Long COVID: A Systematic Review of Preventive Strategies
Abstract
1. Introduction
2. The Pathogenic Mechanism of Long COVID
3. Risk Factors for Long COVID
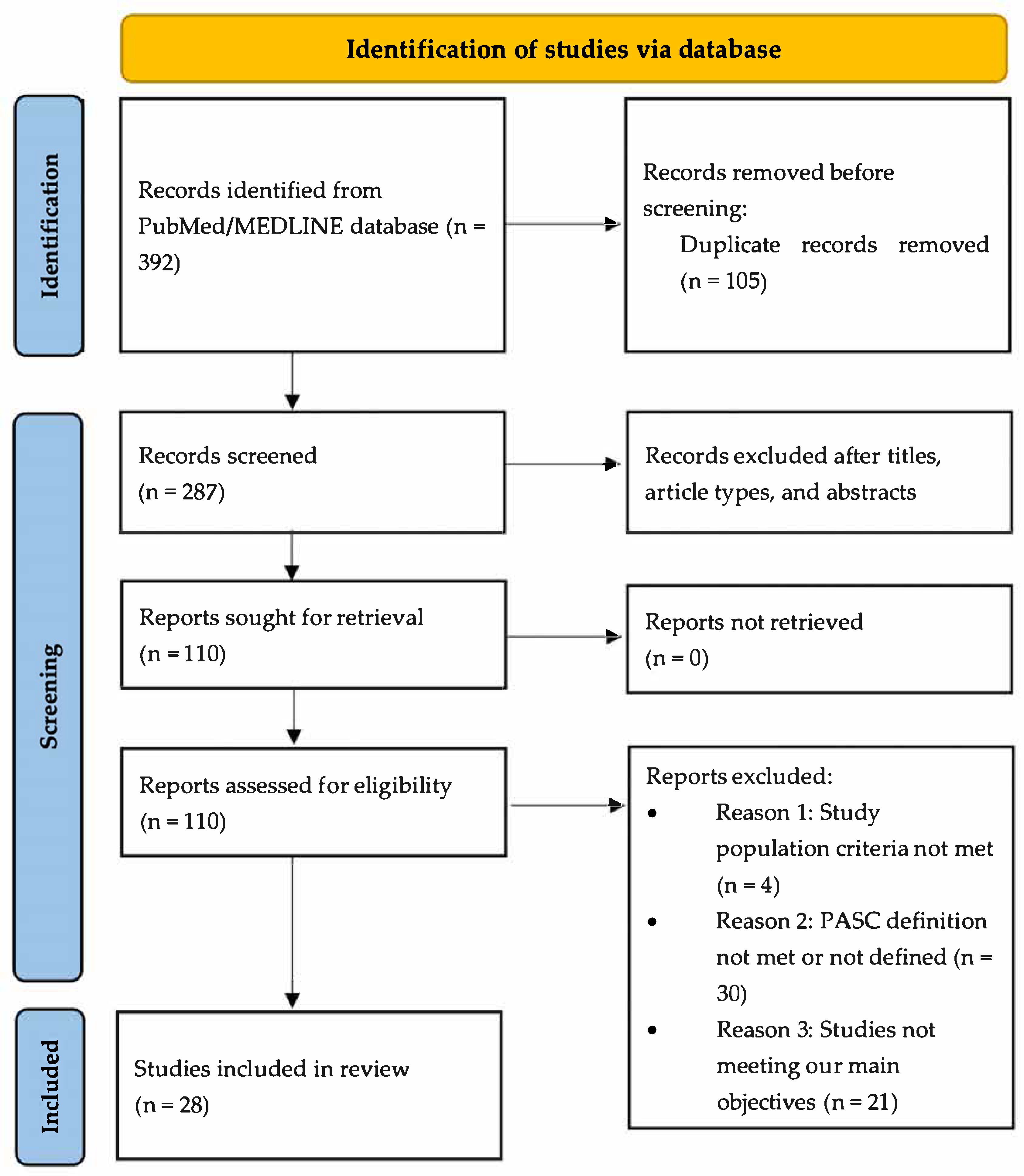
4. Materials and Methods
5. Results
5.1. Impact of COVID-19 Vaccine on Prevention of Long COVID
5.2. The Impact of Antivirals on the Prevention of Long COVID
5.3. The Impact of Other Treatments on the Prevention of Long COVID
6. Discussion
6.1. COVID-19 Vaccines Reduce the Risk of Long COVID
6.2. Equivocal Evidence for Protective Effect of Antivirals and Other Drugs
6.3. Effect of Nutrients and Lifestyle Factors on Long COVID
6.4. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Disease Control and Prevention. COVID-19 Timeline. Available online: https://www.cdc.gov/museum/timeline/covid19.html (accessed on 7 December 2024).
- Ely, E.W.; Brown, L.M.; Fineberg, H.V.; National Academies of Sciences Engineering; Medicine Committee on Examining the Working Definition for Long Covid. Long Covid Defined. N. Engl. J. Med. 2024, 391, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Committee on Examining the Working Definition for Long COVID (Washington District of Columbia). A Long Covid Definition: A Chronic, Systemic Disease State with Profound Consequences; National Academies Press: Washington, DC, USA, 2024. [Google Scholar]
- World Health Organization. Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 7 December 2024).
- Global Burden of Disease Long COVID Collaborators; Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss., O.; Bobkova, P.; Bonsel, G.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar]
- Carlile, O.; Briggs, A.; Henderson, A.D.; Butler-Cole, B.F.C.; Tazare, J.; Tomlinson, L.A.; Marks, M.; Jit, M.; Lin, L.Y.; Bates, C.; et al. Impact of long COVID on health-related quality-of-life: An OpenSAFELY population cohort study using patient-reported outcome measures (OpenPROMPT). Lancet Reg. Health Eur. 2024, 40, 100908. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Lin, K.; Ai, J.; Zhang, W. The efficacy of antivirals, corticosteroids, and monoclonal antibodies as acute COVID-19 treatments in reducing the incidence of long COVID: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.K.N.; Tsang, C.Y.W.; Chan, Y.H.; Telaga, S.A.; Ng, L.Y.A.; Chung, C.M.; Yip, Y.M.; Cheung, P.P. The effect of pre-COVID and post-COVID vaccination on long COVID: A systematic review and meta-analysis. J. Infect. 2024, 89, 106358. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Ma, J.; Zhang, Q.; Shao, J.; Liang, S.; Yu, Y.; Li, W.; Wang, C. The long-term health outcomes, pathophysiological mechanisms and multidisciplinary management of long COVID. Signal Transduct. Target. Ther. 2023, 8, 416. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Lipkin, W.I. ME/CFS and Long COVID share similar symptoms and biological abnormalities: Road map to the literature. Front. Med. 2023, 10, 1187163. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Postacute Sequelae of SARS-CoV-2 Infection in the Pre-Delta, Delta, and Omicron Eras. N. Engl. J. Med. 2024, 391, 515–525. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B.; Aleman, S.; Bach, K.; Boribong, B.P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat. Immunol. 2023, 24, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated with Post-Acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 76, e487–e490. [Google Scholar] [CrossRef]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deveau, T.M.; Munter, S.E.; Ryder, D.; Buck, A.; Beck-Engeser, G.; Chan, F.; Lu, S.; Goldberg, S.A.; Hoh, R.; et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J. Clin. Investig. 2023, 133, e163669. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brunink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Sarubbo, F.; El Haji, K.; Vidal-Balle, A.; Bargay Lleonart, J. Neurological consequences of COVID-19 and brain related pathogenic mechanisms: A new challenge for neuroscience. Brain Behav. Immun. Health 2022, 19, 100399. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rossler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 Is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 2022, 163, 495–506.e8. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Mendes de Almeida, V.; Engel, D.F.; Ricci, M.F.; Cruz, C.S.; Lopes, I.S.; Alves, D.A.; d’Auriol, M.; Magalhaes, J.; Machado, E.C.; Rocha, V.M.; et al. Gut microbiota from patients with COVID-19 cause alterations in mice that resemble post-COVID symptoms. Gut Microbes 2023, 15, 2249146. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Risk Factors Associated with Long COVID Syndrome: A Retrospective Study. Iran. J. Med. Sci. 2021, 46, 428–436. [Google Scholar]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mule, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef]
- Fernandez-de-Las-Penas, C.; Martin-Guerrero, J.D.; Pellicer-Valero, O.J.; Navarro-Pardo, E.; Gomez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalon, J.A.; Cigaran-Mendez, M.; Hernandez-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Gagliardi, M.C.; Tieri, P.; Ortona, E.; Ruggieri, A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.; Colombo, M.G.; Wetzel, A.J.; Martus, P.; Joos, S. Persisting Symptoms After COVID-19. Dtsch. Arztebl. Int. 2022, 119, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Esquivel, J.; Mendoza-Hernandez, M.A.; Guzman-Solorzano, H.P.; Sarmiento-Hernandez, K.A.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; Paz-Michel, B.A.; Murillo-Zamora, E.; Rojas-Larios, F.; Lugo-Trampe, A.; et al. Clinical Characteristics in the Acute Phase of COVID-19 That Predict Long COVID: Tachycardia, Myalgias, Severity, and Use of Antibiotics as Main Risk Factors, While Education and Blood Group B Are Protective. Healthcare 2023, 11, 197. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyere, F.; Stefic, K.; et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association Between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Quan, S.F.; Weaver, M.D.; Czeisler, M.E.; Barger, L.K.; Booker, L.A.; Howard, M.E.; Jackson, M.L.; Lane, R.I.; McDonald, C.F.; Ridgers, A.; et al. Association of Obstructive Sleep Apnea with Post-Acute Sequelae of SARS-CoV-2 Infection. Am. J. Med. 2024, 137, 529–537.e3. [Google Scholar] [CrossRef]
- Wang, S.; Quan, L.; Chavarro, J.E.; Slopen, N.; Kubzansky, L.D.; Koenen, K.C.; Kang, J.H.; Weisskopf, M.G.; Branch-Elliman, W.; Roberts, A.L. Associations of Depression, Anxiety, Worry, Perceived Stress, and Loneliness Prior to Infection with Risk of Post-COVID-19 Conditions. JAMA Psychiatry 2022, 79, 1081–1091. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Abu Hamdh, B.; Nazzal, Z. A prospective cohort study assessing the relationship between long-COVID symptom incidence in COVID-19 patients and COVID-19 vaccination. Sci. Rep. 2023, 13, 4896. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of long covid symptoms after COVID-19 vaccination: Community based cohort study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef]
- Babicki, M.; Kapusta, J.; Pieniawska-Śmiech, K.; Kałuzińska-Kołat, Ż.; Kołat, D.; Mastalerz-Migas, A.; Jankowski, P.; Chudzik, M. Do COVID-19 Vaccinations Affect the Most Common Post-COVID Symptoms? Initial Data from the STOP-COVID Register-12-Month Follow-Up. Viruses 2023, 15, 1370. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Degli Antoni, M.; Minisci, D.; Amadasi, S.; Castelli, F.; Odone, A.; Quiros-Roldan, E. The impact of early therapies for COVID-19 on death, hospitalization and persisting symptoms: A retrospective study. Infection 2023, 51, 1633–1644. [Google Scholar] [CrossRef]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.; Nicklas, J.; Puskarich, M.A.; Cohen, K.; Belani, H.; Anderson, B.; Huling, J.D.; Tignanelli, C.; et al. Outpatient treatment of COVID-19 with metformin, ivermectin, and fluvoxamine and the development of Long Covid over 10-month follow-up. medRxiv 2022. [Google Scholar] [CrossRef]
- Brunvoll, S.H.; Nygaard, A.B.; Fagerland, M.W.; Holland, P.; Ellingjord-Dale, M.; Dahl, J.A.; Soraas, A. Post-acute symptoms 3-15 months after COVID-19 among unvaccinated and vaccinated individuals with a breakthrough infection. Int. J. Infect. Dis. 2023, 126, 10–13. [Google Scholar] [CrossRef]
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.T.H.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: Staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef]
- Catalán, I.P.; Martí, C.R.; Sota, D.P.; Álvarez, A.C.; Gimeno, M.J.E.; Juana, S.F.; Rodríguez, G.H.; Bajo, E.D.; Gaya, N.T.; Blasco, J.U.; et al. Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission. J. Med. Virol. 2022, 94, 205–210. [Google Scholar] [CrossRef]
- Congdon, S.; Narrowe, Z.; Yone, N.; Gunn, J.; Deng, Y.; Nori, P.; Cowman, K.; Islam, M.; Rikin, S.; Starrels, J. Nirmatrelvir/ritonavir and risk of long COVID symptoms: A retrospective cohort study. Sci. Rep. 2023, 13, 19688. [Google Scholar] [CrossRef]
- Davelaar, J.; Jessurun, N.; Schaap, G.; Bode, C.; Vonkeman, H. The effect of corticosteroids, antibiotics, and anticoagulants on the development of post-COVID-19 syndrome in COVID-19 hospitalized patients 6 months after discharge: A retrospective follow up study. Clin. Exp. Med. 2023, 23, 4881–4888. [Google Scholar] [CrossRef] [PubMed]
- Durstenfeld, M.S.; Peluso, M.J.; Lin, F.; Peyser, N.D.; Isasi, C.; Carton, T.W.; Henrich, T.J.; Deeks, S.G.; Olgin, J.E.; Pletcher, M.J.; et al. Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study. J. Med. Virol. 2024, 96, e29333. [Google Scholar] [CrossRef]
- Fatima, S.; Ismail, M.; Ejaz, T.; Shah, Z.; Fatima, S.; Shahzaib, M.; Jafri, H.M. Association between long COVID and vaccination: A 12-month follow-up study in a low- to middle-income country. PLoS ONE 2023, 18, e0294780. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Las-Penas, C.; Franco-Moreno, A.; Ruiz-Ruigomez, M.; Arrieta-Ortubay, E.; Ryan-Murua, P.; Lumbreras-Bermejo, C.; Del-Valle-Loarte, P.; Pellicer-Valero, O.J.; Giordano, R.; Arendt-Nielsen, L.; et al. Is Antiviral Treatment with Remdesivir at the Acute Phase of SARS-CoV-2 Infection Effective for Decreasing the Risk of Long-Lasting Post-COVID Symptoms? Viruses 2024, 16, 947. [Google Scholar] [CrossRef]
- Gebo, K.A.; Heath, S.L.; Fukuta, Y.; Zhu, X.; Baksh, S.; Abraham, A.G.; Habtehyimer, F.; Shade, D.; Ruff, J.; Ram, M.; et al. Early antibody treatment, inflammation, and risk of post-COVID conditions. mBio 2023, 14, e0061823. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, J.; Tang, H.T.; Wong, H.K.; Lyu, A.; Cheung, C.H.; Bian, Z. Prevalence and risk factors of long COVID 6–12 months after infection with the Omicron variant among nonhospitalized patients in Hong Kong. J. Med. Virol. 2023, 95, e28862. [Google Scholar] [CrossRef]
- MacCallum-Bridges, C.; Hirschtick, J.L.; Patel, A.; Orellana, R.C.; Elliott, M.R.; Fleischer, N.L. The impact of COVID-19 vaccination prior to SARS-CoV-2 infection on prevalence of long COVID among a population-based probability sample of Michiganders, 2020–2022. Ann. Epidemiol. 2024, 92, 17–24. [Google Scholar] [CrossRef]
- Nehme, M.; Vetter, P.; Chappuis, F.; Kaiser, L.; Guessous, I.; CoviCare Study, T. Prevalence of Post-Coronavirus Disease Condition 12 Weeks After Omicron Infection Compared with Negative Controls and Association with Vaccination Status. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 76, 1567–1575. [Google Scholar] [CrossRef]
- Nevalainen, O.P.O.; Horstia, S.; Laakkonen, S.; Rutanen, J.; Mustonen, J.M.J.; Kalliala, I.E.J.; Ansakorpi, H.; Kreivi, H.R.; Kuutti, P.; Paajanen, J.; et al. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat. Commun. 2022, 13, 6152. [Google Scholar] [CrossRef]
- Trinh, N.T.; Jödicke, A.M.; Català, M.; Mercadé-Besora, N.; Hayati, S.; Lupattelli, A.; Prieto-Alhambra, D.; Nordeng, H.M. Effectiveness of COVID-19 vaccines to prevent long COVID: Data from Norway. Lancet Respir. Med. 2024, 12, e33–e34. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Xiao, W.; Shi, J.; Chen, W.; Jia, Q.; Zhou, Y.; Wang, R.; Chen, X.; Feng, L. Paxlovid reduces the risk of Long COVID in patients six months after hospital discharge. J. Med. Virol. 2023, 95, e29014. [Google Scholar] [CrossRef]
- Woldegiorgis, M.; Cadby, G.; Ngeh, S.; Korda, R.J.; Armstrong, P.K.; Maticevic, J.; Knight, P.; Jardine, A.; Bloomfield, L.E.; Effler, P.V. Long COVID in a highly vaccinated but largely unexposed Australian population following the 2022 SARS-CoV-2 Omicron wave: A cross-sectional survey. Med. J. Aust. 2024, 220, 323–330. [Google Scholar] [CrossRef]
- Xie, Z.; Stallings-Smith, S.; Patel, S.; Case, S.; Hong, Y.R. COVID-19 booster vaccine uptake and reduced risks for long-COVID: A cross-sectional study of a U.S. adult population. Vaccine 2024, 42, 3529–3535. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Li, Y.; Goldfeld, K.S.; Cobb, G.F.; Sturm-Reganato, C.L.; Ostrosky-Zeichner, L.; Jayaweera, D.T.; Philley, J.V.; Desruisseaux, M.S.; Keller, M.J.; et al. COVID-19 Convalescent Plasma Therapy: Long-Term Implications. Open Forum Infect. Dis. 2024, 11, ofad686. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Penfold, R.S.; Canas, L.D.S.; Sudre, C.; Rjoob, K.; Murray, B.; Molteni, E.; Kerfoot, E.; Cheetham, N.; Pujol, J.C.; et al. SARS-CoV-2 infection following booster vaccination: Illness and symptom profile in a prospective, observational community-based case-control study. J. Infect. 2023, 87, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.A.; Pollett, S.D.; Fries, A.C.; Berjohn, C.M.; Maves, R.C.; Lalani, T.; Smith, A.G.; Mody, R.M.; Ganesan, A.; Colombo, R.E.; et al. Persistent COVID-19 Symptoms at 6 Months After Onset and the Role of Vaccination Before or After SARS-CoV-2 Infection. JAMA Netw. Open 2023, 6, e2251360. [Google Scholar] [CrossRef]
- Nascimento, T.; do Valle Costa, L.; Ruiz, A.D.; Ledo, C.B.; Fernandes, V.P.L.; Cardoso, L.F.; Junior, J.M.V.; Saretta, R.; Kalil-Filho, R.; Drager, L.F. Vaccination status and long COVID symptoms in patients discharged from hospital. Sci. Rep. 2023, 13, 2481. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef]
- Brannock, M.D.; Chew, R.F.; Preiss, A.J.; Hadley, E.C.; Redfield, S.; McMurry, J.A.; Leese, P.J.; Girvin, A.T.; Crosskey, M.; Zhou, A.G.; et al. Long COVID risk and pre-COVID vaccination in an EHR-based cohort study from the RECOVER program. Nat. Commun. 2023, 14, 2914. [Google Scholar] [CrossRef]
- Di Fusco, M.; Sun, X.; Moran, M.M.; Coetzer, H.; Zamparo, J.M.; Alvarez, M.B.; Puzniak, L.; Tabak, Y.P.; Cappelleri, J.C. Impact of COVID-19 and effects of booster vaccination with BNT162b2 on six-month long COVID symptoms, quality of life, work productivity and activity impairment during Omicron. J. Patient Rep. Outcomes 2023, 7, 77. [Google Scholar] [CrossRef]
- Lundberg-Morris, L.; Leach, S.; Xu, Y.; Martikainen, J.; Santosa, A.; Gisslen, M.; Li, H.; Nyberg, F.; Bygdell, M. COVID-19 vaccine effectiveness against post-COVID-19 condition among 589,722 individuals in Sweden: Population based cohort study. BMJ 2023, 383, e076990. [Google Scholar] [CrossRef]
- Malden, D.E.; Liu, I.A.; Qian, L.; Sy, L.S.; Lewin, B.J.; Asamura, D.T.; Ryan, D.S.; Bezi, C.; Williams, J.T.B.; Kaiser, R.; et al. Post-COVID conditions following COVID-19 vaccination: A retrospective matched cohort study of patients with SARS-CoV-2 infection. Nat. Commun. 2024, 15, 4101. [Google Scholar] [CrossRef] [PubMed]
- Sigler, R.; Covarrubias, K.; Chen, B.; Rubarth, R.B.; Torosian, K.; Sanchez, C.R.; Bharti, A.; DeGruttola, V.; Aslam, S. Post-acute sequelae of COVID-19 in solid organ transplant recipients. Transpl. Infect. Dis. 2023, 25, e14167. [Google Scholar] [CrossRef]
- Tannous, J.; Pan, A.P.; Potter, T.; Bako, A.T.; Dlouhy, K.; Drews, A.; Sostman, H.D.; Vahidy, F.S. Real-world effectiveness of COVID-19 vaccines and anti-SARS-CoV-2 monoclonal antibodies against postacute sequelae of SARS-CoV-2: Analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open 2023, 13, e067611. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Harrison, P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 2022, 103, 154–162. [Google Scholar] [CrossRef]
- Boglione, L.; Meli, G.; Poletti, F.; Rostagno, R.; Moglia, R.; Cantone, M.; Esposito, M.; Scianguetta, C.; Domenicale, B.; Di Pasquale, F.; et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: Does remdesivir have a protective effect? QJM Mon. J. Assoc. Physicians 2022, 114, 865–871. [Google Scholar] [CrossRef]
- Fung, K.W.; Baye, F.; Baik, S.H.; McDonald, C.J. Nirmatrelvir and Molnupiravir and Post-COVID-19 Condition in Older Patients. JAMA Intern. Med. 2023, 183, 1404–1406. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Berry, K.; Rajeevan, N.; Li, Y.; Mutalik, P.; Yan, L.; Bui, D.; Cunningham, F.; Hynes, D.M.; Rowneki, M.; et al. Effectiveness of Nirmatrelvir-Ritonavir Against the Development of Post-COVID-19 Conditions Among U.S. Veterans: A Target Trial Emulation. Ann. Intern. Med. 2023, 176, 1486–1497. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern. Med. 2023, 183, 554–564. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of COVID-19: Cohort study. BMJ 2023, 381, e074572. [Google Scholar] [CrossRef]
- Tomasa-Irriguible, T.M.; Monfà, R.; Miranda-Jiménez, C.; Morros, R.; Robert, N.; Bordejé-Laguna, L.; Vidal, S.; Torán-Monserrat, P.; Barriocanal, A.M. Preventive Intake of a Multiple Micronutrient Supplement during Mild, Acute SARS-CoV-2 Infection to Reduce the Post-Acute COVID-19 Condition: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Nutrients 2024, 16, 1631. [Google Scholar] [CrossRef]
- Krishna, B.; Wills, M.; Sithole, N. Long COVID: What is known and what gaps need to be addressed. Br. Med. Bull. 2023, 147, 6–19. [Google Scholar] [CrossRef]
- Lin, D.Y.; Gu, Y.; Xu, Y.; Wheeler, B.; Young, H.; Sunny, S.K.; Moore, Z.; Zeng, D. Association of Primary and Booster Vaccination and Prior Infection With SARS-CoV-2 Infection and Severe COVID-19 Outcomes. JAMA 2022, 328, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. First Oral Antiviral for Treatment of COVID-19 in Adults. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults. (accessed on 7 December 2024).
- Wang, Y.; Su, B.; Alcalde-Herraiz, M.; Barclay, N.L.; Tian, Y.; Li, C.; Wareham, N.J.; Paredes, R.; Xie, J.; Prieto-Alhambra, D. Modifiable lifestyle factors and the risk of post-COVID-19 multisystem sequelae, hospitalization, and death. Nat. Commun. 2024, 15, 6363. [Google Scholar] [CrossRef] [PubMed]
| Study Author | Study Design/Country (Data) | Study Period/Participants (SARS-CoV-2 Variants) | LC Cases | Patient n (F %) | Age (Years), Mean ± SD or Median (IQR) | Vaccinated (%); Vaccine Type and Vaccination Time | Vaccine Impact on LC |
|---|---|---|---|---|---|---|---|
| Ayoubkhani et al. [44] | Prospective cohort/UK | Community based; visit during period of Feb–Sep 2021; COVID-19 at least 12 wks before final visit/(A, Δ) | LC 3–10 mo after COVID-19 | COVID-19, n = 28,356 (F 55.6%) | 45.9 ± 13.6 | Vaccinated (100%): BNT162b2, mRNA-1273, or ChAdOx1 after COVID-19 | Protective, 1st vaccine 12.8% reduction in odds (p < 0.001); 2nd vaccine provided additional 8.8% reduction (p = 0.003) |
| Antonelli et al. [66] | Prospective case–control study/UK | Community based; June–Nov 2021 (Δ) and Dec 2021–Apr 2022 (O)/cases (third dose recipient), controls (second dose recipient) | LC Sx ≥ 12 wks | All COVID-19, Delta: n = 1910 in each group (F 57%); Omicron: n = 7894 in each group (F 60.7%) | Delta: cases 64 ± 12.8, controls 63.7 ± 12.9; Omicron: cases 45.5 ± 16.3, controls 44.3 ± 17.7 | Vaccinated (100%): 3 doses of monovalent in cases, 2 doses in controls) before COVID-19 | No differences between cases and controls in both Δ and O eras, but trend towards protection in vaccinated individuals; when LC, Sx ≥ 4 wks, cases with aOR 0.56 (95% CI: 044–0.70) during Δ era |
| Richard et al. [67] | Prospective cohort/USA | Feb 2020-Dec 2021 (wild-type, A, Δ), data from MHS EPICC study | LC Sx ≥ 3 mo | COVID-19, n = 1832 (F 39%) | 40.5 ± 13.7 (age range 18–44) | Fully vaccinated (22.9%) [2 doses of BNT162b2 or mRNA-1273 or 1 dose of Ad26.COV2.S] before COVID-19 | Trend towards protective effect, RR 0.73 (95%CI: 0.47–1.14) ** (when LC Sx ≥ 28 d, RR 0.72 (0.54–0.96) |
| Nehme et al. [59] | Prospective longitudinal cohort/Switzerland | Outpatients, COVID-19 during period of Dec 2021–Feb 2022 (O) | LC 3 mo after COVID-19 | COVID-19, n = 1807 (F 62.3%); COVID-19 negative, n = 882 (F 63.9%) | COVID-19 positive, 41.6 ± 13.5; COVID-19 negative, 43.7 ± 14.9 | Fully vaccinated (75.5%) [at least 2 doses of mRNA-1273 (61.2%) or BNT162b2 (36.1%)] before COVID-19 | Protective, adjusted prevalence 9.7% vs. 18.1% (p < 0.001) |
| Brunvoll et al. [48] | Prospective cohort (Norweigian COVID-19 cohort)/Norway | COVID-19 during period of Nov 2020–Oct 2021 (wild-type, A, Δ) | LC 3 mo-15 mo after COVID-19 | COVID-19, n = 1420 (F 71%) | Vaccinated, 48.3 ± 11.4; unvaccinated, 45.7 ± 12.3 | Fully vaccinated (25%) [at least 2 doses of mRNA vaccines at least 2 wks before COVID-19] | No differences in all components except for memory problem, fully vaccinated vs. unvaccinated, 11.9% vs. 17.3% (p = 0.02) |
| Abu Hamdh et al. [43] | Prospective cohort/Palestine | COVID-19 during period of Sep 2021– Jan 2022, with FU phone interviews on d 10, 30, 60, 90/(mainly Δ) | LC at 90 d | COVID-19, n = 669 (F 57%) | 35.9 ± 11.5 | Vaccinated (41%) [BNT162b2 (17.8%), Sputnik Light (12.7%), mRNA-1273 (3.7%), Sputnik V (2.4%), ChAdOx1 (2.4%)] before COVID-19 | Protective, ≥1 dose vaccinated vs. unvaccinated, aOR 0.15 (95% CI: 0.09–0.24) |
| Fatima et al. [54] | Prospective cohort/Pakistan | Hospitalized patients with COVID-19 during period of Feb 2021–June 2021/(A) | LC at 12 mo | COVID-19 patients admitted to Aga Khan University hospital, n = 481 (F 38.3%) | 56.9 ± 14.3 | # Fully vaccinated (19%): 2 does of vaccines; partially vaccinated (19.2%): 1 dose before COVID-19 | Protective, fully vaccinated aOR 0.38 (95% CI: 0.20–0.70), partially vaccinated aOR 0.44 (95% CI: 0.24–0.80) |
| Nascimento et al. [68] | Prospective cohort/Brazil | Hospitalized patients with COVID-19 during May 2021 and Feb 2022 (A, Δ, O) | LC at 90 d | COVID-19 patients, n = 412 (35.4%) | 60 (IQR 48–72) | Fully vaccinated (44.9%) [1 dose of Janssen or 2 doses of # other vaccines before COVID-19] | Protective, aOR for fully vaccinated 0.55 (p= 0.007) |
| Català et al. [49] | Staggered retrospective cohort /UK (CPRD); Spain (SIDIAP); Estonia (CORIVA) | Primary care data; registered by Jan or Feb 2021, with FU until Jan 2022 (UK), June 2022 (Spain), Dec 2022 (Estonia)/(A, Δ, O) | LC between 90 d and 365 d after COVID-19 | Over 10 million vaccinated vs. over 10 million unvaccinated (n/a) | n/a | Vaccinated with 1 dose (BNT162b2, ChAdOx1, mRNA-1273, or Ad26.COV2.S) +GOLD (49.7%); * AURUM (49.4); SIDIAP (51.5%); CORIVA (19.4%) before COVID-19 | Protective: +GOLD, sHR 0.54 (95% CI: 0.44–0.67); * AURUM, 0.48 (0.34–0.68); SIDIAP, 0.71 (0.55–0.91); CORIVA, 0.59 (0.40–0.87) |
| Trinh et al. [61] | Staggered retrospective cohort/Norway (Norwegian linked health registries) | Primary care data; vaccination roll out from Jan to Aug 2021, FU up to 1 yr/(A, Δ, O) | LC between 90 d and 365 d after COVID-19 | Over 2.3 million vaccinated vs. over 1.5 million unvaccinated (n/a) | n/a | Vaccinated with at least 1 dose (60.7%) [BNT162b2, mRNA-1273, or ChAdOx1] at least 14 d before COVID-19 | Protective, sHR 0.64 (95% CI: 0.55–0.74) |
| Luo et al. [57] | Retrospective cohort/Hong Kong | Outpatient setting; COVID-19 during period of Dec 2021–May 2022/(mainly O) | LC at 6–12 mo after COVID-19 | COVID-19, n = 6242 (F 66.9%) | 47 (IQR 36–60) | Boosted (57.5%; 3 or more BNT162b2 or CoronaVac) vs. less than 3 doses before COVID-19 | Not protective, aOR 1.11 (95% CI: 0.99–1.24) |
| MacCallum-Bridges et al. [58] | Population-based retrospective cohort/USA | Outpatient setting; COVID-19 during period of March 2020–May 2022/(wild-type, A, Δ, O) | LC at 90 d after COVID-19 | COVID-19, n = 4695 (F 54.0%) | age 18–29 (25.7%); age 30–49 (37.6%); age 50–64 (24.2%); 65+ (13.5%) | Fully vaccinated (27.9%) [2 doses of BNT162b2, mRNA-1273, ChAdOx1, or Sinovac or 1 dose of Ad26.COV2.S] before COVID-19 | Protective, aPR 0.42 (95% CI: 0.34–0.53) |
| Babicki et al. [45] | Retrospective cohort/Poland (STOP-COVID registry) | Unspecified study period, FU visits at 3 mo and 12 mo after COVID-19/(n/a) | LC at 1 yr after COVID-19 | COVID-19, n = 801 (F 65.4%) | 53.5 ± 12.8 | Fully vaccinated (83%) [2 doses of BNT162b2, mRNA-1273, or ChAdOx1 or 1 dose of Ad26.COV2.S], 73.6% vaccinated after COVID-19, 9.4% before COVID-19 | No differences except headache (17.4% vs. 29.4%, p = 0.001), arthralgia (5.4% vs. 10.3%, p = 0.032), and dysregulation of HTN (11.6% vs. 18.4%, p = 0.030) were more common in unvaccinated |
| Woldegiorgis et al. [63] | Cross-sectional survey/Australia | COVID-19 during period of July–Aug 2022, FU in 90 d/(O) | LC at 90 d after COVID-19 | COVID-19, n = 11,697 (F 52.0%) | age 18–29 (20.9%); age 30–39 (21.0%); age 40–49 (18.7%); 50–59 (18.0%); 60–69 (11.6%); 70+ (9.7%) | #Vaccinated, 0–2 (6%); 3 doses (76.3%); 4 doses (17.7%) before COVID-19 | Protective, 3 doses aRR 1.3 (95% CI: 1.1–1.5); 0–2 doses aRR 1.4 (1.2–1.8) compared to 4 or more vaccine doses |
| Xie et al. [64] | Cross-sectional survey/USA | Outpatient setting; 2022 National Health Interview Survey/(O) | LC 3 mo or longer post-infection | COVID-19, n = 8757 (weighted 87,509,670) (F 53.3%) | age 18–29 (23.8%); age 30–39 (21.3%); age 40–49 (18.2%); age 50–64 (23.2%); 65+ (13.5%) | # Vaccinated, 1 dose (17.3%); initial series (33.3%); booster (27.2%) before COVID-19 | Protective, booster vs. unvaccinated, aOR 0.75 (95% CI: 0.61–0.93) |
| Study Author | Study Design/Country | Study Period/Participants/(SARS-CoV-2 Variants) | LC Cases | Patient n (F %) | Age, Mean ± SD or Median (IQR) | Antivirals (Treated %) | Antiviral Impact on LC |
|---|---|---|---|---|---|---|---|
| Nevalainen et al. [60] | Randomized trial/Finland | Hospitalized patients with COVID-19 during period of July 2020–Jan 2021/(wild type, A) | LC 1 yr after COVID-19 | Treated, 114 (F 35.1%); untreated, 94 (F 36.2%). | Treated, 57.2 ± 13.5; untreated, 59.7 ± 13.2 | Remdesivir (200 mg on 1st day, then 100 mg daily for maximum of 10 days) | No differences between remdesivir-treated and untreated with wide CI |
| Boglione et al. [78] | Prospective cohort/Italy | Hospitalized patients with COVID-19 during period of March 2020–Jan 2021(wild type, A) | LC symptoms ≥ 12 wks | Total of 449 (F 22%); Remdesvir-treated, 163; untreated, 165 | 65 (IQR 56–75.5) | Remdesivir | Protective, OR: 0.64 (95% CI: 0.41–0.78) |
| Fernández-de-las-Peñas et al. [55] | Retrospective, case–control study/Madrid, Spain | Hospitalized patients with COVID-19 during period of Sep 2020–March 2021/(wild type, A) | LC 3 mo or later following COVID-19 | Treated, 216 (F 43.5%); untreated, 216 (F 43.5%) | Treated, 55.4 ± 12.6; untreated, 55.6 ± 12.7 | Remdesivir (200 mg on 1st day, then 100 mg daily for maximum of 10 days) | Protective, OR: 0.401 (95 CI: 0.26–0.63) |
| Durstenfeld et al. [53] | Prospective cohort/USA | Vaccinated outpatients with their first SARS-CoV-2 positive result between March and Aug 2022/(O) | LC 90 d or later following COVID-19 | Treated, 353 (F 53.3%); untreated, 1258 (F 64.9%) | Treated, 62.1 ± 12.7; untreated, 55.1 ± 13.6 | Nirmatrelvir/ritonavir | No association, aOR: 1.15 (95% CI: 0.80–1.64) |
| Wang et al. [62] | Prospective cohort/Shanghai, China | Admitted with COVID-19 and then discharged between April and June 2022/(O) | LC 6 mo after being discharged | COVID-19, 634 (F 54.4%) | 74.1 ± 11.4 | Nirmatrelvir/ritonavir | Protective, OR: 0.35 (95 CI: 0.21–0.60) |
| Congdon et al. [51] | Retrospective cohort/New York, USA | Phone interviews between May and Nov 2022; COVID-19 four mo before the phone interview/(O) | LC 4 mo after COVID-19 | Treated, 250 (F 66.4%); untreated, 250 (F 73.6%); hospitalized (1%) | 50.6 | Nirmatrelvir/ritonavir | No reduction in overall risk of LC (incidence of 44% vs. 50%, p = 0.21; aOR: 0.83, 95% CI: 0.57–1.2). |
| Bertuccio et al. [46] | Retrospective cohort/Italy | Outpatients with mild to moderate COVID-19 during period of April 2021–March 2022/(A, 3.5%; Δ, 2.2%; O 94.3%) | LC 3 mo after COVID-19 | COVID-19, 649 (F 48.4%) | 67 (IQR 54–76) | 77 with antivirals (molnupiravir, 41.6%; nirmatrelvir/ritonavir, 13.0%; remdesivir, 45.5%); 141 with mAbs (bamlanivimab/ etesevimab, 44.7%; casirivimab, 16.3%; sotrovimab, 39.0%) | Antiviral protective, aOR of 0.43 (95% CI: 0.21–0.87) for any symptoms; mAbs protective, aOR of 0.48 (0.25–0.92) for neuro-behavioral symptoms |
| Study Author | Study Design/Country (Data) | Study Period/Participants/ (SARS-CoV-2 Variants) | LC Cases | Patient n (F %) | Age (Years), Mean ± SD or Median (IQR) | Treatment (Treated %) | Treatment Impact on LC |
|---|---|---|---|---|---|---|---|
| Gebo et al. [56] | Randomized clinical trial/USA | Outpatients with COVID-19 between June 2020 and Oct 2021/(wild type, A, Δ) | LC 90 d after CCP | 882 (F 57.4%) | 43 ± n/a | CCP | No association, aOR: 0.75 (95% CI: 0.46–1.23) |
| Yoon et al. [65] | Secondary analysis of randomized clinical trial/USA (CONTAIN-RCT) | Hospitalized with COVID-19 between April 2020 and March 2021/(wild type, A) | LC 18 mo post-randomization | 281 (F 44.5%) | 59 (IQR 50–67) | CCP | No association, aOR: 0.95 (95% CI: 0.54–1.67) |
| Bramante et al. [47] | Randomized clinical trial/USA | Enrolled from Dec 2020 to Jan 2022/(wild type, A, Δ, O) | LC 10 mo after randomization | 1125 (F 56%) | 45 (IQR 37–54) | # Metformin, ivermectin, fluvoxamine | Only metformin was protective; HR: 0.58 (95% CI: 0.38–0.88); ivermectin, HR: 0.99 (0.59–1.64); fluvoxamine, HR: 1.36 (0.79–2.39) |
| Davelaar et al. [52] | Retrospective cohort/Netherlands | Hospitalized with COVID-19 between March 2020 and Sep 2021/(wild type, A, Δ) | LC 6 mo after being discharged | 123 (F 38.2%) | 62.1 ± 9.5 | Corticosteroids | Protective, aOR: 0.32 (95% CI: 0.11–0.90) |
| Catalán et al. [50] | Retrospective cohort/Spain | Telephone survey between March 2021 and April 2021 for patients hospitalized with COVID-19 one yr earlier/(wild type) | LC 1 yr after being discharged | 76 (F 38%) | Treated, 68.5 (IQR 60.2–75.5); untreated, 61.5 (IQR 52.7–72.5) | Corticosteroids | Protective: headache (6.3% vs. 25%, p = 0.032); dysphagia (11.4% vs. 0%, p = 0.049); depression (22.7% vs. 3.1%, p = 0.016), chest pain (11.4% vs. 0%, p= 0.049); bodily pain (* SF-36: 100 vs. 75, p = 0.017), mental health (* SF-36: 86 vs. 76, p = 0.027) |
| Tomasa-Irriguible et al. [83] | Randomized clinical trial/Catalonia, Spain | Outpatients with COVID-19 between Sep 2021 and Feb 2023/(Δ, O) | LC after 6 mo | 246 (F 68.3%) | 46.8 ± 16.3 | Multiple micronutrient supplement | No reduction in incidence of LC (intervention, 27.7% vs. placebo, 25%; p = 0.785) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.O.; Nanda, N. Long COVID: A Systematic Review of Preventive Strategies. Infect. Dis. Rep. 2025, 17, 56. https://doi.org/10.3390/idr17030056
Park SO, Nanda N. Long COVID: A Systematic Review of Preventive Strategies. Infectious Disease Reports. 2025; 17(3):56. https://doi.org/10.3390/idr17030056
Chicago/Turabian StylePark, Sun O., and Neha Nanda. 2025. "Long COVID: A Systematic Review of Preventive Strategies" Infectious Disease Reports 17, no. 3: 56. https://doi.org/10.3390/idr17030056
APA StylePark, S. O., & Nanda, N. (2025). Long COVID: A Systematic Review of Preventive Strategies. Infectious Disease Reports, 17(3), 56. https://doi.org/10.3390/idr17030056






