Impact of COVID-19 Vaccination on Hospitalization and Mortality: A Comparative Analysis of Clinical Outcomes During the Early Phase of the Pandemic
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, B.S. Rapid COVID-19 Vaccine Development. Science 2020, 368, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 Vaccines in Development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Morgenstern, C.; Rawson, T.; Hinsley, W.; Perez-Guzman, P.N.; Bhatt, S.; Ferguson, N.M. Socioeconomic and Temporal Heterogeneity in SARS-CoV-2 Exposure and Disease in England from May 2020 to February 2023. MedRxiv 2025. [Google Scholar] [CrossRef]
- Bilal, U.; Mullachery, P.H.; Schnake-Mahl, A.; Rollins, H.; McCulley, E.; Kolker, J.; Barber, S.; Diez Roux, A.V. Heterogeneity in Spatial Inequities in COVID-19 Vaccination Across 16 Large US Cities. Am. J. Epidemiol. 2022, 191, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 nCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and Effectiveness of mRNA BNT162b2 Vaccine against SARS-CoV-2 Infections and COVID-19 Cases, Hospitalisations, and Deaths Following a Nationwide Vaccination Campaign in Israel: An Observational Study Using National Surveillance Data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk Factors and Disease Profile of Post-Vaccination SARS-CoV-2 Infection in UK Users of the COVID Symptom Study App: A Prospective, Community-Based, Nested, Case-Control Study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological Mechanisms of Vaccine-Induced Protection against COVID-19 in Humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems Vaccinology of the BNT162b2 mRNA Vaccine in Humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Zaeck, L.M.; GeurtsvanKessel, C.H.; De Vries, R.D. COVID-19 Vaccine Effectiveness and Evolving Variants: Understanding the Immunological Footprint. Lancet Respir. Med. 2023, 11, 395–396. [Google Scholar] [CrossRef]
- Varea-Jiménez, E.; Aznar Cano, E.; Vega-Piris, L.; Martínez Sánchez, E.V.; Mazagatos, C.; García San Miguel Rodríguez-Alarcón, L.; Casas, I.; Sierra Moros, M.J.; Iglesias-Caballero, M.; Vazquez-Morón, S.; et al. Comparative Severity of COVID-19 Cases Caused by Alpha, Delta or Omicron SARS-CoV-2 Variants and Its Association with Vaccination. Enfermedades Infecc. y Microbiol. Clínica 2024, 42, 187–194. [Google Scholar] [CrossRef]
- Hoang, T.N.A.; Byrne, A.; Quach, H.-L.; Bannister-Tyrrell, M.; Vogt, F. How Well Do Different COVID-19 Vaccines Protect against Different Viral Variants? A Systematic Review and Meta-Analysis. Trans. R. Soc. Trop. Med. Hyg. 2025, 119, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Moll, M.E.; Mata-Tijerina, V.L.; Gutiérrez-Salazar, C.C.; Silva-Ramírez, B.; Peñuelas-Urquides, K.; González-Escalante, L.; Escobedo-Guajardo, B.L.; Cruz-Luna, J.E.; Corrales-Pérez, R.; Gómez-García, S.; et al. The Impact of Comorbidity Status in COVID-19 Vaccines Effectiveness before and after SARS-CoV-2 Omicron Variant in Northeastern Mexico: A Retrospective Multi-Hospital Study. Front. Public Health 2024, 12, 1402527. [Google Scholar] [CrossRef] [PubMed]
- Lyons, Z.; DiBiase, L.; Sickbert-Bennett, E.; Weber, D.J. 420. Prevalence of Comorbidities and COVID-19 Vaccination among COVID-19 Deaths. Open Forum Infect. Dis. 2023, 10, ofad500.490. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; The CITIID-NIHR BioResource COVID-19 Collaboration; Principal Investigators; et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Yanez, N.D.; Weiss, N.S.; Romand, J.-A.; Treggiari, M.M. COVID-19 Mortality Risk for Older Men and Women. BMC Public Health 2020, 20, 1742. [Google Scholar] [CrossRef]
- UCSF. Institute For Global Health Sciences. La respuesta de México al COVID-19: Estudio de caso. Available online: https://globalhealthsciences.ucsf.edu/wp-content/uploads/2024/02/la_respuesta_de_mexico_al_covid_esp.pdf (accessed on 16 April 2025).
- Batista-Roche, J.L.; Mirabent-Casals, M.; Gómez-Gil, B.; Berlanga-Robles, C.; García-Gasca, A. Variantes de SARS-CoV-2 y Los Casos Asociados a Cuatro Olas Epidemiológicas En Sinaloa, México. TIP RECQB 2022, 25, e474. [Google Scholar] [CrossRef]
- Antonio-Villa, N.E.; Bello-Chavolla, O.Y.; Fermín-Martínez, C.A.; Fernández-Chirino, L.; Ramírez-García, D. The Evolving Landscape of SARS-CoV-2 Vaccination in Mexico: Real-World Evidence in Mexican Pensioners. Lancet Reg. Health—Am. 2023, 27, 100624. [Google Scholar] [CrossRef] [PubMed]
- Carnalla, M.; Basto-Abreu, A.; Stern, D.; Bautista-Arredondo, S.; Shamah-Levy, T.; Alpuche-Aranda, C.M.; Rivera-Dommarco, J.; Barrientos-Gutiérrez, T. Acceptance, Refusal and Hesitancy of COVID-19 Vaccination in Mexico: Ensanut 2020 COVID-19. Salud Publica Mex. 2021, 63, 598–606. [Google Scholar] [CrossRef]
- Gaitán-Rossi, P.; Mendez-Rosenzweig, M.; García-Alberto, E.; Vilar-Compte, M. Barriers to COVID-19 Vaccination among Older Adults in Mexico City. Int. J. Equity Health 2022, 21, 85. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-nCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- WHO. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection. Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed on 21 May 2025).
- Ranucci, M.; Parati, G.; Di Dedda, U.; Bussotti, M.; Agricola, E.; Menicanti, L.; Bombace, S.; De Martino, F.; Giovinazzo, S.; Zambon, A.; et al. When Outcomes Diverge: Age and Cardiovascular Risk as Determinants of Mortality and ICU Admission in COVID-19. J. Clin. Med. 2022, 11, 4099. [Google Scholar] [CrossRef] [PubMed]
- Mazereel, V.; Van Assche, K.; Detraux, J.; De Hert, M. COVID-19 Vaccination for People with Severe Mental Illness: Why, What, and How? Lancet Psychiatry 2021, 8, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Shoham, S.; Batista, C.; Ben Amor, Y.; Ergonul, O.; Hassanain, M.; Hotez, P.; Kang, G.; Kim, J.H.; Lall, B.; Larson, H.J.; et al. Vaccines and Therapeutics for Immunocompromised Patients with COVID-19. eClinicalMedicine 2023, 59, 101965. [Google Scholar] [CrossRef]
- Kohler, H.; Bäuerle, A.; Schweda, A.; Weismüller, B.; Fink, M.; Musche, V.; Robitzsch, A.; Pfeiffer, C.; Benecke, A.-V.; Dörrie, N.; et al. Increased COVID-19-Related Fear and Subjective Risk Perception Regarding COVID-19 Affects Behavior in Individuals with Internal High-Risk Diseases. J. Prim. Care Community Health 2021, 12, 2150132721996898. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, Y.; Yin, X.; Zhao, Y.; Wang, Z.; Tan, F.; Gong, E.; Shao, R. Study on Medical Care-Seeking Behaviors and Chronic Comorbidities Based on the Ecology of the Medical Care Model. Big Data Anal. Healthc. 2024, 2, 25–33. Available online: https://www.whioce.com/journal/BDAH/2/1/10.10086/amcmr.v1i11.79 (accessed on 16 April 2025).
- Speth, M.M.; Singer-Cornelius, T.; Oberle, M.; Gengler, I.; Brockmeier, S.J.; Sedaghat, A.R. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol. Head Neck Surg. 2020, 163, 114–120. [Google Scholar] [CrossRef]
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-Informed COVID-19 Vaccine Prioritization Strategies by Age and Serostatus. Science 2021, 371, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Conacyt. Datos COVID-19 México. Available online: https://datos.covid-19.conacyt.mx/index.php (accessed on 21 May 2025).
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and Long-Term Disruption of Glycometabolic Control after SARS-CoV-2 Infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef]
- Paleiron, N.; Mayet, A.; Marbac, V.; Perisse, A.; Barazzutti, H.; Brocq, F.-X.; Janvier, F.; Dautzenberg, B.; Bylicki, O. Impact of Tobacco Smoking on the Risk of COVID-19: A Large Scale Retrospective Cohort Study. Nicotine Tob. Res. 2021, 23, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, K.J.; Tassiopoulos, K.N.; Jeffrey, C.; Stranges, S.; Martin, J. Using Causal Diagrams within the Grading of Recommendations, Assessment, Development and Evaluation Framework to Evaluate Confounding Adjustment in Observational Studies. J. Clin. Epidemiol. 2024, 175, 111532. [Google Scholar] [CrossRef]
- Lederer, D.J.; Bell, S.C.; Branson, R.D.; Chalmers, J.D.; Marshall, R.; Maslove, D.M.; Ost, D.E.; Punjabi, N.M.; Schatz, M.; Smyth, A.R.; et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann. ATS 2019, 16, 22–28. [Google Scholar] [CrossRef]
- Marjot, T.; Webb, G.J.; Barritt, A.S.; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and Liver Disease: Mechanistic and Clinical Perspectives. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef]
- Marjot, T.; Webb, G.J.; Barritt, A.S.; Ginès, P.; Lohse, A.W.; Moon, A.M.; Pose, E.; Trivedi, P.; Barnes, E. SARS-CoV-2 Vaccination in Patients with Liver Disease: Responding to the next Big Question. Lancet Gastroenterol. Hepatol. 2021, 6, 156–158. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052. [Google Scholar] [CrossRef]
- Lu, Y.; Lindaas, A.; Matuska, K.; Izurieta, H.S.; McEvoy, R.; Menis, M.; Shi, X.; Steele, W.R.; Wernecke, M.; Chillarige, Y.; et al. Real-World Effectiveness of mRNA COVID-19 Vaccines Among US Nursing Home Residents Aged ≥65 Years in the Pre-Delta and High Delta Periods. Open Forum Infect. Dis. 2024, 11, ofae051. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Rahai, N.; Beck, E.; Beebe, E.; Conroy, B.; Esposito, D.; Govil, P.; Kopel, H.; Lu, T.; Mansi, J.; et al. Evaluating the Effectiveness of mRNA-1273.815 Against COVID-19 Hospitalization Among Adults Aged ≥ 18 Years in the United States. Infect. Dis. Ther. 2025, 14, 199–216. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Chickery, S.; Webber, A.; Ong, T.C.; Rowley, E.A.K.; DeSilva, M.B.; Dascomb, K.; Irving, S.A.; Klein, N.P.; Grannis, S.J.; et al. Interim Estimates of 2024–2025 COVID-19 Vaccine Effectiveness Among Adults Aged ≥18 Years—VISION and IVY Networks, September 2024–January 2025. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.M.; Abdelaziz, O.H.; Shamsseldain, H.E.; Eltrawy, H.H. Functional Outcomes in Post COVID-19 Patients with Persistent Dyspnea: Multidisciplinary Approach. Int. J. Cardiovasc. Imaging 2023, 39, 1115–1122. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital Admission and Emergency Care Attendance Risk for SARS-CoV-2 Delta (B.1.617.2) Compared with Alpha (B.1.1.7) Variants of Concern: A Cohort Study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.M.; Baptista, H.; Oliveira, A.; Jardim, B.; de Castro Neto, M. Beyond Comorbidities, Sex and Age Have No Effect on COVID-19 Health Care Demand. Sci. Rep. 2022, 12, 7356. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N. Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar] [CrossRef]
- UN Research Roadmap for the COVID-19 Recovery. Available online: https://www.un.org/en/coronavirus/communication-resources/un-research-roadmap-covid-19-recovery (accessed on 16 April 2025).
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Inoue, K.; Sakamaki, K.; Komukai, S.; Ito, Y.; Goto, A.; Shinozaki, T. Methodological Tutorial Series for Epidemiological Studies: Confounder Selection and Sensitivity Analyses to Unmeasured Confounding from Epidemiological and Statistical Perspectives. J. Epidemiol. 2025, 35, 3–10. [Google Scholar] [CrossRef]
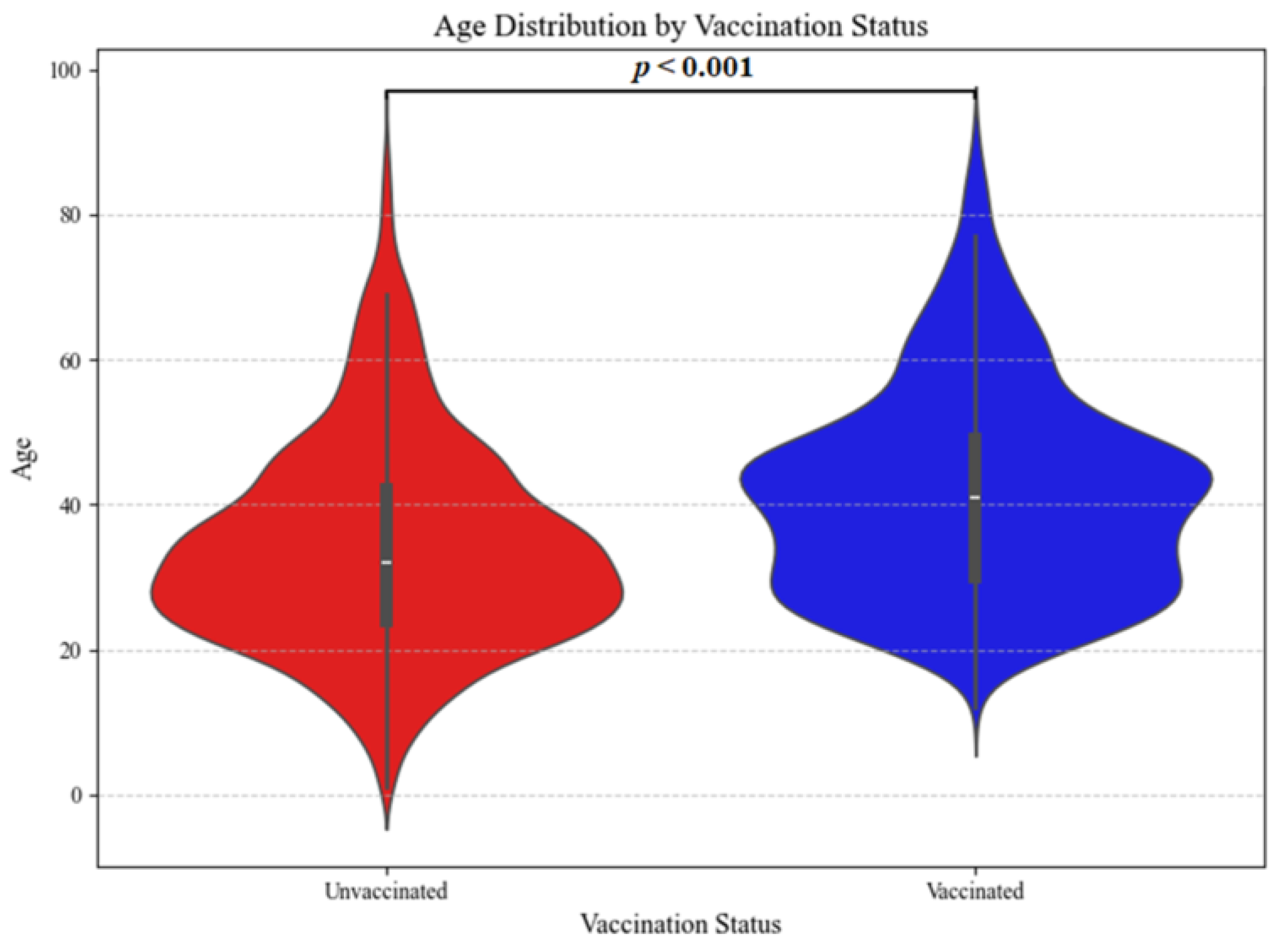


| Characteristic | Unvaccinated n (%) n = 3170 | Vaccinated n (%) n = 1455 | p † |
|---|---|---|---|
| Age (min–max) | 32 (1–91) | 41 (12–91) | 0.0001 |
| Men | 1653 (52.1%) | 691 (47.5%) | |
| Women | 1517 (47.9%) | 764 (52.5%) | 0.003 |
| Hospitalization | 76 (2.4%) | 28 (1.9%) | 0.314 |
| Mortality | 34 (1.1%) | 13 (0.9%) | 0.573 |
| Hypertension | 344 (10.85%) | 237 (16.28%) | 0.0001 |
| Diabetes mellitus | 257 (8.10%) | 179 (12.30%) | 0.0001 |
| Obesity | 222 (7.0%) | 140 (9.62%) | 0.002 |
| Smoking | 111 (3.50%) | 50 (3.43%) | 0.911 |
| Pneumonia | 107 (3.37%) | 46 (3.16%) | 0.706 |
| Asthma | 57 (1.79%) | 23 (1.58%) | 0.599 |
| Immunosuppression | 32 (1.00%) | 22 (1.51%) | 0.140 |
| Cardiovascular disease | 26 (0.82%) | 9 (0.61%) | 0.462 |
| Renal disease | 25 (0.78%) | 11 (0.75%) | 0.907 |
| Neurological disorder | 24 (0.75%) | 5 (0.34%) | 0.098 |
| HIV δ | 8 (0.25%) | 8 (0.54%) | 0.174 |
| Cancer δ | 7 (0.22%) | 5 (0.34%) | 0.535 |
| Chronic liver disease δ | 2 (0.12%) | 5 (0.34%) | 0.035 |
| Hemolytic anemia δ | 4 (0.12%) | 1 (0.06%) | 1 |
| Tuberculosis δ | 3 (0.09%) | 0 (0%) | 0.556 |
| Symptom | Unvaccinated n (%) n = 3170 | Vaccinated n (%) n = 1455 | p † |
|---|---|---|---|
| Fever | 1881 (59.34%) | 1026 (70.52%) | 0.000 |
| Cough | 1823 (57.51%) | 1079 (74.16%) | 0.000 |
| Headache | 2004 (63.22%) | 1150 (79.04%) | 0.000 |
| Sore throat | 1601 (50.50%) | 943 (64.81%) | 0.000 |
| General malaise | 1358 (42.84%) | 730 (50.17%) | 0.000 |
| Myalgia | 1692 (53.38%) | 963 (66.19%) | 0.000 |
| Arthralgia | 1575 (49.68%) | 915 (62.89%) | 0.000 |
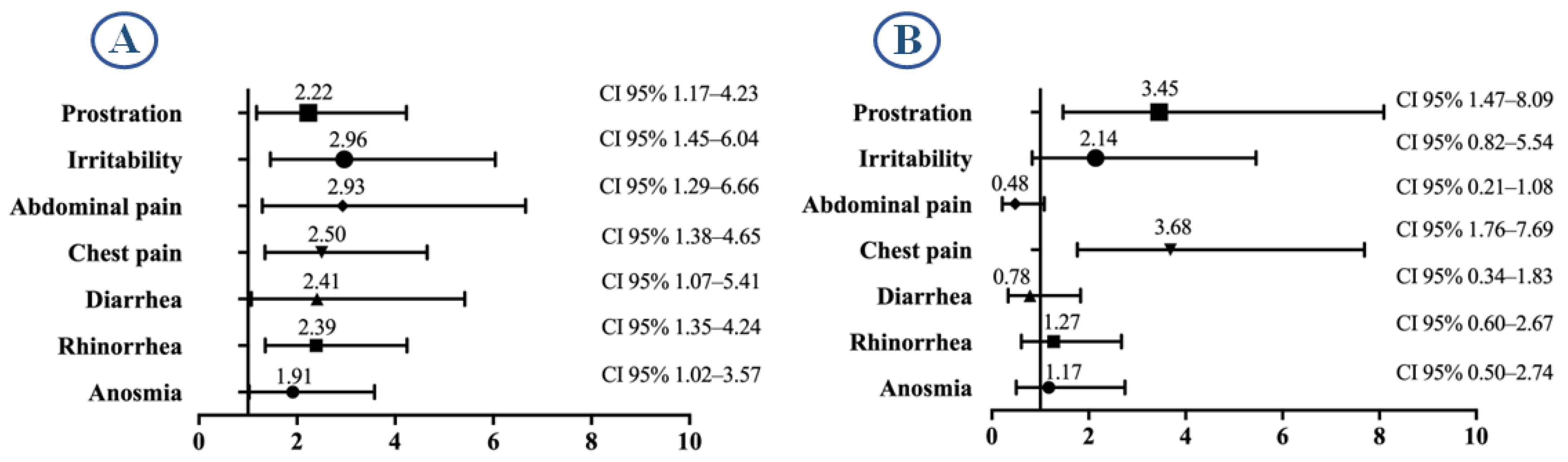
| Prostration | 404 (12.74%) | 175 (12.03%) | 0.5246 |
| Rhinorrhea | 1454 (45.87%) | 916 (62.96%) | 0.000 |
| Chills | 1166 (36.78%) | 647 (44.47%) | 0.000 |
| Abdominal pain | 459 (14.48%) | 235 (16.15%) | 0.1516 |
| Conjunctivitis | 196 (6.18%) | 143 (9.83%) | 0.000 |
| Dyspnea | 279 (8.80%) | 171 (11.75%) | 0.002 |
| Cyanosis | 12 (0.38%) | 4 (0.27%) | 0.7736 |
| Diarrhea | 516 (16.28%) | 302 (20.76%) | 0.0002 |
| Chest pain | 428 (13.50%) | 246 (16.91%) | 0.0027 |
| Tachypnea | 12 (0.38%) | 4 (0.27%) | 0.7736 |
| Irritability | 267 (8.42%) | 134 (9.21%) | 0.4083 |
| Rhinitis | 87 (2.74%) | 50 (3.44%) | 0.2319 |
| Anosmia | 762 (24.04%) | 410 (28.18%) | 0.003 |
| Dysgeusia | 783 (24.70%) | 396 (27.22%) | 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garduño-Orbe, B.; Palma-Ramírez, P.S.; López-Ortiz, E.; García-Morales, G.; Sánchez-Rebolledo, J.M.; Emigdio-Loeza, A.; Gómez-García, A.; López-Ortiz, G. Impact of COVID-19 Vaccination on Hospitalization and Mortality: A Comparative Analysis of Clinical Outcomes During the Early Phase of the Pandemic. Infect. Dis. Rep. 2025, 17, 74. https://doi.org/10.3390/idr17040074
Garduño-Orbe B, Palma-Ramírez PS, López-Ortiz E, García-Morales G, Sánchez-Rebolledo JM, Emigdio-Loeza A, Gómez-García A, López-Ortiz G. Impact of COVID-19 Vaccination on Hospitalization and Mortality: A Comparative Analysis of Clinical Outcomes During the Early Phase of the Pandemic. Infectious Disease Reports. 2025; 17(4):74. https://doi.org/10.3390/idr17040074
Chicago/Turabian StyleGarduño-Orbe, Brenda, Paola Selene Palma-Ramírez, Eduardo López-Ortiz, Gabriela García-Morales, Juan Manuel Sánchez-Rebolledo, Alexis Emigdio-Loeza, Anel Gómez-García, and Geovani López-Ortiz. 2025. "Impact of COVID-19 Vaccination on Hospitalization and Mortality: A Comparative Analysis of Clinical Outcomes During the Early Phase of the Pandemic" Infectious Disease Reports 17, no. 4: 74. https://doi.org/10.3390/idr17040074
APA StyleGarduño-Orbe, B., Palma-Ramírez, P. S., López-Ortiz, E., García-Morales, G., Sánchez-Rebolledo, J. M., Emigdio-Loeza, A., Gómez-García, A., & López-Ortiz, G. (2025). Impact of COVID-19 Vaccination on Hospitalization and Mortality: A Comparative Analysis of Clinical Outcomes During the Early Phase of the Pandemic. Infectious Disease Reports, 17(4), 74. https://doi.org/10.3390/idr17040074






