyas, J.; Duan, Z.; El-Serag, H.B. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology 2014, 60, 98–105. [Google Scholar] [CrossRef]
- Wasitthankasem, R.; Posuwan, N.; Vichaiwattana, P.; Theamboonlers, A.; Klinfueng, S.; Vuthitanachot, V.; Thanetkongtong, N.; Saelao, S.; Foonoi, M.; Fakthongyoo, A.; et al. Decreasing Hepatitis C Virus Infection in Thailand in the Past Decade: Evidence from the 2014 National Survey. PLoS ONE 2016, 11, e0149362. [Google Scholar] [CrossRef]
- Sunanchaikarn, S.; Theamboonlers, A.; Chongsrisawat, V.; Yoocharoen, P.; Tharmaphornpilas, P.; Warinsathien, P.; Sinlaparatsamee, S.; Paupunwatana, S.; Chaiear, K.; Khwanjaipanich, S.; et al. Seroepidemiology and genotypes of hepatitis C virus in Thailand. Asian Pac. J. Allergy Immunol. 2007, 25, 175–182. [Google Scholar]
- Jatapai, A.; Nelson, K.E.; Chuenchitra, T.; Kana, K.; Eiumtrakul, S.; Sunantarod, E.; Rangsin, R. Prevalence and risk factors for hepatitis C virus infection among young Thai men. Am. J. Trop. Med. Hyg. 2010, 83, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Pratedrat, P.; Nilyanimit, P.; Wasitthankasem, R.; Posuwan, N.; Auphimai, C.; Hansoongnern, P.; Pimsing, N.; Ngamnimit, S.; Thongmai, C.; Phaengkha, W.; et al. Qualitative hepatitis C virus RNA assay identifies active infection with sufficient viral load for treatment among Phetchabun residents in Thailand. PLoS ONE 2023, 18, e0268728. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Geng, N.; Zhu, W.; Liu, H.; Bai, H. Prevalence and characteristics of hepatitis C virus infection in Shenyang City, Northeast China, and prediction of HCV RNA positivity according to serum anti-HCV level: Retrospective review of hospital data. Virol. J. 2020, 17, 36. [Google Scholar] [CrossRef]
- Kanistanon, D.; Neelamek, M.; Dharakul, T.; Songsivilai, S. Genotypic distribution of hepatitis C virus in different regions of Thailand. J. Clin. Microbiol. 1997, 35, 1772–1776. [Google Scholar] [CrossRef]
- Wasitthankasem, R.; Vongpunsawad, S.; Siripon, N.; Suya, C.; Chulothok, P.; Chaiear, K.; Rujirojindakul, P.; Kanjana, S.; Theamboonlers, A.; Tangkijvanich, P.; et al. Genotypic distribution of hepatitis C virus in Thailand and Southeast Asia. PLoS ONE 2015, 10, e0126764. [Google Scholar] [CrossRef]
- Wasitthankasem, R.; Pimsingh, N.; Treesun, K.; Posuwan, N.; Vichaiwattana, P.; Auphimai, C.; Thongpan, I.; Tongsima, S.; Vongpunsawad, S.; Poovorawan, Y. Prevalence of Hepatitis C Virus in an Endemic Area of Thailand: Burden Assessment toward HCV Elimination. Am. J. Trop. Med. Hyg. 2020, 103, 175–182. [Google Scholar] [CrossRef]
- Lynch, S.M.; Wu, G.Y. Hepatitis C Virus: A Review of Treatment Guidelines, Cost-effectiveness, and Access to Therapy. J. Clin. Transl. Hepatol. 2016, 4, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Midgard, H.; Weir, A.; Palmateer, N.; Lo Re, V., III; Pineda, J.A.; Macías, J.; Dalgard, O. HCV epidemiology in high-risk groups and the risk of reinfection. J. Hepatol. 2016, 65, S33–S45. [Google Scholar] [CrossRef]
- Vo-Quang, E.; Pawlotsky, J.M. ‘Unusual’ HCV genotype subtypes: Origin, distribution, sensitivity to direct-acting antiviral drugs and behaviour on antiviral treatment and retreatment. Gut 2024, 73, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Guntipalli, P.; Pakala, R.; Kumari Gara, S.; Ahmed, F.; Bhatnagar, A.; Endaya Coronel, M.K.; Razzack, A.A.; Solimando, A.G.; Thompson, A.; Andrews, K.; et al. Worldwide prevalence, genotype distribution and management of hepatitis C. Acta Gastroenterol. Belg. 2021, 84, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, M.; Nour, M.; El-Raey, F.; Nagdy, H.; Almansoury, Y.; El-Kassas, M. Gender differences in prevalence of hepatitis C virus infection in Egypt: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 2499. [Google Scholar] [CrossRef]
- Di Martino, V.; Lebray, P.; Myers, R.P.; Pannier, E.; Paradis, V.; Charlotte, F.; Moussalli, J.; Thabut, D.; Buffet, C.; Poynard, T. Progression of liver fibrosis in women infected with hepatitis C: Long-term benefit of estrogen exposure. Hepatology 2004, 40, 1426–1433. [Google Scholar] [CrossRef]
- Barbaglia, M.N.; Harris, J.M.; Smirnov, A.; Burlone, M.E.; Rigamonti, C.; Pirisi, M.; Minisini, R.; Magri, A. 17β-Oestradiol Protects from Hepatitis C Virus Infection through Induction of Type I Interferon. Viruses 2022, 14, 1806. [Google Scholar] [CrossRef]
- Wasitthankasem, R.; Vichaiwattana, P.; Siripon, N.; Posuwan, N.; Auphimai, C.; Klinfueng, S.; Thanetkongtong, N.; Vuthitanachot, V.; Saiyatha, S.; Thongmai, C.; et al. Birth-cohort HCV screening target in Thailand to expand and optimize the national HCV screening for public health policy. PLoS ONE 2018, 13, e0202991. [Google Scholar] [CrossRef]
- Cho, E.J.; Jeong, S.H.; Han, B.H.; Lee, S.U.; Yun, B.C.; Park, E.T. Hepatitis C virus (HCV) genotypes and the influence of HCV subtype 1b on the progression of chronic hepatitis C in Korea: A single center experience. Clin. Mol. Hepatol. 2012, 18, 219–224. [Google Scholar] [CrossRef]
- Wu, N.; Rao, H.Y.; Yang, W.B.; Gao, Z.L.; Yang, R.F.; Fei, R.; Gao, Y.H.; Jin, Q.; Wei, L. Impact of hepatitis C virus genotype 3 on liver disease progression in a Chinese national cohort. Chin. Med. J. Engl. 2020, 133, 253–261. [Google Scholar] [CrossRef]
- Chan, A.; Patel, K.; Naggie, S. Genotype 3 Infection: The Last Stand of Hepatitis C Virus. Drugs 2017, 77, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Daw, M.A.; El-Bouzedi, A.; Dau, A.A. Geographic distribution of HCV genotypes in Libya and analysis of risk factors involved in their transmission. BMC Res. Notes 2015, 8, 367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chakravarti, A.; Dogra, G.; Verma, V.; Srivastava, A.P. Distribution pattern of HCV genotypes & its association with viral load. Indian J. Med. Res. 2011, 133, 326–331. [Google Scholar] [PubMed]
- Goletti, S.; Zuyten, S.; Goeminne, L.; Verhofstede, C.; Rodriguez-Villalobos, H.; Bodeus, M.; Stärkel, P.; Horsmans, Y.; Kabamba-Mukadi, B. Comparison of Sanger sequencing for hepatitis C virus genotyping with a commercial line probe assay in a tertiary hospital. BMC Infect. Dis. 2019, 19, 738. [Google Scholar] [CrossRef]

| Characteristics | Value |
|---|---|
| Median age (years, IQR); n = 934 | 57 (49–65) |
| Male, n/N (%) | 1121/1729 (65) |
| Female, n/N (%) | 608/1729 (35) |
| Median HCV viral load (log10 IU/mL, IQR); n = 1377 | 6.15 (5.46–6.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kham-Kjing, N.; Phruekthayanon, S.; Krueyot, T.; Phutthakham, P.; Intarasoot, S.; Tragoolpua, K.; Preechasuth, K.; Carraway, T.S.; Kongyai, N.; Khamduang, W. Molecular Epidemiology of Hepatitis C Virus Genotypes in Northern Thailand: A Retrospective Study from 2016 to 2024. Infect. Dis. Rep. 2025, 17, 73. https://doi.org/10.3390/idr17040073
Kham-Kjing N, Phruekthayanon S, Krueyot T, Phutthakham P, Intarasoot S, Tragoolpua K, Preechasuth K, Carraway TS, Kongyai N, Khamduang W. Molecular Epidemiology of Hepatitis C Virus Genotypes in Northern Thailand: A Retrospective Study from 2016 to 2024. Infectious Disease Reports. 2025; 17(4):73. https://doi.org/10.3390/idr17040073
Chicago/Turabian StyleKham-Kjing, Nang, Sirithip Phruekthayanon, Thipsuda Krueyot, Panaddar Phutthakham, Sorasak Intarasoot, Khajornsak Tragoolpua, Kanya Preechasuth, Tanawan Samleerat Carraway, Natedao Kongyai, and Woottichai Khamduang. 2025. "Molecular Epidemiology of Hepatitis C Virus Genotypes in Northern Thailand: A Retrospective Study from 2016 to 2024" Infectious Disease Reports 17, no. 4: 73. https://doi.org/10.3390/idr17040073
APA StyleKham-Kjing, N., Phruekthayanon, S., Krueyot, T., Phutthakham, P., Intarasoot, S., Tragoolpua, K., Preechasuth, K., Carraway, T. S., Kongyai, N., & Khamduang, W. (2025). Molecular Epidemiology of Hepatitis C Virus Genotypes in Northern Thailand: A Retrospective Study from 2016 to 2024. Infectious Disease Reports, 17(4), 73. https://doi.org/10.3390/idr17040073







