Spatial, Social and Serological Factors in the Prevalence and Risk of Leprosy in Areas of High Endemicity: An Integrative Review
Abstract
:1. Introduction
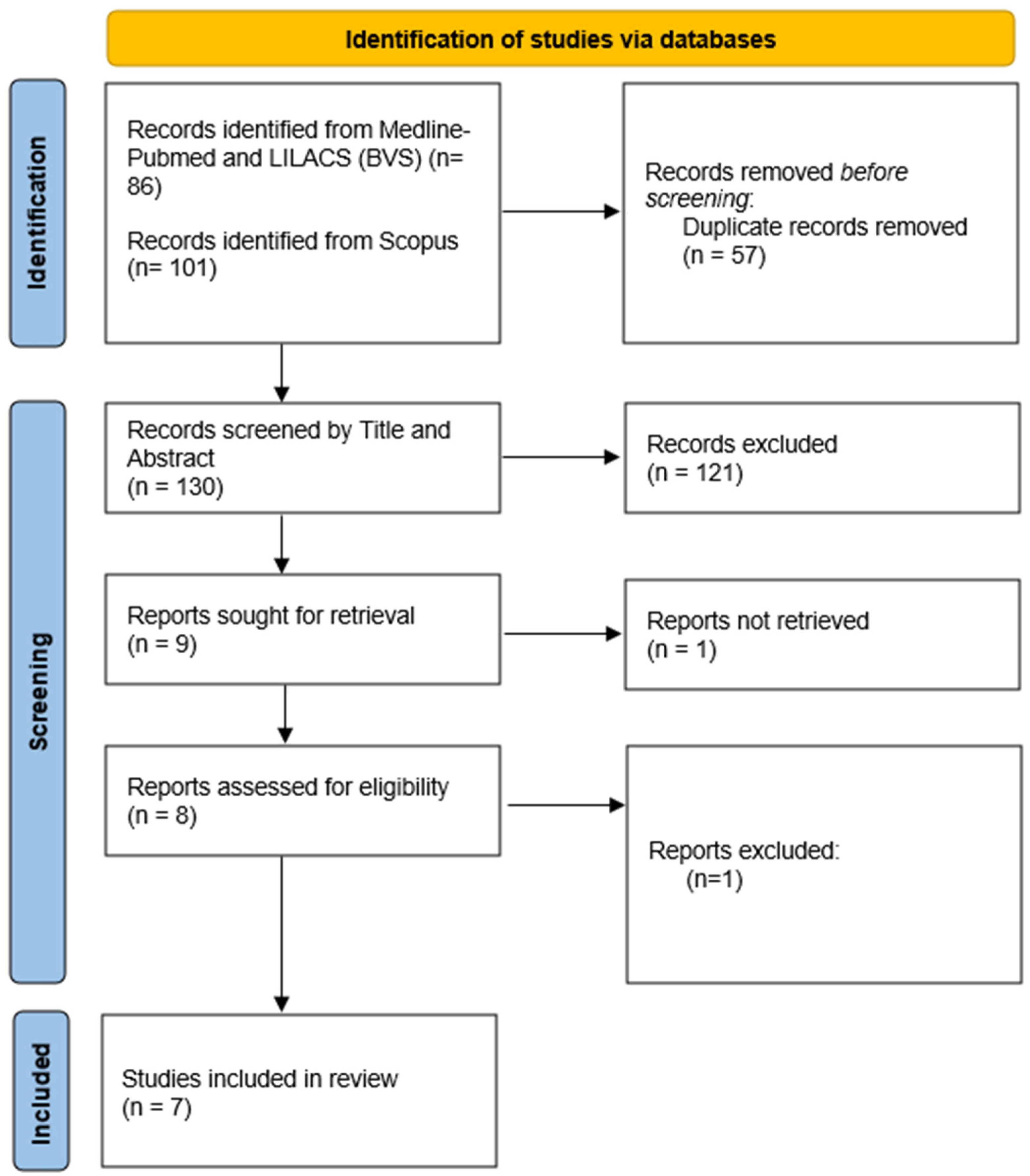
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| M. leprae | Mycobacterium leprae |
| GIS | Geographic Information Systems |
| QCRI | Qatar Computing Research Institute |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
References
- Ridley, D.S.; Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 1966, 34, 255–273. Available online: http://www.ncbi.nlm.nih.gov/pubmed/5950347 (accessed on 3 November 2024).
- Britton, W.J.; Lockwood, D.N.J. Leprosy. Lancet 2004, 363, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Hanseníase no Brasil: Caracterização das Incapacidades Físicas; Ministry of Health: Brasília, Brazil, 2020. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/hanseniase_brasil_caracterizacao_incapacidades_fisicas.pdf (accessed on 23 January 2025).
- Penna, G.O.; Wand-del-Rey de Oliveira, M.; Penna, M.L. Dermatological diseases of compulsory notification in Brazil. An. Bras. Dermatol. 2011, 86, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Froes, L.A.R.; Sotto, M.N.; Trindade, M.A.B. Leprosy: Clinical and immunopathological characteristics. An. Bras. Dermatol. 2022, 97, 338–347. [Google Scholar] [CrossRef]
- de Araujo, D.M.; Silva, E.C.d.S.e.; Gomes, H.V.d.S.; Carbogim, F.d.C.; Junior, G.F.X.; Coelho, A.d.C.O. Leprosy and impacts on the quality of life of people with physical disabilities: A scoping review. Rev. Bras. Enferm. 2024, 77 (Suppl. S3), e20230101. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Boletim Epidemioloógico de Hanseníase. 2023; Ministry of Health: Brasília, Brazil, 2023. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2023/boletim_hanseniase-2023_internet_completo.pdf (accessed on 13 January 2025).
- Niitsuma, E.N.A.; Bueno, I.d.C.; Arantes, E.O.; Carvalho, A.P.M.; Junior, G.F.X.; Fernandes, G.d.R.; Lana, F.C.F. Factors associated with leprosy in contacts: Systematic review and meta-analysis. Rev. Bras. Epidemiol. 2021, 24, e210039. [Google Scholar] [CrossRef]
- Rodrigues, R.N.; Barbosa, J.C.; Alencar, C.H. High-risk areas of leprosy in Brazil between 2001–2015. Rev. Bras. Enferm. 2020, 73, e20180583. [Google Scholar] [CrossRef]
- Silva, C.L.M.; Fonseca, S.C.; Kawa, H.; Palmer, D.d.O.Q. Spatial distribution of leprosy in Brazil: A literature review. Rev. Soc. Bras. Med. Trop. 2017, 50, 439–449. [Google Scholar] [CrossRef]
- Machado, L.M.G.; dos Santos, E.S.; Cavaliero, A.; Steinmann, P.; Ignotti, E. Spatio-temporal analysis of leprosy risks in a municipality in the state of Mato Grosso-Brazilian Amazon: Results from the leprosy post-exposure prophylaxis program in Brazil. Infect. Dis. Poverty 2022, 11, 21. [Google Scholar] [CrossRef]
- Barreto, J.G.; Bisanzio, D.; Guimarães, L.d.S.; Spencer, J.S.; Vazquez-Prokopec, G.M.; Kitron, U.; Salgado, C.G. Spatial analysis spotlighting early childhood leprosy transmission in a hyperendemic municipality of the Brazilian Amazon region. PLoS Negl. Trop. Dis. 2014, 8, e2665. [Google Scholar] [CrossRef]
- Spencer, J.S.; Duthie, M.S.; Geluk, A.; Balagon, M.F.; Kim, H.J.; Wheat, W.H.; Chatterjee, D.; Jackson, M.; Li, W.; Kurihara, J.N.; et al. Identification of serological biomarkers of infection, disease progression and treatment efficacy for leprosy. Mem. Inst. Oswaldo Cruz 2012, 107 (Suppl. S1), 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bührer-Sékula, S.; Smits, H.L.; Gussenhoven, G.C.; van Leeuwen, J.; Amador, S.; Fujiwara, T.; Klatser, P.R.; Oskam, L. Simple and fast lateral flow test for classification of leprosy patients and identification of contacts with high risk of developing leprosy. J. Clin. Microbiol. 2003, 41, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Luz, L.; Saavedra, D.P.; Fogaça, M.B.T.; Bührer-Sékula, S.; Stefani, M.M.d.A. Challenges and advances in serological and molecular tests to aid leprosy diagnosis. Exp. Biol. Med. 2023, 248, 2083–2094. [Google Scholar] [CrossRef]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, J.W.; Dias, G.H.; Nobre, M.L.; Dias, M.C.D.S.; Araújo, S.F.; Barbosa, J.D.; da Trindade-Neto, P.B.; Blackwell, J.M.; Jeronimo, S.M.B. Geographic information systems and applied spatial statistics are efficient tools to study Hansen’s disease (leprosy) and to determine areas of greater risk of disease. Am. J. Trop. Med. Hyg. 2010, 82, 306–314. [Google Scholar] [CrossRef]
- de Sousa, D.B.; Souza-Santos, R.; da Cunha, M.D.; Sobral, A. Hot spots of leprosy in the endemic area of São Luís, Maranhão State, Northeastern Brazil. J. Infect. Public Health 2020, 13, 228–234. [Google Scholar] [CrossRef]
- Lima, L.N.G.C.; Frota, C.C.; Mota, R.M.S.; Almeida, R.L.F.; Pontes, M.A.d.A.; Gonçalves, H.d.S.; Rodrigues, L.C.; Kendall, C.; Kerr, L. Widespread nasal carriage of Mycobacterium leprae among a healthy population in a hyperendemic region of northeastern Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 898–905. [Google Scholar] [CrossRef]
- Linhares, M.S.C.; Kerr, L.R.F.S.; Kendall, C.; de Almeida, R.L.F.; Klovdahl, A.; Frota, C.C. Spatial distribution pattern of new leprosy cases under 15 years of age and their contacts in Sobral, Ceará, Brazil. Cienc. Saude Coletiva 2022, 27, 1641–1652. [Google Scholar] [CrossRef]
- Nicchio, M.V.; Araujo, S.; Martins, L.C.; Pinheiro, A.V.; Pereira, D.C.; Borges, A.; Antunes, D.E.; Barreto, J.G.; Goulart, I.M.B. Spatial and temporal epidemiology of Mycobacterium leprae infection among leprosy patients and household contacts of an endemic region in Southeast Brazil. Acta Trop. 2016, 163, 38–45. [Google Scholar] [CrossRef]
- da Silva, M.B.; Li, W.; Bouth, R.C.; Gobbo, A.R.; Messias, A.C.C.; Moraes, T.M.P.; Jorge, E.V.O.; Barreto, J.G.; Filho, F.B.; Conde, G.A.B.; et al. Latent leprosy infection identified by dual RLEP and anti-PGL-I positivity: Implications for new control strategies. PLoS ONE 2021, 16, e0251631. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.d.C.; Barreto, J.G.; Bueno, I.d.C.; Costa, B.O.; Lana, F.C.F. Combined use of serological markers and spatial analysis in the epidemiological surveillance of leprosy. Rev. Panam. Salud Publica 2021, 45, 1. [Google Scholar] [CrossRef] [PubMed]
- Nobre, M.L.; Santos, L.F.d.P.; Dias, G.H.; da Silva, J.A.M.; Amorim, F.M.; da Cunha, D.C.S.; Jeronimo, S.M.B.; Aires, C.A.M. Anti-PGL-I serology as a strategy for Hansen’s disease control in an endemic area in Brazil. Lepr. Rev. 2025, 96, 2024108. [Google Scholar] [CrossRef]
| Virtual Health Library (BVS—Medline and LILACS) | (“Leprosy” OR “Leprosy” OR “Lepra” OR “Mycobacterium leprae”) AND (“Prevalence” OR “Prevalencia” OR “Infection rate”) AND (“Serology” OR “Serologia” OR “Serología” OR “Anti-PGL-1” OR “ML Flow”) AND (“Risk of becoming ill” OR “Riesgo de enfermedad” OR “Disease risk” OR “Incidência” OR “Incidence” OR “Incidencia” OR “Susceptibility” OR “Susceptibilidad”) AND (“Análise espacial” OR “Análisis espacial” OR “Spatial analysis” OR “Sistema de Información Geográfica” OR “Geographic Information System” OR “Cluster analysis” OR “Análisis de conglomerados” OR “Spatial autocorrelation” OR “Autocorrelación espacial” OR “Spatial patterns” OR “Patrones espaciales”) AND (“Determinantes sociais” OR “Determinantes sociales” OR “Social determinants” OR “Factores socioeconómicos” OR “Socioeconomic factors” OR “Pobreza” OR “Poverty” OR “Saneamiento” OR “Sanitation”) |
| Scopus | (“Hanseníase” OR “Leprosy” OR “Lepra” OR “Mycobacterium leprae”) AND (“Prevalência” OR “Prevalence” OR “Prevalencia” OR “Infection rate”) AND (“Serology” OR “Serologia” OR “Serología” OR “Anti-PGL-1” OR “ML Flow”) AND (“Risk of illness” OR “Riesgo de enfermedad” OR “Disease risk” OR “Incidência” OR “Incidence” OR “Incidencia” OR “Susceptibility” OR “Susceptibilidad”) AND (“Análise espacial” OR “Análisis espacial” OR “Spatial analysis” OR “Sistema de Información Geográfica” OR “Geographic Information System” OR “Cluster analysis” OR “Análisis de conglomerados” OR “Spatial autocorrelation” OR “Autocorrelación espacial” OR “Spatial patterns” OR “Patrones espaciales”) AND (“Determinantes sociais” OR “Determinantes sociales” OR “Social determinants” OR “Factores socioeconómicos” OR “Socioeconomic factors” OR “Pobreza” OR “Poverty” OR “Saneamiento” OR “Sanitation”) AND PUBYEAR > 2009 AND PUBYEAR < 2025 AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”) OR LIMIT-TO (LANGUAGE, “Portuguese”) OR LIMIT-TO (LANGUAGE, “Spanish”)) AND (LIMIT-TO (PUBSTAGE, “final”)) AND (LIMIT-TO (OA, “all”))) |
| Title | Authors | Year | Methodological Approach | Key Results | Contribution of the Study to Answering the Research Question |
|---|---|---|---|---|---|
| Geographic information systems and applied spatial statistics are efficient tools to study Hansen’s disease (leprosy) and to determine areas of greater risk of disease. | [18] | 2010 | Cluster analysis and GIS | It identified two high-risk areas with statistical significance, with a relationship between the distribution of leprosy and socioeconomic variables of poverty. The use of GIS and spatial analysis proved effective in mapping clusters of the disease, indicating areas for targeting control interventions. | It contributes by identifying high-risk areas and correlating them with socioeconomic factors, supporting specific interventions in the regions most affected by leprosy. |
| Hot spots of leprosy in the endemic area of São Luís, Maranhão state, northeastern Brazil | [19] | 2019 | Spatial analysis and socioeconomic indicators | It found positive correlations between cases and the number of residents, open sewers and low income, while sidewalks and schooling had a negative correlation. Seropositivity among contacts was 23.19%. It has a heterogeneous spatial pattern, with hyperendemic clusters. | The study advances the understanding of the social and spatial factors that influence the prevalence of leprosy, showing the importance of infrastructure and socioeconomic conditions in transmission. |
| Widespread nasal carriage of Mycobacterium leprae among a healthy population in a hyperendemic region of northeastern Brazil | [20] | 2015 | Cross-sectional study | The prevalence of RLEP positivity was high among cases (69.2%) and contacts (66.9%), with dissemination of the bacillus among the healthy population. Factors associated with leprosy included male gender, low income, previous contact and age. It defined areas of multibacillary case clusters. | It reinforces the importance of molecular and social factors, suggesting surveillance of contacts and endemic areas to control transmission in vulnerable communities. |
| Spatial distribution pattern of new leprosy cases under 15 years of age and their contacts in Sobral, Ceará, Brazil | [21] | 2021 | Spatial analysis and household contact | It detected leprosy cases in children under 15 in hyperendemic areas, with higher seropositivity rates among household contacts. It identified clusters of subclinical infection in low-income areas, suggesting that transmission is related to the proximity of seropositive individuals. | It highlights the role of social factors and physical proximity in transmission, contributing to interventions in vulnerable families and areas. |
| Spatial and temporal epidemiology of Mycobacterium leprae infection among leprosy patients and household contacts of an endemic region in Southeast Brazil | [22] | 2016 | Serology (anti-PGL-I and NDO-LID) and spatial analysis | It observed high seropositivity (42%) in contacts for the NDO-LID rapid test, with three groups of subclinical infection identified. The spatial and serological evaluation proposed priority areas for surveillance and prevention. | It contributes by linking serology and spatial analysis, proposing targeted surveillance in high-risk areas, strengthening leprosy control in endemic locations. |
| Latent leprosy infection identified by dual RLEP and anti-PGL-I positivity: Implications for new control strategies | [23] | 2021 | Serology (PGL-I) and molecular analysis (RLEP) in multiple groups | He found high seropositivity (46%) for contacts with active leprosy, with a higher risk of progression to clinical disease. He suggested chemoprophylaxis for double-positive household contacts to reduce transmission. | It reinforces the importance of identifying serologically and molecularly positive contacts to prevent the spread of leprosy in endemic areas. |
| Combined use of serological markers and spatial analysis in the epidemiological surveillance of leprosy | [24] | 2021 | Serology (anti-PGL-I) and territorial analysis | Seropositivity was higher among family members and neighbors of positive schoolchildren. It found a higher rate in low-income households and areas with few rooms. The territorial analysis indicated a possible hidden endemic and identified vulnerable sectors for active screening. | It integrates serological and spatial factors, suggesting screening activities for early diagnosis and control in areas with a high concentration of seropositivity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lages, D.d.S.; Maciel, I.C.L.; Vidal, S.L.; Lana, F.C.F. Spatial, Social and Serological Factors in the Prevalence and Risk of Leprosy in Areas of High Endemicity: An Integrative Review. Infect. Dis. Rep. 2025, 17, 57. https://doi.org/10.3390/idr17030057
Lages DdS, Maciel ICL, Vidal SL, Lana FCF. Spatial, Social and Serological Factors in the Prevalence and Risk of Leprosy in Areas of High Endemicity: An Integrative Review. Infectious Disease Reports. 2025; 17(3):57. https://doi.org/10.3390/idr17030057
Chicago/Turabian StyleLages, Daniele dos Santos, Isabela Cristina Lana Maciel, Sarah Lamas Vidal, and Francisco Carlos Félix Lana. 2025. "Spatial, Social and Serological Factors in the Prevalence and Risk of Leprosy in Areas of High Endemicity: An Integrative Review" Infectious Disease Reports 17, no. 3: 57. https://doi.org/10.3390/idr17030057
APA StyleLages, D. d. S., Maciel, I. C. L., Vidal, S. L., & Lana, F. C. F. (2025). Spatial, Social and Serological Factors in the Prevalence and Risk of Leprosy in Areas of High Endemicity: An Integrative Review. Infectious Disease Reports, 17(3), 57. https://doi.org/10.3390/idr17030057






