A Comprehensive Review of Progress in Preventing Urinary Infections Associated with the Use of Urinary Catheters: A Dual Analysis of Publications and Patents
Abstract
1. Introduction
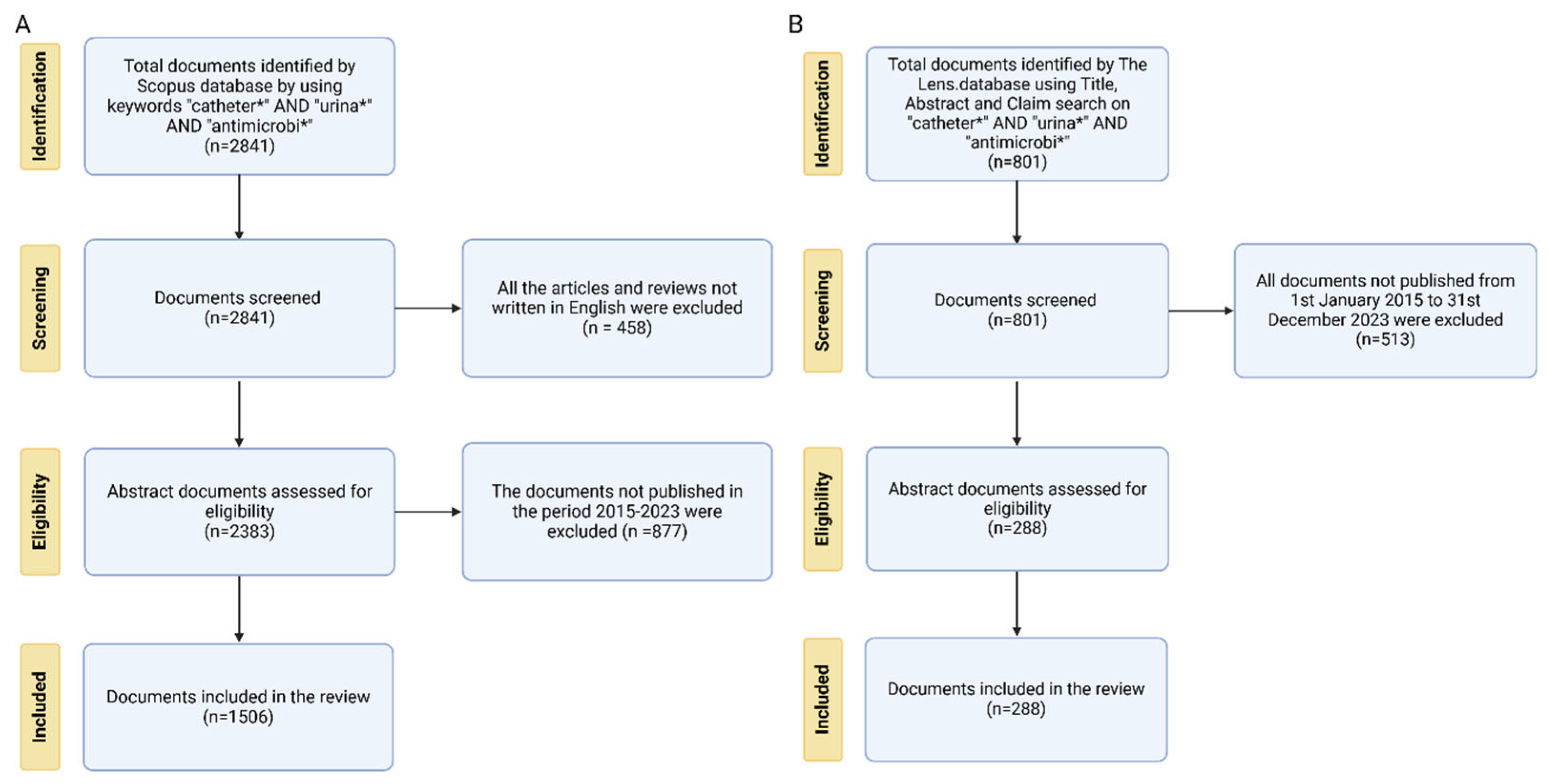
2. Materials and Methods
2.1. Bibliometric Database Collection and Analysis
2.2. Patent Database Collection and Analysis
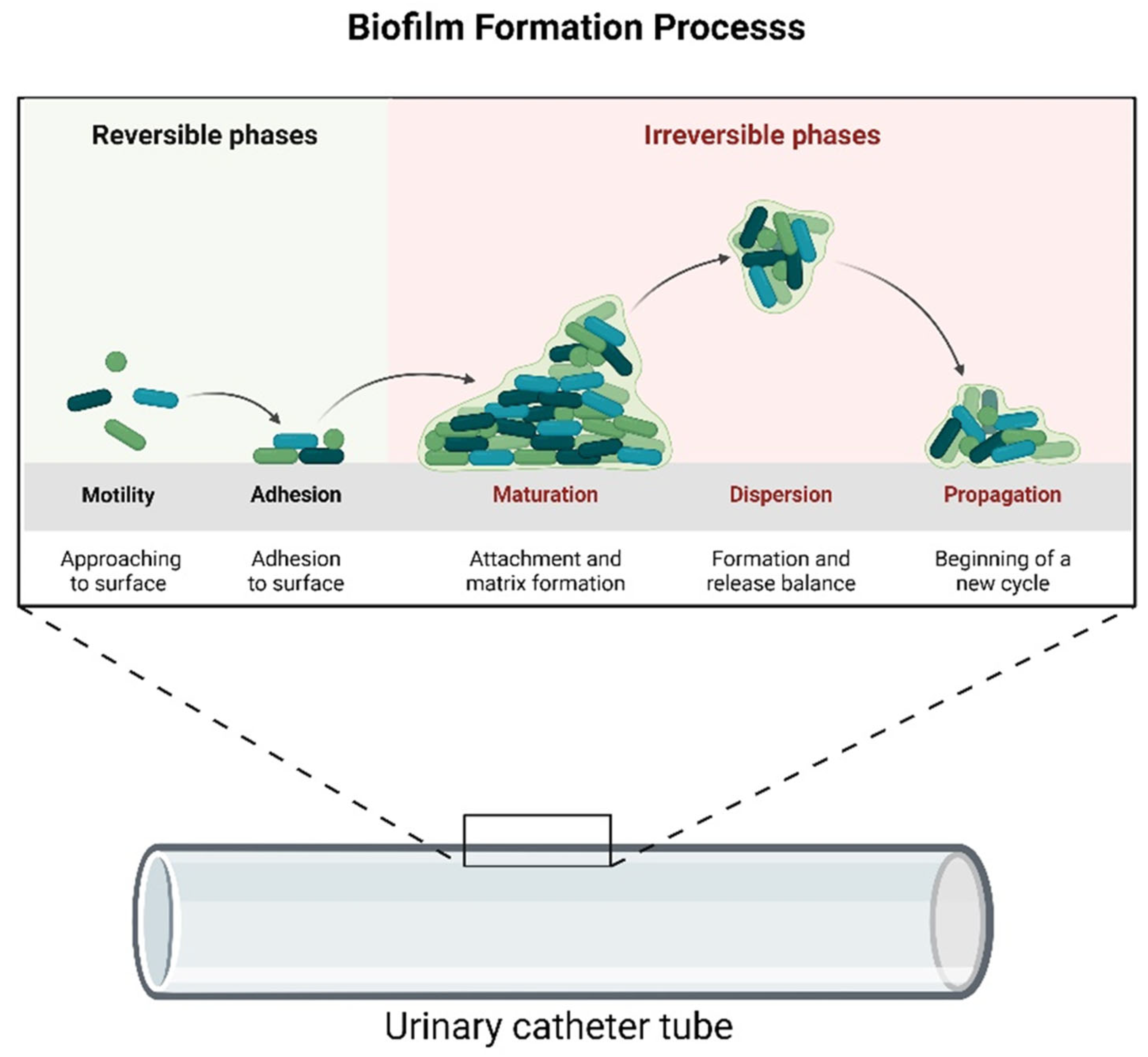
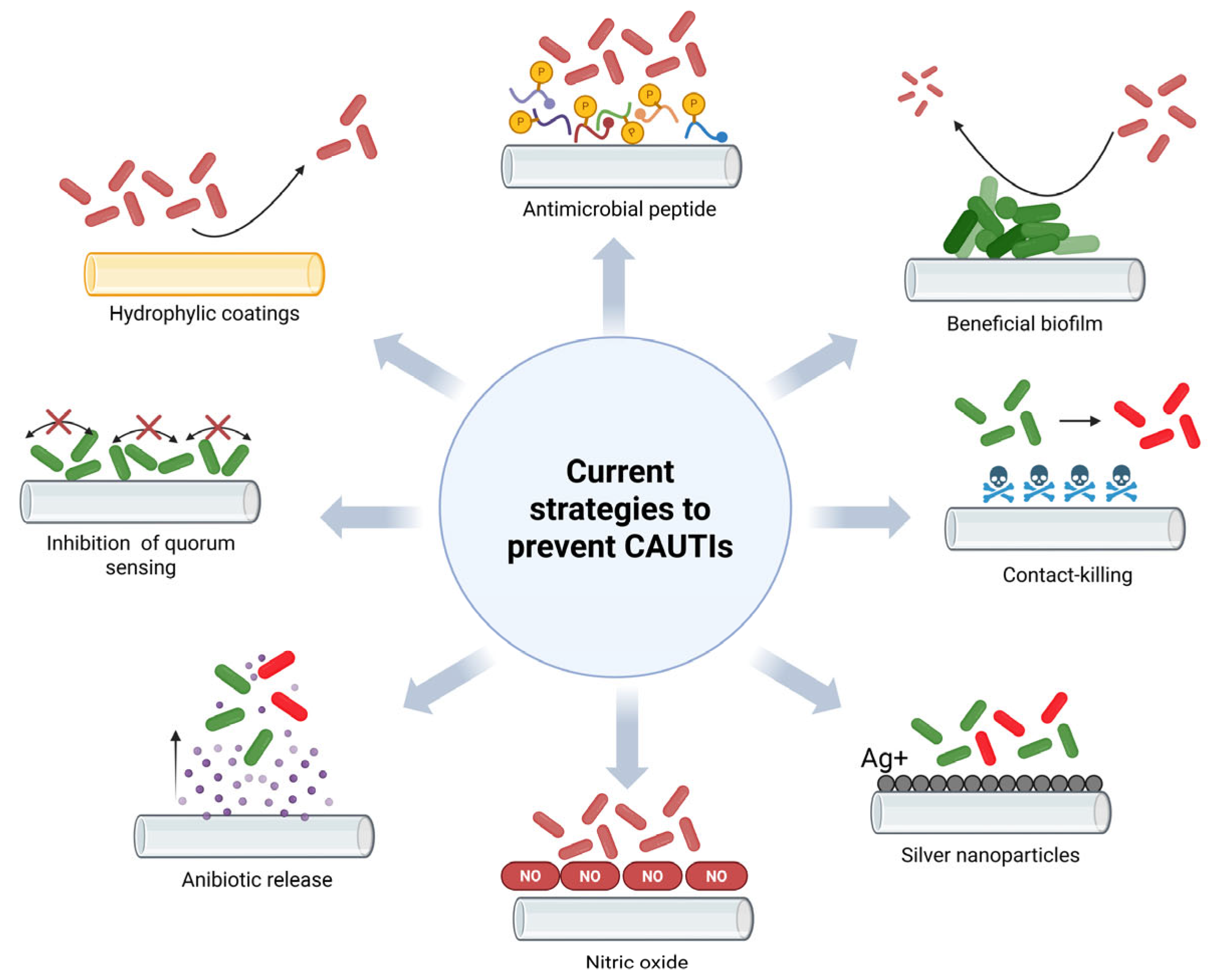
3. Results and Discussion
3.1. Bibliometric Data Analysis
3.2. Predominant Journals, Highest Cited Articles, and Main Thematic Areas
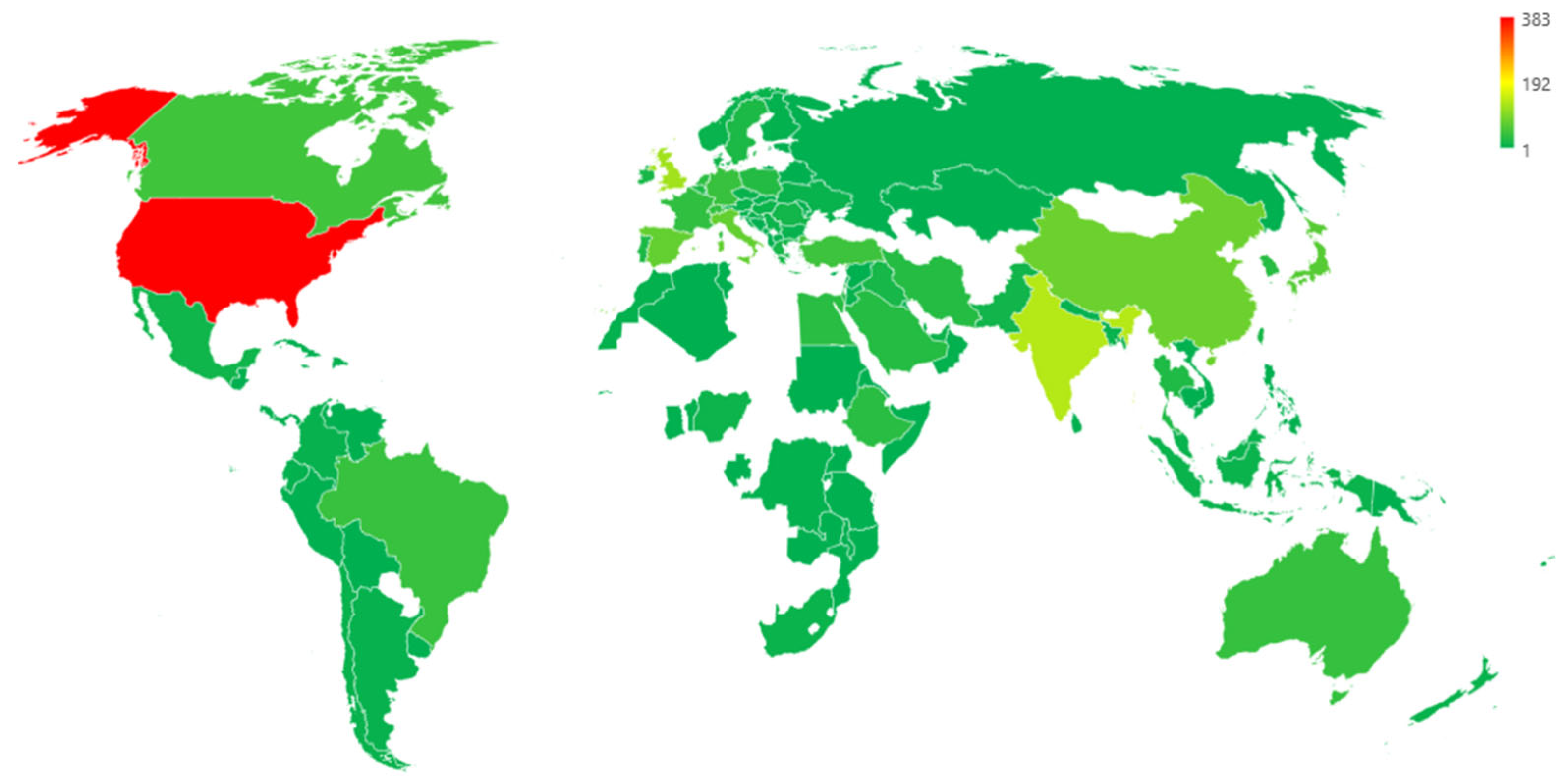
3.3. Funding Sponsors and Geographical Distribution of Academic Publications
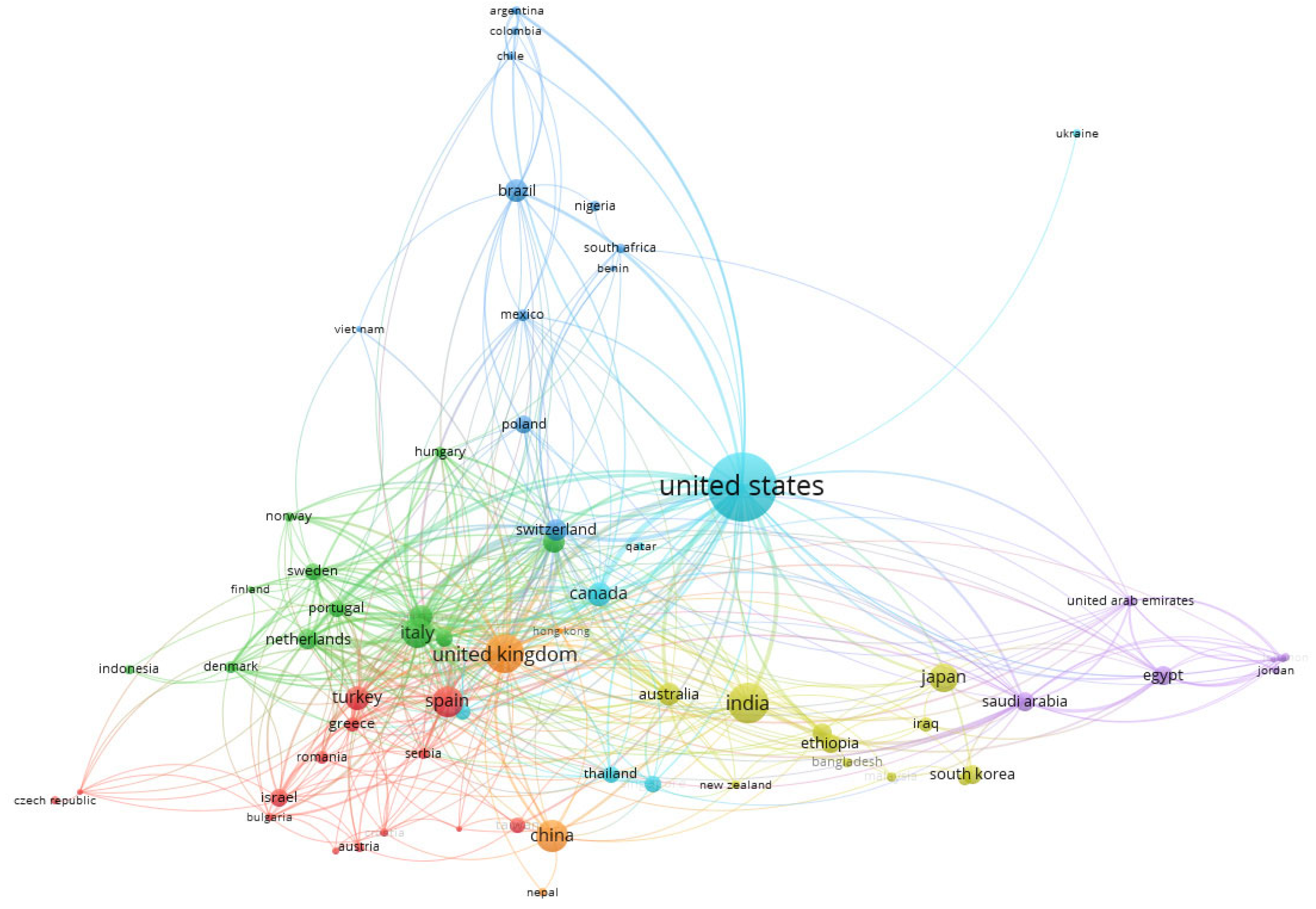
3.4. Co-Authorship of Countries and Authors
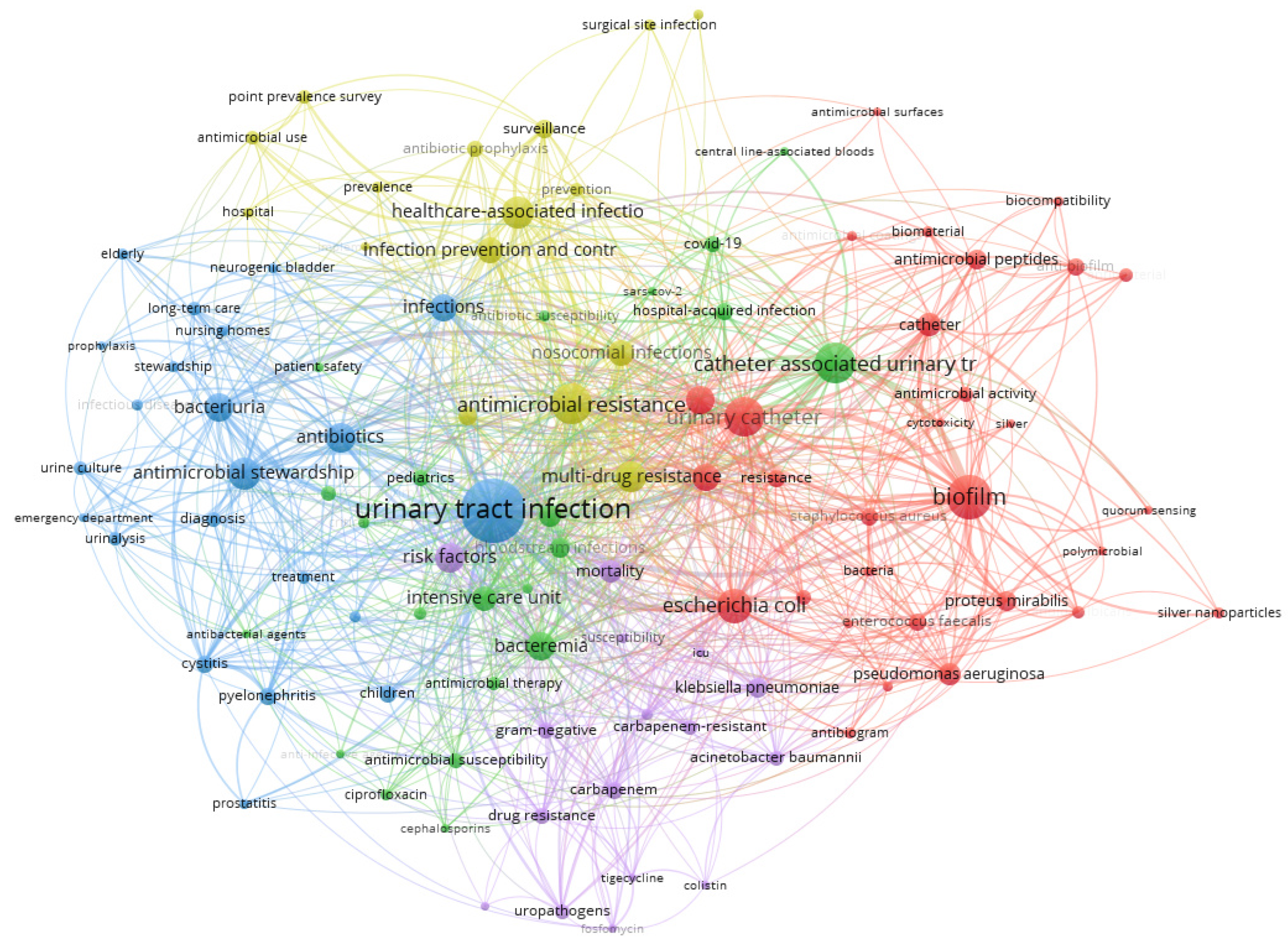
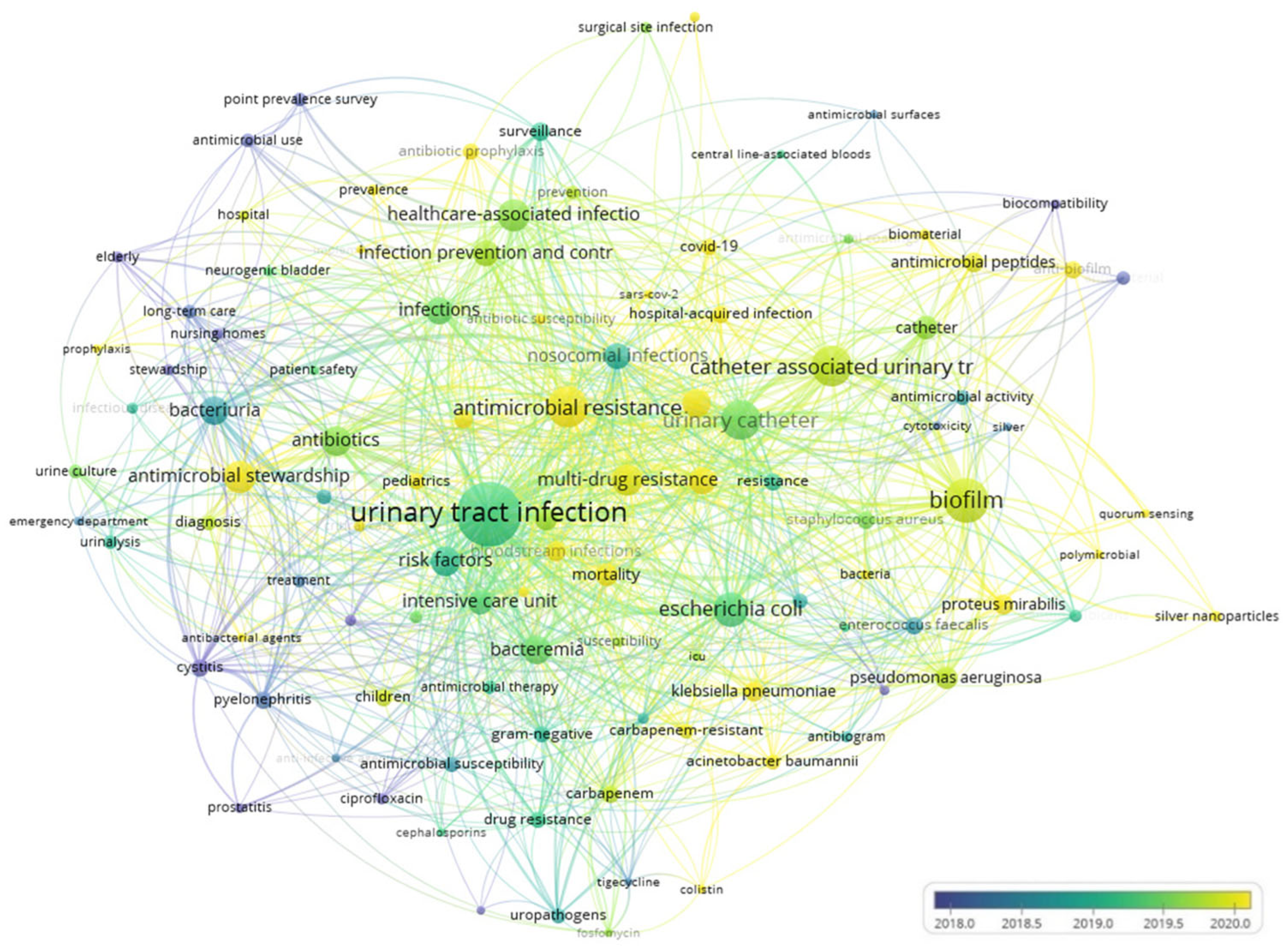
3.5. Co-Occurrence Analysis of the Top Keywords in the Recovered Articles
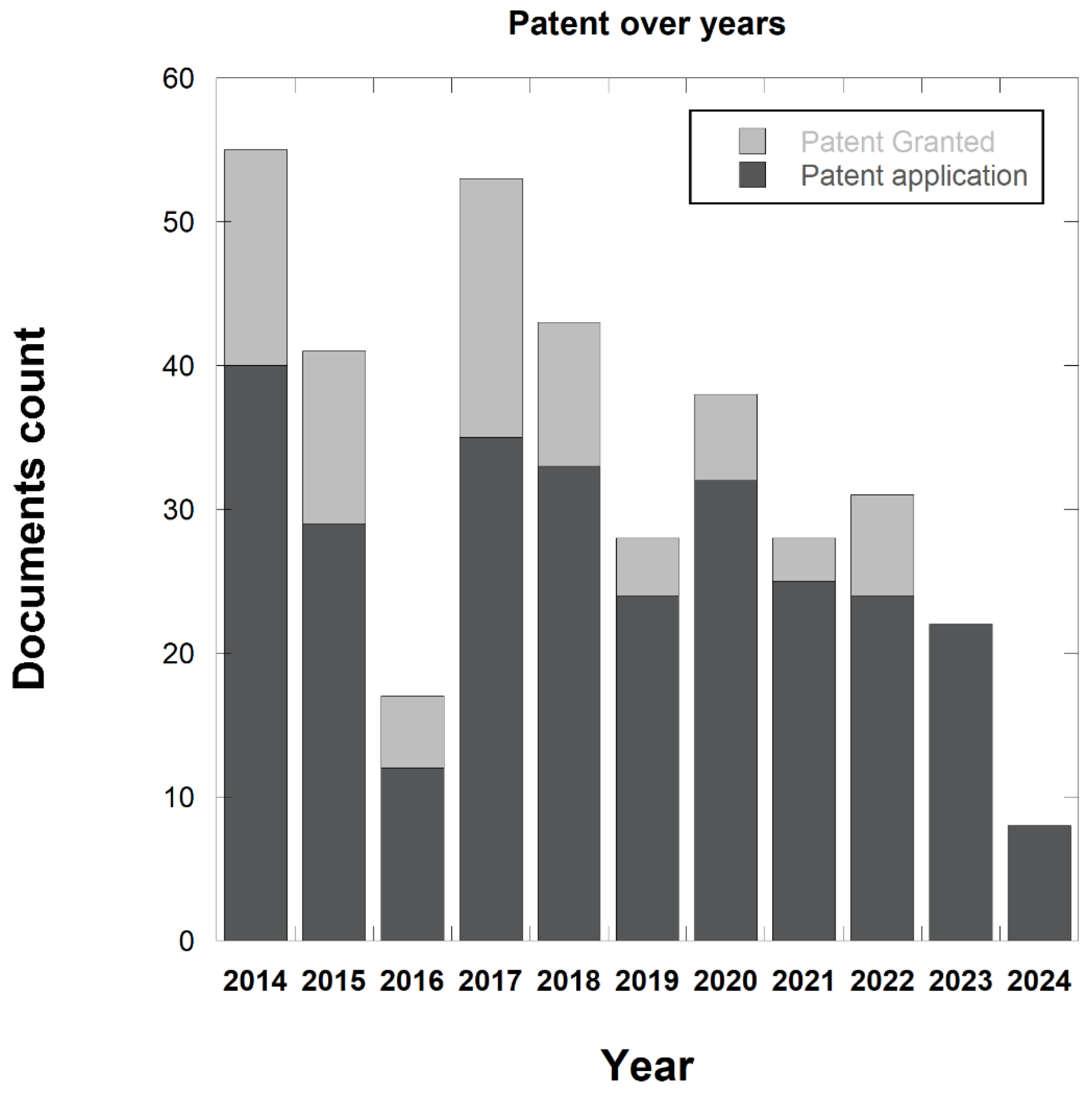
3.6. Patent Database Analysis
3.7. Top 10 Patent Applicants
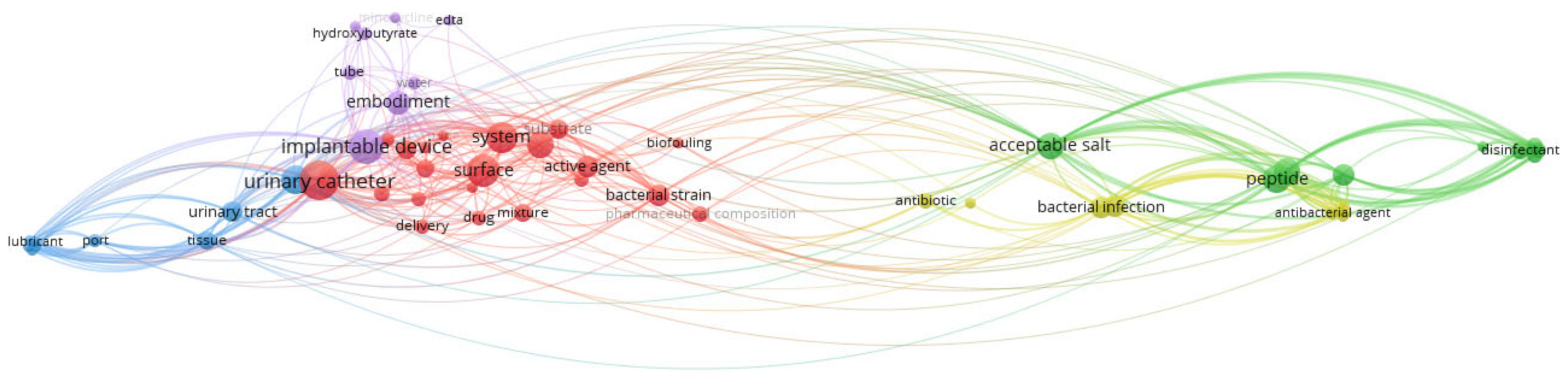
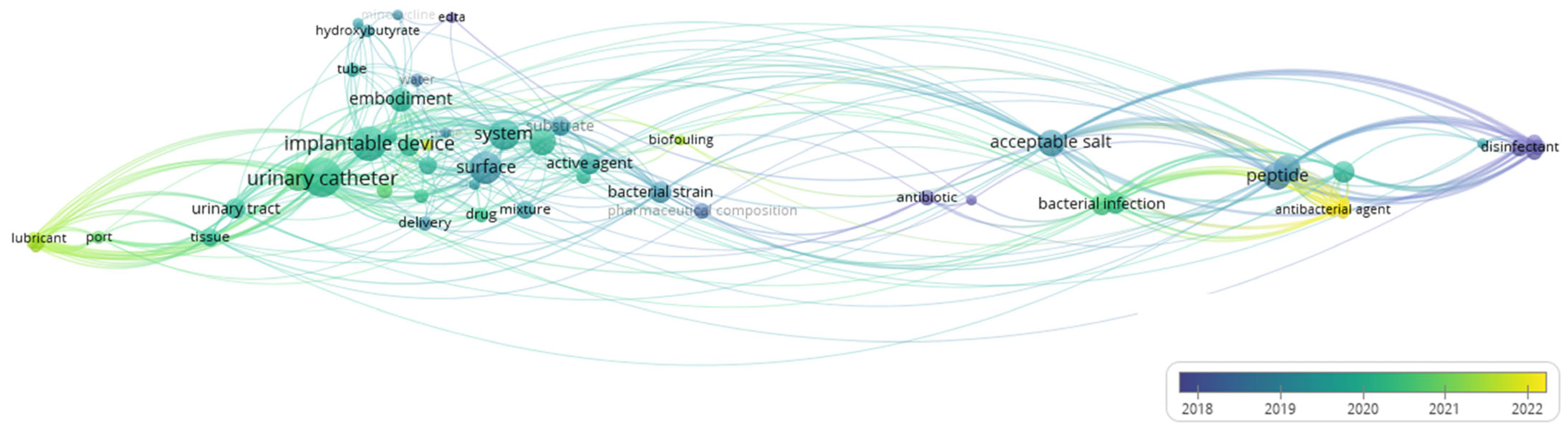
3.8. Co-Occurrence Analysis of the Top Keywords of the Recovered Patents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feneley, R.C.L.; Hopley, I.B.; Wells, P.N.T. Urinary Catheters: History, Current Status, Adverse Events and Research Agenda. J. Med. Eng. Technol. 2015, 39, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Falkiner, F. The Insertion and Management of Indwelling Urethral Catheters—Minimizing the Risk of Infection. J. Hosp. Infect. 1993, 25, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P. Hand Hygiene: Back to the Basics of Infection Control. Indian J. Med. Res. 2011, 134, 611. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.; Magaš, M.; Škrobonja, I.; Barać, N. Catheter associated urinary tract infections—Targeting zero infections. Med. Flum. 2020, 56, 444–451. [Google Scholar] [CrossRef]
- Maharjan, G.; Khadka, P.; Siddhi Shilpakar, G.; Chapagain, G.; Dhungana, G.R. Catheter-Associated Urinary Tract Infection and Obstinate Biofilm Producers. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7624857. [Google Scholar] [CrossRef]
- Maki, D.G.; Tambyah, P.A. Engineering out the Risk of Infection with Urinary Catheters. Emerg. Infect. Dis. 2001, 7, 342–347. [Google Scholar] [CrossRef]
- Serpico, L.; Dello Iacono, S.; De Stefano, L.; De Martino, S.; Battisti, M.; Dardano, P.; Pedatella, S.; De Nisco, M. pH-Sensitive Release of Antioxidant Se-Glycoconjugates through a Flexible Polymeric Patch. Eur. Polym. J. 2022, 178, 111486. [Google Scholar] [CrossRef]
- Alrebish, S.A.; Yusufoglu, H.S.; Alotibi, R.F.; Abdulkhalik, N.S.; Ahmed, N.J.; Khan, A.H. Epidemiology of Healthcare-Associated Infections and Adherence to the HAI Prevention Strategies. Healthcare 2022, 11, 63. [Google Scholar] [CrossRef]
- Baenas, D.F.; Saad, E.J.; Diehl, F.A.; Musso, D.; González, J.G.; Russo, V.; Vilaró, M.; Albertini, R.A. Nosocomial urinary tract infection: An analysis beyond urinary catheterization. Rev. Chil. Infectol. 2018, 35, 246–252. [Google Scholar] [CrossRef]
- Melzer, M.; Welch, C. Does the Presence of a Urinary Catheter Predict Severe Sepsis in a Bacteraemic Cohort? J. Hosp. Infect. 2017, 95, 376–382. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Ferguson, J.K.; Anderson, M.; Sear, J.; Barnett, A. Length of Stay and Mortality Associated with Healthcare-Associated Urinary Tract Infections: A Multi-State Model. J. Hosp. Infect. 2016, 93, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mody, L.; Meddings, J.; Edson, B.S.; McNamara, S.E.; Trautner, B.W.; Stone, N.D.; Krein, S.L.; Saint, S.; Perl, T.M. Enhancing Resident Safety by Preventing Healthcare-Associated Infection: A National Initiative to Reduce Catheter-Associated Urinary Tract Infections in Nursing Homes. Clin. Infect. Dis. 2015, 61, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Rubi, H.; Mudey, G.; Kunjalwar, R. Catheter-Associated Urinary Tract Infection (CAUTI). Cureus 2022, 14, e30385. [Google Scholar] [CrossRef]
- Clarke, K.; Hall, C.L.; Wiley, Z.; Chernetsky Tejedor, S.; Kim, J.S.; Reif, L.; Witt, L.; Jacob, J.T. Catheter-Associated Urinary Tract Infections in Adults: Diagnosis, Treatment, and Prevention. J. Hosp. Med. 2020, 15, 552–556. [Google Scholar] [CrossRef]
- Alothman, A.; Al Thaqafi, A.; Al Ansary, A.; Zikri, A.; Fayed, A.; Khamis, F.; Al Salman, J.; Al Dabal, L.; Khalife, N.; AlMusawi, T.; et al. Prevalence of Infections and Antimicrobial Use in the Acute-Care Hospital Setting in the Middle East: Results from the First Point-Prevalence Survey in the Region. Int. J. Infect. Dis. 2020, 101, 249–258. [Google Scholar] [CrossRef]
- Chen, W.; Xiang, S.; Cao, Y. Risk factors and pathogen distribution of urinary tract infection after ureteral stenting in urological patients. Chin. J. Clin. Infect. Dis. 2019, 12, 344–349. [Google Scholar] [CrossRef]
- Letica-Kriegel, A.S.; Salmasian, H.; Vawdrey, D.K.; Youngerman, B.E.; Green, R.A.; Furuya, E.Y.; Calfee, D.P.; Perotte, R. Identifying the Risk Factors for Catheter-Associated Urinary Tract Infections: A Large Cross-Sectional Study of Six Hospitals. BMJ Open 2019, 9, e022137. [Google Scholar] [CrossRef]
- AL-Hazmi, H. Role of Duration of Catheterization and Length of Hospital Stay on the Rate of Catheter-Related Hospital-Acquired Urinary Tract Infections. RRU 2015, 7, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Storme, O.; Saucedo, J.T.; Garcia-Mora, A.; Dehesa-Dávila, M.; Naber, K.G. Risk Factors and Predisposing Conditions for Urinary Tract Infection. Ther. Adv. Urol. 2019, 11, 19–28. [Google Scholar] [CrossRef]
- Lee, D.S.; Choe, H.-S.; Kim, H.Y.; Yoo, J.M.; Bae, W.J.; Cho, Y.H.; Kim, S.W.; Han, C.H.; Bae, S.R.; Jang, H.; et al. Role of Age and Sex in Determining Antibiotic Resistance in Febrile Urinary Tract Infections. Int. J. Infect. Dis. 2016, 51, 89–96. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Brauer, A.L.; Humby, M.S.; Shao, J.; Chakraborty, S. Prospective Assessment of Catheter-Associated Bacteriuria Clinical Presentation, Epidemiology, and Colonization Dynamics in Nursing Home Residents. JCI Insight 2021, 6, e144775. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, R.; Murt, A. Epidemiology of Urological Infections: A Global Burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.M.; Moat, J.; Jameson, E. CAUTI’s next Top Model—Model Dependent Klebsiella Biofilm Inhibition by Bacteriophages and Antimicrobials. Biofilm 2020, 2, 100038. [Google Scholar] [CrossRef] [PubMed]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating Strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef]
- Navarro, S.; Sherman, E.; Colmer-Hamood, J.A.; Nelius, T.; Myntti, M.; Hamood, A.N. Urinary Catheters Coated with a Novel Biofilm Preventative Agent Inhibit Biofilm Development by Diverse Bacterial Uropathogens. Antibiotics 2022, 11, 1514. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial Exo-Polysaccharides in Biofilms: Role in Antimicrobial Resistance and Treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Wi, Y.M.; Patel, R. Understanding Biofilms and Novel Approaches to the Diagnosis, Prevention, and Treatment of Medical Device-Associated Infections. Infect. Dis. Clin. North. Am. 2018, 32, 915–929. [Google Scholar] [CrossRef]
- Ch’ng, J.-H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-Associated Infection by Enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- Kreve, S.; Reis, A.C.D. Bacterial Adhesion to Biomaterials: What Regulates This Attachment? A Review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm Dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, D.; Gu, Q.; Miao, J.; Cen, X.; Golodok, R.P.; Savich, V.V.; Ilyushchenko, A.P.; Zhou, Z.; Wang, R. One-Step Coordination of Metal-Phenolic Networks as Antibacterial Coatings with Sustainable and Controllable Copper Release for Urinary Catheter Applications. RSC Adv. 2022, 12, 15685–15693. [Google Scholar] [CrossRef]
- Alves, D.; Vaz, A.T.; Grainha, T.; Rodrigues, C.F.; Pereira, M.O. Design of an Antifungal Surface Embedding Liposomal Amphotericin B through a Mussel Adhesive-Inspired Coating Strategy. Front. Chem. 2019, 7, 431. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nanocomposite Coating for Urinary Catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdusemed, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review On The Recent Use of Phage-Based Strategies With Other Antibiofilm Agents. NSA 2021, 14, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, Z.; Li, S.; Yuan, X. Antimicrobial Strategies for Urinary Catheters. J. Biomed. Mater. Res. Part A 2019, 107, 445–467. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Tzanov, T. Simultaneous Ultrasound-Assisted Hybrid Polyzwitterion/Antimicrobial Peptide Nanoparticles Synthesis and Deposition on Silicone Urinary Catheters for Prevention of Biofilm-Associated Infections. Nanomaterials 2021, 11, 3143. [Google Scholar] [CrossRef]
- Yu, K.; Lo, J.C.Y.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.W.; Lange, D.; Kizhakkedathu, J.N. Anti-Adhesive Antimicrobial Peptide Coating Prevents Catheter Associated Infection in a Mouse Urinary Infection Model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef]
- Akcam, F.Z.; Kaya, O.; Temel, E.N.; Buyuktuna, S.A.; Unal, O.; Yurekli, V.A. An Investigation of the Effectiveness against Bacteriuria of Silver-Coated Catheters in Short-Term Urinary Catheter Applications: A Randomized Controlled Study. J. Infect. Chemother. 2019, 25, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, A.T.; Brisson, B.A.; Singh, A.; Weese, J.S. In Vitro Evaluation of the Impact of Silver Coating on Escherichia Coli Adherence to Urinary Catheters. Can. Vet. J. 2015, 56, 490–494. [Google Scholar] [PubMed]
- Ahamad, I.; Bano, F.; Anwer, R.; Srivastava, P.; Kumar, R.; Fatma, T. Antibiofilm Activities of Biogenic Silver Nanoparticles Against Candida albicans. Front. Microbiol. 2022, 12, 741493. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Elkhooly, T.A.; Elsherbiny, S.M.; Reicha, F.M.; Shokeir, A.A. Facile Coating of Urinary Catheter with Bio–Inspired Antibacterial Coating. Heliyon 2019, 5, e02986. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Xing, M.; Ge, L.; Wang, M.; Li, Q.; Li, X.; Ouyang, J. Nanosilver Particles in Medical Applications: Synthesis, Performance, and Toxicity. IJN 2014, 9, 2399–2407. [Google Scholar] [CrossRef]
- Goda, R.M.; El-Baz, A.M.; Khalaf, E.M.; Alharbi, N.K.; Elkhooly, T.A.; Shohayeb, M.M. Combating Bacterial Biofilm Formation in Urinary Catheter by Green Silver Nanoparticle. Antibiotics 2022, 11, 495. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W.; Xu, W.; Xu, S. Fluorescent Ag Nanoclusters Templated by Carboxymethyl-β-Cyclodextrin (CM-β-CD) and Their in Vitro Antimicrobial Activity. Mater. Sci. Eng. C 2013, 33, 656–662. [Google Scholar] [CrossRef]
- Dias, L.D.; Duarte, L.S.; Naves, P.L.F.; Napolitano, H.B.; Bagnato, V.S. Self-Disinfecting Urethral Catheter to Overcome Urinary Infections: From Antimicrobial Photodynamic Action to Antibacterial Biochemical Entities. Microorganisms 2022, 10, 2484. [Google Scholar] [CrossRef]
- Fisher, L.E.; Hook, A.L.; Ashraf, W.; Yousef, A.; Barrett, D.A.; Scurr, D.J.; Chen, X.; Smith, E.F.; Fay, M.; Parmenter, C.D.J.; et al. Biomaterial Modification of Urinary Catheters with Antimicrobials to Give Long-Term Broadspectrum Antibiofilm Activity. J. Control. Release 2015, 202, 57–64. [Google Scholar] [CrossRef]
- Faustino, C.M.C.; Lemos, S.M.C.; Monge, N.; Ribeiro, I.A.C. A Scope at Antifouling Strategies to Prevent Catheter-Associated Infections. Adv. Colloid. Interface Sci. 2020, 284, 102230. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Liu, H.; Yang, X.; Shan, Y.; Xu, X.; Shang, S.; Song, Z. Dual-Functional Antimicrobial Coating Based on a Quaternary Ammonium Salt from Rosin Acid with in Vitro and in Vivo Antimicrobial and Antifouling Properties. Chem. Eng. J. 2019, 374, 564–575. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, S.; Yang, J.; Ma, A. Advancing Strategies of Biofouling Control in Water-Treated Polymeric Membranes. Polymers 2022, 14, 1167. [Google Scholar] [CrossRef]
- Huang, Z.; Ghasemi, H. Hydrophilic Polymer-Based Anti-Biofouling Coatings: Preparation, Mechanism, and Durability. Adv. Colloid. Interface Sci. 2020, 284, 102264. [Google Scholar] [CrossRef] [PubMed]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B. Bacteriophages and Phage-Derived Proteins—Application Approaches. CMC 2015, 22, 1757–1773. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, G.; Legese, H.; Woldu, Y.; Araya, T.; Hagos, K.; Gebreyesuswasihun, A. Bacteriological Profile, Risk Factors and Antimicrobial Susceptibility Patterns of Symptomatic Urinary Tract Infection among Students of Mekelle University, Northern Ethiopia. BMC Infect. Dis. 2019, 19, 950. [Google Scholar] [CrossRef]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative Strategies Toward the Disassembly of the EPS Matrix in Bacterial Biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Henly, E.L.; Norris, K.; Rawson, K.; Zoulias, N.; Jaques, L.; Chirila, P.G.; Parkin, K.L.; Kadirvel, M.; Whiteoak, C.; Lacey, M.M.; et al. Impact of Long-Term Quorum Sensing Inhibition on Uropathogenic Escherichia Coli. J. Antimicrob. Chemother. 2021, 76, 909–919. [Google Scholar] [CrossRef]
- Rathinam, P.; Vijay Kumar, H.S.; Viswanathan, P. Eugenol Exhibits Anti-Virulence Properties by Competitively Binding to Quorum Sensing Receptors. Biofouling 2017, 33, 624–639. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Gov, Y.; Ghiselli, R.; Del Prete, M.S.; Mocchegiani, F.; Saba, V.; Orlando, F.; Scalise, G.; Balaban, N.; et al. RNA III Inhibiting Peptide Inhibits In Vivo Biofilm Formation by Drug-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 1979–1983. [Google Scholar] [CrossRef]
- Pant, J.; Goudie, M.J.; Chaji, S.M.; Johnson, B.W.; Handa, H. Nitric Oxide Releasing Vascular Catheters for Eradicating Bacterial Infection. J. Biomed. Mater. Res. 2018, 106, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Z.; Wang, J.; Lopez, A.I.; Li, S.; Kumar, A.; Yu, F.; Chen, H.; Cai, C.; Zhang, L. Probiotic E. Coli Nissle 1917 Biofilms on Silicone Substrates for Bacterial Interference against Pathogen Colonization. Acta Biomater. 2017, 50, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.E. Antimicrobial Peptides. Surg. Infect. 2018, 19, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A. Statistical Bibliography or Bibliometrics. J. Doc. 1969, 25, 348–349. [Google Scholar]
- Zheng, M.; Fu, H.-Z.; Ho, Y.-S. Research Trends and Hotspots Related to Ammonia Oxidation Based on Bibliometric Analysis. Env. Sci. Pollut. Res. 2017, 24, 20409–20421. [Google Scholar] [CrossRef]
- Osareh, F. Bibliometrics, Citation Analysis and Co-Citation Analysis: A Review of Literature I. Libri 1996, 46, 149–158. [Google Scholar] [CrossRef]
- Choi, D.; Song, B. Exploring Technological Trends in Logistics: Topic Modeling-Based Patent Analysis. Sustainability 2018, 10, 2810. [Google Scholar] [CrossRef]
- Kim, G.; Bae, J. A Novel Approach to Forecast Promising Technology through Patent Analysis. Technol. Forecast. Soc. Change 2017, 117, 228–237. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Li, C.; Chen, B.; Sun, Y. Using Bibliometric Analysis to Understand the Recent Progress in Agroecosystem Services Research. Ecol. Econ. 2019, 156, 293–305. [Google Scholar] [CrossRef]
- Cammarano, A.; Dello Iacono, S.; Meglio, C.; Nicolais, L. Advances in Transdermal Drug Delivery Systems: A Bibliometric and Patent Analysis. Pharmaceutics 2023, 15, 2762. [Google Scholar] [CrossRef]
- Serpico, L.; Dello Iacono, S.; Cammarano, A.; De Stefano, L. Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing. Gels 2023, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Cammarano, A.; Iacono, S.D.; Battisti, M.; De Stefano, L.; Meglio, C.; Nicolais, L. A Systematic Review of Microneedles Technology in Drug Delivery through a Bibliometric and Patent Overview. Heliyon 2024, 10, e40658. [Google Scholar] [CrossRef] [PubMed]
- Speziali, M.G. Cellulose Technologies Applied to Biomedical Purposes from the Patentometric Point of View. Cellulose 2020, 27, 10095–10117. [Google Scholar] [CrossRef]
- Jain, R.; Tripathi, M.; Agarwal, V.; Murthy, J. Patent Data Analytics for Technology Benchmarking: R-Based Implementation. World Pat. Inf. 2020, 60, 101952. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Matta, R.; Schaeffer, A.J. The Top 100 Cited Articles in Pediatric Urology: A Bibliometric Analysis. J. Pediatr. Urol. 2021, 17, 709.e1–709.e12. [Google Scholar] [CrossRef]
- Speziali, M.G.; Livio, D.F.; Tarabal, V.S.; Granjeiro, P.A. Technology Landscape and a Short Patentometric Review for Antibiofilm Technologies. World Pat. Inf. 2023, 72, 102158. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Bukar, U.A.; Sayeed, M.S.; Razak, S.F.A.; Yogarayan, S.; Amodu, O.A.; Mahmood, R.A.R. A Method for Analyzing Text Using VOSviewer. MethodsX 2023, 11, 102339. [Google Scholar] [CrossRef]
- Santha Kumar, R.; Kaliyaperumal, K. A Scientometric Analysis of Mobile Technology Publications. Scientometrics 2015, 105, 921–939. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of Co-Infections and Superinfections in Hospitalized Patients with COVID-19: A Retrospective Cohort Study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.R. The Use of Multiple Indicators in the Assessment of Basic Research. Scientometrics 1996, 36, 343–362. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated Infections, Medical Devices and Biofilms: Risk, Tolerance and Control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial Infections: Epidemiology, Prevention, Control and Surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas Aeruginosabiofilms to Antimicrobial Agents-How P. aeruginosaCan Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Benin, A.L.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; et al. Antimicrobial-Resistant Pathogens Associated with Pediatric Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network, 2015-2017. Infect. Control Hosp. Epidemiol. 2020, 41, 19–30. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, E75–E83. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A Review of the Recent Advances in Antimicrobial Coatings for Urinary Catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, Pathogenesis and Prevention—A Journey to Break the Wall: A Review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L. Biofilm-Related Disease. Expert. Rev. Anti-Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L. Analysis of Risk Factors for Infection after Transrectal Ultrasound-Guided Prostate Biopsy and Analysis of the Value of Preoperative Prophylactic Antimicrobial Use. JOMH 2023, 19, 60. [Google Scholar] [CrossRef]
- INICC Members; Rosenthal, V.D.; Todi, S.K.; Álvarez-Moreno, C.; Pawar, M.; Karlekar, A.; Zeggwagh, A.A.; Mitrev, Z.; Udwadia, F.E.; Navoa-Ng, J.A.; et al. Impact of a Multidimensional Infection Control Strategy on Catheter-Associated Urinary Tract Infection Rates in the Adult Intensive Care Units of 15 Developing Countries: Findings of the International Nosocomial Infection Control Consortium (INICC). Infection 2012, 40, 517–526. [Google Scholar] [CrossRef]
- Losito, A.R.; Raffaelli, F.; Del Giacomo, P.; Tumbarello, M. New Drugs for the Treatment of Pseudomonas Aeruginosa Infections with Limited Treatment Options: A Narrative Review. Antibiotics 2022, 11, 579. [Google Scholar] [CrossRef]
- Juarez, G.E.; Mateyca, C.; Galvan, E.M. Proteus Mirabilis Outcompetes Klebsiella Pneumoniae in Artificial Urine Medium through Secretion of Ammonia and Other Volatile Compounds. Heliyon 2020, 6, e03361. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus Mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell. Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alzahrani, A.J.; Tobaiqy, M.; Alresasi, A.M.; Bu-Shehab, I.; Al-Hadary, I.; Alhmeed, N.; Alismail, M.; et al. Antimicrobial Susceptibility of Gram-Positive and Gram-Negative Bacteria: A 5-Year Retrospective Analysis at a Multi-Hospital Healthcare System in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 43. [Google Scholar] [CrossRef]
- Nair, S.V.; Baranwal, G.; Chatterjee, M.; Sachu, A.; Vasudevan, A.K.; Bose, C.; Banerji, A.; Biswas, R. Antimicrobial Activity of Plumbagin, a Naturally Occurring Naphthoquinone from Plumbago Rosea, against Staphylococcus aureus and Candida albicans. Int. J. Med. Microbiol. 2016, 306, 237–248. [Google Scholar] [CrossRef]
- Zare-Farashbandi, F.; Geraei, E.; Siamaki, S. Study of Co-Authorship Network of Papers in the Journal of Research in Medical Sciences Using Social Network Analysis. J. Res. Med. Sci. 2014, 19, 41–46. [Google Scholar]
- Chen, G.; Xiao, L. Selecting Publication Keywords for Domain Analysis in Bibliometrics: A Comparison of Three Methods. J. Informetr. 2016, 10, 212–223. [Google Scholar] [CrossRef]
- Pottier, P.; Lagisz, M.; Burke, S.; Drobniak, S.M.; Downing, P.A.; Macartney, E.L.; Martinig, A.R.; Mizuno, A.; Morrison, K.; Pollo, P.; et al. Title, Abstract and Keywords: A Practical Guide to Maximize the Visibility and Impact of Academic Papers. Proc. R. Soc. B. 2024, 291, 20241222. [Google Scholar] [CrossRef] [PubMed]
- Gaston, J.R.; Andersen, M.J.; Johnson, A.O.; Bair, K.L.; Sullivan, C.M.; Guterman, L.B.; White, A.N.; Brauer, A.L.; Learman, B.S.; Flores-Mireles, A.L.; et al. Enterococcus Faecalis Polymicrobial Interactions Facilitate Biofilm Formation, Antibiotic Recalcitrance, and Persistent Colonization of the Catheterized Urinary Tract. Pathogens 2020, 9, 835. [Google Scholar] [CrossRef]
- Mandakhalikar, K.D.; Wang, R.; Rahmat, J.N.; Chiong, E.; Neoh, K.G.; Tambyah, P.A. Restriction of in Vivo Infection by Antifouling Coating on Urinary Catheter with Controllable and Sustained Silver Release: A Proof of Concept Study. BMC Infect. Dis. 2018, 18, 370. [Google Scholar] [CrossRef]
- Stefani, S.; Campanile, F.; Santagati, M.; Mezzatesta, M.L.; Cafiso, V.; Pacini, G. Insights and Clinical Perspectives of Daptomycin Resistance in Staphylococcus aureus: A Review of the Available Evidence. Int. J. Antimicrob. Agents 2015, 46, 278–289. [Google Scholar] [CrossRef]
- Benny, A.T.; Rathinam, P.; Dev, S.; Mathew, B.; Radhakrishnan, E. Perillaldehyde Mitigates Virulence Factors and Biofilm Formation of Pseudomonas Aeruginosa Clinical Isolates, by Acting on the Quorum Sensing Mechanism in Vitro. J. Appl. Microbiol. 2022, 133, 385–399. [Google Scholar] [CrossRef]
- Moureau, N. Hydrophilic Biomaterial Intravenous Hydrogel Catheter for Complication Reduction in PICC and Midline Catheters. Expert. Rev. Med. Devices 2024, 21, 207–216. [Google Scholar] [CrossRef]
- Page, S.; Hazen, D.; Kelley, K.; Singh, R.; Rodgers, R.B.; Brewer, B.; Sadowski, J.; Desai, A.; Beeler, C.; Webb, D.; et al. Changing the Culture of Urine Culturing: Utilizing Agile Implementation to Improve Diagnostic Stewardship in the ICU. Am. J. Infect. Control 2020, 48, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.; Chhibber, S.; Harjai, K. Antimicrobial and Antifouling Efficacy of Urinary Catheters Impregnated with a Combination of Macrolide and Fluoroquinolone Antibiotics against Pseudomonas Aeruginosa. Biofouling 2016, 32, 795–806. [Google Scholar] [CrossRef]
- Bekele, T.; Tesfaye, A.; Sewunet, T.; Waktola, H.D. Pseudomonas Aeruginosa Isolates and Their Antimicrobial Susceptibility Pattern among Catheterized Patients at Jimma University Teaching Hospital, Jimma, Ethiopia. BMC Res. Notes 2015, 8, 488. [Google Scholar] [CrossRef]
- Maniam, L.; Vellasamy, K.M.; Ong, T.A.; Teh, C.S.J.; Jabar, K.A.; Mariappan, V.; Narayanan, V.; Vadivelu, J.; Pallath, V. Genotypic Characteristics of Uropathogenic Escherichia Coli Isolated from Complicated Urinary Tract Infection (cUTI) and Asymptomatic Bacteriuria—A Relational Analysis. PeerJ 2023, 11, e15305. [Google Scholar] [CrossRef] [PubMed]
- Garcell, H.G.; Al-Ajmi, J.; Arias, A.V.; Abraham, J.C.; Garmendia, A.M.F.; Hernandez, T.M.F. Catheter-Associated Urinary Tract Infection and Urinary Catheter Utilization Ratio over 9 Years, and the Impact of the COVID-19 Pandemic on the Incidence of Infection in Medical and Surgical Wards in a Single Facility in Western Qatar. Qatar Med. J. 2023, 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Hiyama, Y.; Uehara, T.; Ichihara, K.; Hashimoto, J.; Fujii, S.; Shinagawa, M.; Takahashi, S.; Masumori, N. The Efficacy of Faropenem for Patients with Acute Cystitis Caused by Extended Spectrum β-Lactamase Producing Escherichia Coli. J. Infect. Chemother. 2017, 23, 336–338. [Google Scholar] [CrossRef]
- Baten, E.; Van Der Aa, F.; Goethuys, H.; Slabbaert, K.; Arijs, I.; Van Renterghem, K. Antimicrobial Prophylaxis in Transurethral Resection of the Prostate: Results of a Randomized Trial. J. Urol. 2021, 205, 1748–1752. [Google Scholar] [CrossRef] [PubMed]
- Latour, K.; Lepeleire, J.D.; Jans, B.; Buntinx, F.; Catry, B. Diagnosis, Prevention and Control of Urinary Tract Infections: A Survey of Routine Practices in Belgian Nursing Homes. J. Infect. Prevent. 2020, 21, 182–188. [Google Scholar] [CrossRef]
- Agarwal, R.; Mohapatra, S.; Rath, G.P.; Kapil, A. Active Surveillance of Health Care Associated Infections in Neurosurgical Patients. J. Clin. Diagn. Res. 2017, 11, DC01–DC04. [Google Scholar] [CrossRef]
- Takaya, S.; Hayakawa, K.; Matsunaga, N.; Moriyama, Y.; Katanami, Y.; Tajima, T.; Tanaka, C.; Kimura, Y.; Saito, S.; Kusama, Y.; et al. Surveillance Systems for Healthcare-Associated Infection in High and Upper-Middle Income Countries: A Scoping Review. J. Infect. Chemother. 2020, 26, 429–437. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.Y.; Shan, X.; Han, X.L.; Tian, S.G.; Chen, F.Y.; Su, X.T.; Sun, Y.S.; Huang, L.Y.; Han, L.; et al. A Point-Prevalence Survey of Healthcare-Associated Infection in Fifty-Two Chinese Hospitals. J. Hosp. Infect. 2017, 95, 105–111. [Google Scholar] [CrossRef]
- Suebsubanant, M. Clinical Outcomes and Associated Factors for Mortality among Pediatric Patients with Carbapenem-Resistant Acinetobacter Baumannii. J. Med. Assoc. Thai 2023, 106, 534–543. [Google Scholar] [CrossRef]
- Baveja, U.K.; Govil, D.; Wadhwa, T.; Mehta, Y. Susceptibility of Multi-Drug-Resistant Organisms (MDROs), Isolated from Cases of Urinary Tract Infection to Fosfomycin (The New Antibiotic) Vis-a-Vis Other Antimicrobial Agents. J. Commun. Dis. 2018, 50, 34–37. [Google Scholar] [CrossRef]
- Atamna-Mawassi, H.; Huberman-Samuel, M.; Hershcovitz, S.; Karny-Epstein, N.; Kola, A.; Cortés, L.E.L.; Leibovici, L.; Yahav, D. Interventions to Reduce Infections Caused by Multidrug Resistant Enterobacteriaceae (MDR-E): A Systematic Review and Meta-Analysis. J. Infect. 2021, 83, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.N.; Thi Le, H.T.; Tran, T.D.; Vu, L.T.; Ho, T.H. Clinical Epidemiology Characteristics and Antibiotic Resistance Associated with Urinary Tract Infections Caused by E. Coli. Int. J. Nephrol. 2022, 2022, 2552990. [Google Scholar] [CrossRef] [PubMed]
- Limem, S.; Harun, R.B.; Dubois, M.; Martin, D.P.; Rizk, S. Methods for 3D Printing of Poly-4-Hydroxybutyrate and Copolymers. Tepha US20190375149A1, 12 December 2019. [Google Scholar]
- Eltorai, A.E.M. Method, System, and Devices of Safe, Antimicrobial Light-Emitting Catheters, Tubes, and Instruments. Luminary Catheters US12246189B2, 6 June 2019. [Google Scholar]
- Erbey, J.R.; Tucker, B.J.; Upperco, J.L. Coated Urinary Catheter or Ureteral Stent and Method. Roivios US11541205B2, 30 November 2018. [Google Scholar]
- Knapp, T.E.; Nishtala, V. Enhanced Pre-Wetted Intermittent Catheter with Lubricious Coating. Bard Inc. AU2014248744B2, 12 March 2014. [Google Scholar]
- Littleton, K.R.; Detlor, L.R.; Rego, A. Method for Coating Catheters with a Layer of Antimicrobial Agent. Pursuit Vascular Inc. US9352142B2, 22 May 2015. [Google Scholar]
- Eltorai, A.E.M.; Dicesare, P. Antimicrobial Light-Emitting Device and Method of Reducing Catheter-Associated Urinary Tract Infections. Lumen Catheters US12115385B2, 28 June 2022. [Google Scholar]
- Rosenblatt, J.; Raad, I. Antimicrobial Catheters. University of Texas US11738119B2, 8 July 2020. [Google Scholar]
- Wiita, G.D. Antimicrobial Shield and Barrier for Urinary Catheter. Poiesis Medical US2018/0311469A1, 27 April 2018. [Google Scholar]
- Kalt, J.; Lee, H. Drug Delivery Devices and Methods for Use with a Urinary Catheter. Taris Biomedical US2020/0345976A1, 8 November 2018. [Google Scholar]
- Limaye, A.; Taylor, M. Catheter with Inherent Antimicrobial Properties. Becton Dickinson and Company US2023/0321326A1, 11 April 2022. [Google Scholar]
- Panesar, S. Hydrophilic Urinary Catheter Products with Microcapsules of Anti-Bacterial Agents. Hollister WO2023/215683A1, 25 April 2023. [Google Scholar]
- Bakaletz, L.O.; Goodman, S.D. Peptides and Antibodies for the Removal of Biofilms. The Research Institute at Nationwide Children’s Hospital EP3328429A4, 1 August 2016. [Google Scholar]
- Bakaletz, L.O.; Goodman Steven, D. Hu Specific Interfering Agents. The Research Institute at Nationwide Children’s Hospital WO2017/066719A2, 14 October 2016. [Google Scholar]
- Schwartz, U.W. Antimicrobial Preparations, Methods for Preparing the Same and Uses Thereof to Combat Microorganisms. Ipabc US10470459B2, 28 May 2015. [Google Scholar]
- Deber, C.M. Cationic Antimicrobial Peptides. Hospital for Sick Children US2015/0290278A1, 12 December 2019. [Google Scholar]
- Smyth, H.; Bahamondez-Canas, T.; Tewes, F.; Heersema, L. Antibiofilm Formulations and Use Thereof. University of Texas WO2019/104213A1, 21 November 2018. [Google Scholar]
- Hayouka, Z. Methods for Disrupting Biofilms. Yissum Research Development Company US2019/0038701A1, 2 February 2017. [Google Scholar]
- Mysore, V.G.T.; Pirttilä, A.M. Antimicrobial Peptides, Their Variants and Uses. Oulun Yliopisto US11096985B2, 13 January 2020. [Google Scholar]
- Kizhakkedathu, J.; Lange, D.; Yu, K.; Hancock, R. Polymeric Antifouling Coating with Antimicrobial Peptides. University of British Columbia US2024/0301218A1, 2 June 2022. [Google Scholar]
- Goodman, S.D.; Bakaletz, L.O. Combination Therapies for the Treatment and Prevention of Biofilms. The Research Institute at Nationwide Children’s Hospital WO2022/010942A3, 6 July 2021. [Google Scholar]
- Nicolau, E.; Ortiz, G.V. Compositions Including Antimicrobial Polymer-Peptide Conjugates and Uses Thereof. University of Puerto Rico WO2021/154703A1, 26 January 2021. [Google Scholar]
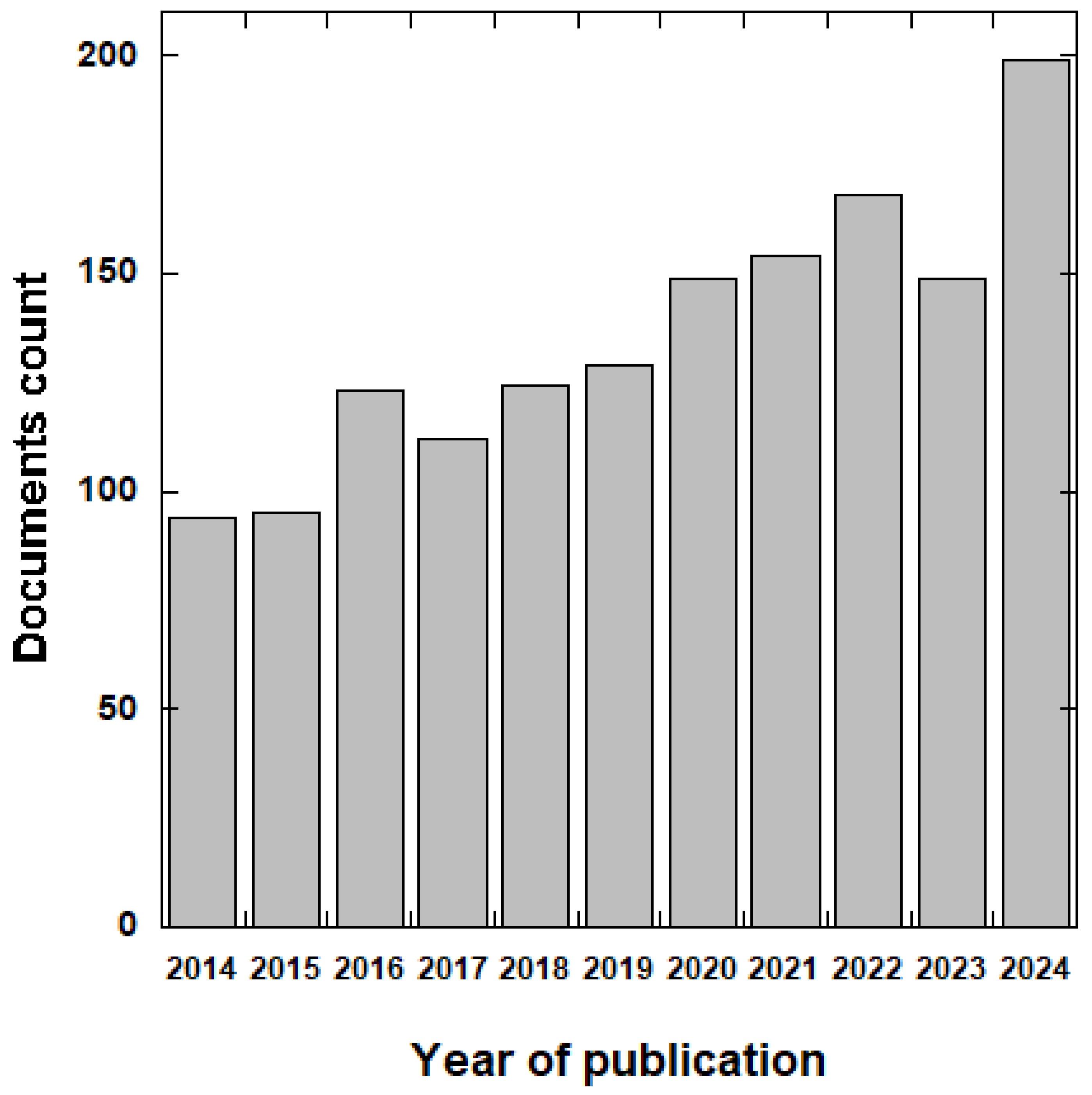
| Year | Number of Publications | % of Publications | AGR |
|---|---|---|---|
| 2014 | 94 | 6.24 | 0.00 |
| 2015 | 95 | 6.31 | 1.06 |
| 2016 | 123 | 8.17 | 29.47 |
| 2017 | 112 | 7.44 | −8.94 |
| 2018 | 125 | 8.30 | 11.61 |
| 2019 | 129 | 8.57 | 3.20 |
| 2020 | 149 | 9.89 | 15.50 |
| 2021 | 154 | 10.23 | 3.36 |
| 2022 | 172 | 11.42 | 11.69 |
| 2023 | 154 | 10.23 | −10.47 |
| 2024 | 199 | 13.21 | 29.22 |
| Rank | Source | Total Publications | IF (2023) | Total Citations |
|---|---|---|---|---|
| 1 | Antibiotics | 47 | 4.3 | 592 |
| 2 | BMC Infectious Diseases | 30 | 3.4 | 510 |
| 3 | Infection Control and Hospital Epidemiology | 27 | 3 | 1930 |
| 4 | Journal Of Hospital Infection | 25 | 3.9 | 674 |
| 5 | Infection and Drug Resistance | 23 | 2.9 | 380 |
| 6 | American Journal of Infection Control | 22 | 4.9 | 477 |
| 7 | PLOS One | 17 | 2.9 | 346 |
| 8 | European Journal of Clinical Microbiology and Infectious Diseases | 17 | 3.7 | 292 |
| 9 | Antimicrobial Resistance and Infection Control | 17 | 4.8 | 327 |
| 10 | Journal Of Infection and Chemotherapy | 15 | 1.9 | 346 |
| Title | PY | Journal | Citations | Ref. |
|---|---|---|---|---|
| Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management | 2015 | Clinical Microbiology Reviews | 3444 | [83] |
| Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention | 2016 | Infection Control and Hospital Epidemiology | 962 | [84] |
| Healthcare-Associated infections, medical devices and biofilms: Risk, tolerance and control | 2015 | Journal of Medical Microbiology | 572 | [85] |
| Nosocomial infections: Epidemiology, prevention, control and surveillance | 2017 | Asian Pacific Journal of Tropical Biomedicine | 479 | [86] |
| Tolerance and resistance of pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics | 2019 | Frontiers in Microbiology | 468 | [87] |
| Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network | 2020 | Infection Control and Hospital Epidemiology | 435 | [88] |
| Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America | 2019 | Clinical Infectious Diseases | 406 | [89] |
| A review of the recent advances in antimicrobial coatings for urinary catheters | 2017 | Acta Biomaterialia | 372 | [90] |
| Biofilm, pathogenesis, and prevention a journey to break the wall: a review | 2016 | Archives of Microbiology | 349 | [91] |
| Biofilm-related disease | 2018 | Expert Review of Anti-Infective Therapy | 328 | [92] |
| Subject Area | Document Count |
|---|---|
| Medicine | 1153 |
| Immunology and Microbiology | 275 |
| Biochemistry, Genetics, and Molecular Biology | 205 |
| Pharmacology, Toxicology, and Pharmaceutics | 189 |
| Materials Science | 91 |
| Engineering | 71 |
| Chemical Engineering | 52 |
| Chemistry | 50 |
| Nursing | 29 |
| Veterinary | 38 |
| Funding Sponsor | Country | Documents Count |
|---|---|---|
| National Institutes of Health | United States | 80 |
| U.S. Department of Health and Human Services | United States | 60 |
| European Commission | European Union | 30 |
| European Regional Development Fund | European Union | 30 |
| National Institute of Allergy and Infectious Diseases | United States | 26 |
| National Natural Science Foundation of China | China | 26 |
| Centers for Disease Control and Prevention | United States | 19 |
| Ministerio de Economía y Competitividad | Spain | 18 |
| National Institute of Diabetes and Digestive and Kidney Diseases | United States | 17 |
| Instituto de Salud Carlos III | Spain | 16 |
| Keywords | ||||
|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 |
| biofilm | Catheter-associated urinary tract infection | urinary tract infection | antimicrobial resistance | risk factors |
| urinary catheter | bacteremia | bacteriuria | multi-drug resistance | mortality |
| Escherichia coli | intensive care unit | antibiotics | healthcare-associated infections | carbapenem |
| antimicrobials | sepsis | antimicrobial stewardship | infection prevention and control | klebsiella pneumoniae |
| antibiotic resistance | bloodstream infections | infections | nosocomial infections | drug resistance |
| catheter | hospital-acquired infection | cystitis | surveillance | gram-negative |
| Pseudomonas aeruginosa | pediatrics | pyelonephritis | antibiotic stewardship | uropathogens |
| Proteus mirabilis | COVID-19 | diagnosis | antibiotic prophylaxis | Acinetobacter baumannii |
| Staphylococcus aureus | antimicrobial susceptibility | treatment | prevention | fluoroquinolones |
| Enterococcus faecalis | pathogens | children | antimicrobial use | carbapenem-resistant |
| Applicant | % Documents | Country |
|---|---|---|
| University of Texas | 6.80 | USA |
| Polyphor | 4.07 | Switzerland |
| Bard | 4.07 | USA |
| Tepha medical device | 3.53 | USA |
| Hollister Inc. | 2.44 | USA |
| Akeso biomedical | 2.17 | USA |
| Griffith Donald | 2.17 | USA |
| University of Zurich | 1.90 | Switzerland |
| Alps South | 1.74 | Italy |
| University of Stanford | 1.63 | USA |
| Keywords | ||||
|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 |
| Antimicrobial coating | Gram-negative bacteria | Urinary stent device | Cytotoxicity | Implantable device |
| Hydrophilic coating | Escherichia coli | Negative pressure | Parallel library | Tube |
| Bacterial strain | Klebsiella Pneumonia | Protective surface area | Phage therapy | Embodiment |
| Surface | Antimicrobial peptide | Lubricant | Therapeutic use | P4HB |
| Urinary catheter | Peptide | Coating | Antibacterial agent | Hydroxybutyrate |
| Biofouling | Formulation | Port | Bacterial infection | Minocycline |
| Polymer | Disinfectant | Urinary tract | Disease | EDTA |
| Delivery | Acceptable salt | Tissue | Antibiotic | Water |
| Application Number | Application Date | Title | Owners | Note | Ref. |
|---|---|---|---|---|---|
| US 2019/0168023 A1 | 5 December 2017 | Method, System, and Devices of Safe, Antimicrobial Light-Emitting Catheters, Tubes, and Instruments | Lumen Catheters LLC | The device consists of a thin, flexible tube with an optically transparent wall. It incorporates a light transmitter, which emits UV light that is effective in killing or inactivating bacteria, viruses, and other pathogens. | [124] |
| US 2019/0091442 A1 | 30 November 2018 | Coated Ureteral Catheter or Ureteral Stent and Method | Strataca Systems Limited | The device comprises a urinary catheter or stent with a protective surface area crucial for maintaining proper drainage and preventing the occlusion of drainage holes. It is coated with a specific material for lubrication, antimicrobial properties, and pH buffering. | [125] |
| US 9694113 B2 | 7 April 2015 | Enhanced Pre-Wetted Intermittent Catheter with Lubricious Coating | Bard Inc. | The catheter assembly comprises a tube-like conduit. Its distal end includes at least one opening for receiving fluid from the patient’s body. The conduit and the sleeve may be arranged in a helical coil configuration. The outer surface of the conduit may feature an antimicrobial coating, to help in the inhibition of the growth of bacteria or other microorganisms on the catheter surface. | [126] |
| US 10709819 B2 | 21 September 2017 | Method For Coating Catheters with a Layer of Antimicrobial Agent | Valencide LLC | A catheter made of highly flexible elastomeric material with an antimicrobial coating designed to reduce the risk of device-associated infections in the urinary tract, respiratory system, and bloodstream. This coating is intended to release iodine in a controlled manner over time, thereby inhibiting the growth of bacteria and other microorganisms on the catheter surface. | [127] |
| US 2022/0323787 A1 | 28 June 2022 | Antimicrobial Light-Emitting Device and Method of Reducing Catheter-Associated Urinary Tract Infections | Lumen Catheters LLC | This invention relates to an antimicrobial urinary catheter device that uses safe, antimicrobial light to disinfect the distal urethra, urethral meatus, and the area surrounding an indwelling urinary catheter. The illumination source is designed to emit light that can eradicate pathogens without being harmful for the patient. | [128] |
| US 11738119 B2 | 8 July 2020 | Antimicrobial Catheters | University Of Texas | An antimicrobial catheter made up of a low durometer aliphatic polyether polyurethane impregnated with a first antimicrobial agent (e.g., minocycline and rifampin) and coated with a second antimicrobial agent (e.g., chlorhexidine, gendine, or gardine). | [129] |
| US 2018/0311469 A1 | 27 April 2018 | Antimicrobial Shield and Barrier for Urinary Catheter | Poiesis Medical LLC | A flexible protective barrier with antimicrobial, antiseptic, and antibacterial properties is designed to interact with a urinary catheter. The shield, which is adaptable, is structured to smoothly slide onto the catheter. It consists of one or more sections that are shaped to conform to and adhere to the urinary meatus and/or the surrounding skin or tissues of the targeted patient. Additionally, a flexible and expandable drape, aligned with the catheter, is connected to the shield, adding an extra layer of protection to prevent the entry of bacteria, fungi, and contaminants into the urinary tract. | [130] |
| US 2020/0345976 A1 | 08 November 2018 | Drug Delivery Devices and Methods for Use with a Urinary Catheter | Taris Biomedical LLC | The urinary catheter is connected to a drug-delivery device. The flexible elongated body, along with the drug delivery lumen, is inserted into the patient’s urethra. The drug, contained within the drug chamber of the reservoir, is then delivered through the drug delivery lumen into the urinary tract. | [131] |
| US 2023/0321326 A1 | 11 April 2022 | Catheter with Inherent Antimicrobial Properties | Becton Dickinson Co. | The catheter is constructed using polyhydroxyalkanoates PHA, a biopolymer known for its antimicrobial properties. PHA materials, such as poly-4-hydroxybutyrate (P4HB) and copolymers of P4HB, are particularly useful for conferring antimicrobial characteristics to the catheter. | [132] |
| WO 2023/215683 A1 | 25 April 2023 | Hydrophilic Urinary Catheter Products with Microcapsules of Anti-Bacterial Agents | Hollister Inc. | The provided urinary catheter has a hydrophilic coating and incorporates anti-microbial microcapsules that release an antimicrobial agent upon irradiation. | [133] |
| Application Number | Application Date | Title | Owners | Note | Ref. |
|---|---|---|---|---|---|
| US 2019/0000971 A1 | 1 August 2016 | Peptides and Antibodies for The Removal of Biofilms | Research Institute at Nationwide Children’s Hospital | Isolated or recombinant polypeptides are used in vaccinating individuals with chronic or recurrent biofilm-related diseases. The polypeptides stimulate the immune system to generate antibodies that target bacteria within the biofilm, disrupting its construction and hindering its maintenance. The antibodies generated by the immune system can prevent or clear bacterial infections by interfering with biofilm formation and function. | [134] |
| WO 2017/066719 A2 | 14 October 2016 | Hu Specific Interfering Agents | Research Institute at Nationwide Children’s Hospital | The method involves the administration of interfering agents able to inhibit the binding of an HU protein to microbial DNA. HU proteins play a key role in binding DNA within the biofilm structure. The interfering agents compete with HU proteins for binding sites on microbial DNA, disrupting the stability of the biofilm. | [135] |
| WO 2015/181558 A1 | 28 May 2015 | Antimicrobial Preparations, Methods for Preparing the Same and Uses Thereof to Combat Microorganisms | Ipabc Ltd. | The patent involves the preparation of antimicrobial substances containing crystalline particles of either an antimicrobial peptide or an antimicrobial polyene. The application of this purpose is to combat microorganisms. The uses of these preparations include combating a wide range of microorganisms, showcasing their versatility in antimicrobial treatments. | [136] |
| US 2015/0290278 A1 | 15 April 2015 | Cationic Antimicrobial Peptides | The Hospital for Sick Children | The method combines a specific peptide and an antibiotic to treat infections synergistically. The peptide has a hydrophobic sequence (Z) with an average hydrophobicity value of at least 0.3 on the Liu-Deber scale, which contributes to the antimicrobial activity. | [137] |
| WO 2019/104213 A1 | 21 November 2018 | Antibiofilm Formulations and Use Thereof | University of Texas | An approach to treat biofilm infections is addressed, presenting a comprehensive array of compositions and methods specifically tailored to address this challenging medical issue. By incorporating modified antibiotics and a diverse range of excipients, the method aims to achieve a more effective response with respect to traditional antibiotic therapies. | [138] |
| US 11103547 B2 | 2 February 2017 | Methods for Disrupting Biofilms | Yissum Research Development Company of the Hebrew University of Jerusalem Ltd. | The invention involves the administration of a pharmaceutical composition to subjects in need of treatment, or for the prevention of biofilm-associated infections. The composition comprises a mixture of random-sequence peptides along with a pharmaceutically acceptable carrier. | [139] |
| US 2020/0138901 A1 | 13 January 2020 | Antimicrobial Peptides, their Variants and Uses | Chain Antimicrobials Oy | Introduction of novel AMPs with a broad spectrum of action in controlling microbial growth and infections. The versatility of the peptides extends to various industries, offering solutions for microbial control and preservation in diverse settings. | [140] |
| WO 2022/251963 A1 | 2 June 2022 | Polymeric Antifouling Coating with Antimicrobial Peptides | Uni of British Columbia | Compositions and methods for coating substrates with polymeric binders and AMPs, to limit biofouling and protein binding, offering solutions for reducing contamination in various applications and improving performance. | [141] |
| WO 2022/010942 A2 | 6 July 2021 | Combination Therapies for the Treatment and Prevention of Biofilms | Res Inst Nationwide Childrens Hospital | A novel approach to treat and prevent biofilms and associated disorders by combining HMGB (high-mobility group box) polypeptides with anti-DNABII antibodies. HMGB polypeptides are known for their ability to disrupt biofilms, while anti-DNABII antibodies specifically target DNABII proteins, which are crucial for biofilm stability. The specific amino acid sequences in the anti-DNABII antibody ensure effective targeting and binding to DNABII proteins, enhancing the therapeutic efficacy of the composition. | [142] |
| WO 2021/154703 A1 | 26 June 2021 | Compositions Including Antimicrobial Polymer-Peptide Conjugates and Uses Thereof | University of Puerto Rico | A peptide conjugate comprising a PEG arm conjugated to an AMP with a specific amino acid sequence. | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrado, B.; Cammarano, A.; Dello Iacono, S.; Renzi, E.; Moretta, R.; Mercurio, M.E.; Ascione, L.; Cummaro, A.; Meglio, C.; Nicolais, L. A Comprehensive Review of Progress in Preventing Urinary Infections Associated with the Use of Urinary Catheters: A Dual Analysis of Publications and Patents. Infect. Dis. Rep. 2025, 17, 64. https://doi.org/10.3390/idr17030064
Corrado B, Cammarano A, Dello Iacono S, Renzi E, Moretta R, Mercurio ME, Ascione L, Cummaro A, Meglio C, Nicolais L. A Comprehensive Review of Progress in Preventing Urinary Infections Associated with the Use of Urinary Catheters: A Dual Analysis of Publications and Patents. Infectious Disease Reports. 2025; 17(3):64. https://doi.org/10.3390/idr17030064
Chicago/Turabian StyleCorrado, Brunella, Aniello Cammarano, Stefania Dello Iacono, Emilia Renzi, Rosalba Moretta, Maria Emilia Mercurio, Laura Ascione, Annunziata Cummaro, Caterina Meglio, and Luigi Nicolais. 2025. "A Comprehensive Review of Progress in Preventing Urinary Infections Associated with the Use of Urinary Catheters: A Dual Analysis of Publications and Patents" Infectious Disease Reports 17, no. 3: 64. https://doi.org/10.3390/idr17030064
APA StyleCorrado, B., Cammarano, A., Dello Iacono, S., Renzi, E., Moretta, R., Mercurio, M. E., Ascione, L., Cummaro, A., Meglio, C., & Nicolais, L. (2025). A Comprehensive Review of Progress in Preventing Urinary Infections Associated with the Use of Urinary Catheters: A Dual Analysis of Publications and Patents. Infectious Disease Reports, 17(3), 64. https://doi.org/10.3390/idr17030064






