The PJI-TNM Classification as Predictor for Revision-Free Implant Survival Rates in Patients with Periprosthetic Joint Infection of the Hip or Knee Joint
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Demographics and Infection Characteristics
3.2. Classification According to the PJI-TNM Classification
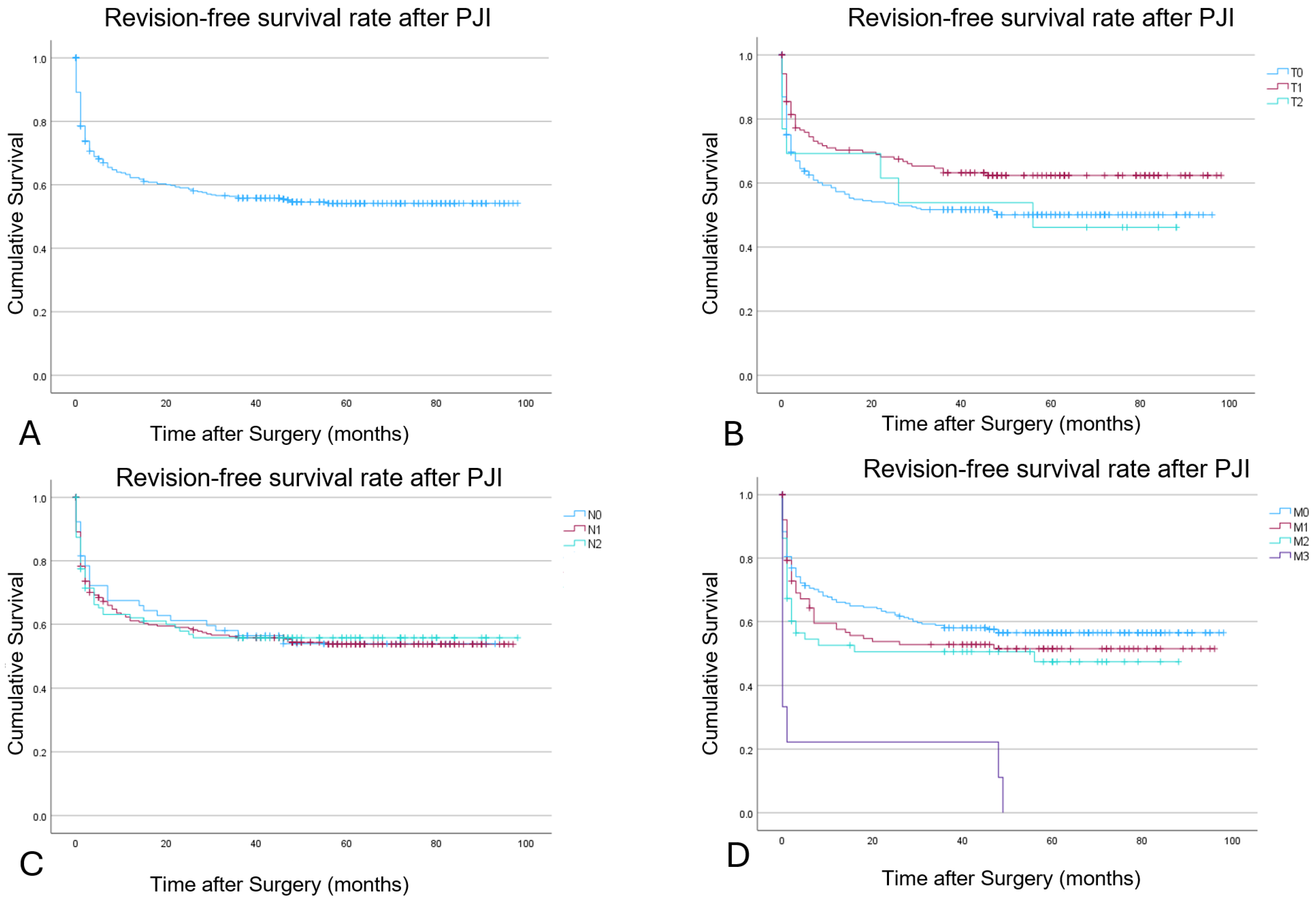
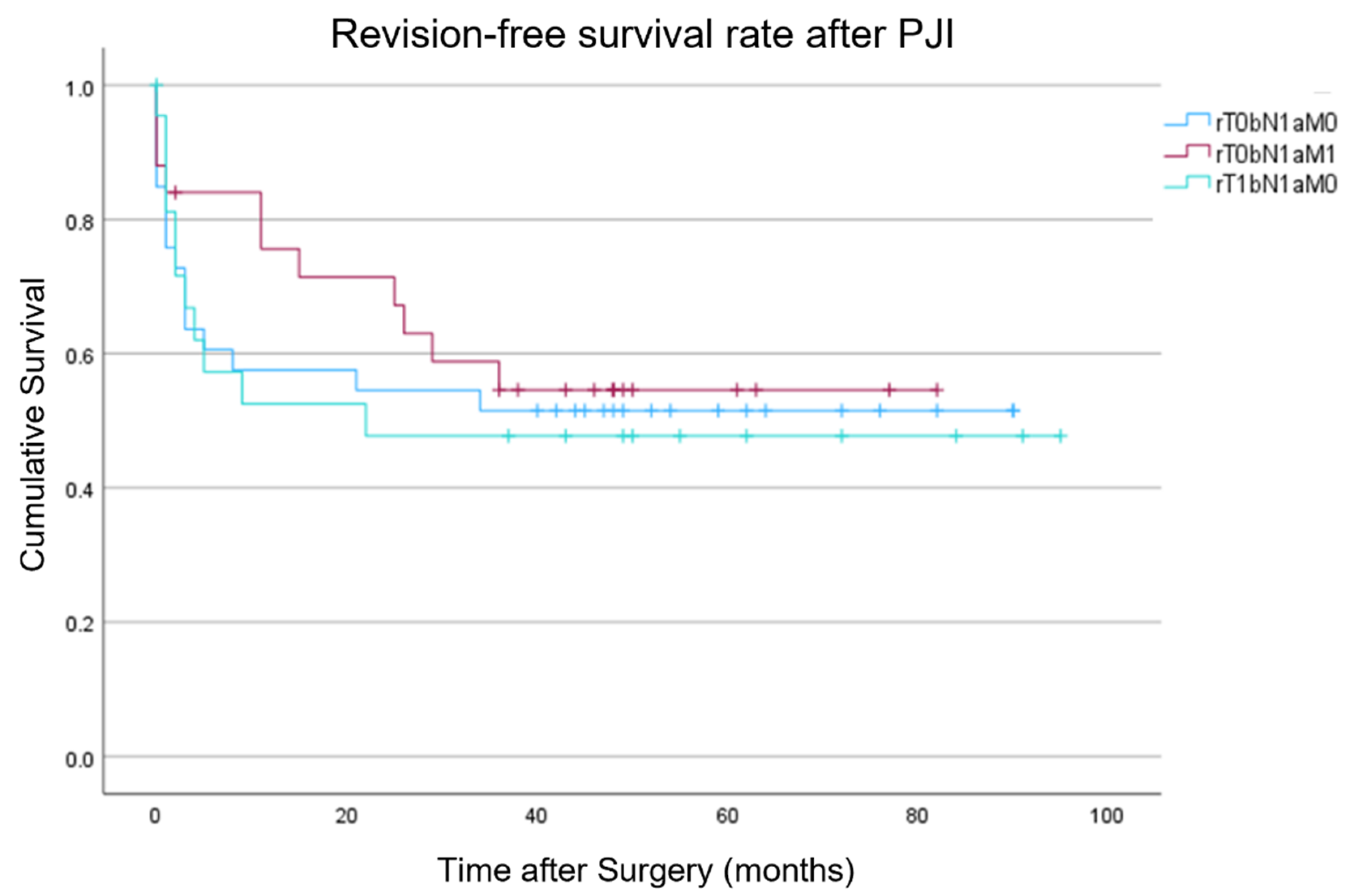
3.3. Outcome Evaluation
3.4. Microbiological and Histopathological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Culture Data | Total by Species | |
|---|---|---|
| Staphylococcus aureus | 66 | 19.94% |
| Staphylococcus epidermidis | 86 | 25.98% |
| Staphylococcus haemolyticus | 6 | 1.81% |
| Staphylococcus hominis | 8 | 2.42% |
| Staphylococcus lugdunensis | 14 | 4.23% |
| Staphylococcus mitis | 1 | 0.30% |
| Staphylococcus pasteuri | 1 | 0.30% |
| Staphylococcus saprophyticus | 1 | 0.30% |
| Staphylococcus warnerii | 4 | 1.21% |
| Streptococcus agalactiae | 12 | 3.63% |
| Streptococcus anginosus | 4 | 1.21% |
| Streptococcus dysgalactiae | 8 | 2.42% |
| Streptococcus gordonii | 1 | 0.30% |
| Streptococcus intermedius | 1 | 0.30% |
| Streptococcus mitis | 8 | 2.42% |
| Streptococcus oralis | 1 | 0.30% |
| Streptococcus parasanguinis | 2 | 0.60% |
| Streptococcus pneumoniae | 1 | 0.30% |
| Streptococcus pyogenes | 3 | 0.91% |
| Streptococcus salivarius | 2 | 0.60% |
| Streptococcus Salivarius | 4 | 1.21% |
| Bacillus licheniformis | 1 | 0.30% |
| Bacillus simplex | 1 | 0.30% |
| Brevibacterium luteolum | 1 | 0.30% |
| Corynebacterium aurimucosum | 1 | 0.30% |
| Corynebacterium striatum | 1 | 0.30% |
| Corynebacterium urealyticum | 1 | 0.30% |
| Dermabacter hominis | 1 | 0.30% |
| Erysipelothrix rhusiopathiae | 1 | 0.30% |
| Kocuria kristinae | 1 | 0.30% |
| Micrococcus luteus | 4 | 1.21% |
| Propionibacterium acnes | 14 | 4.23% |
| Acitenobacter baumanii | 1 | 0.30% |
| Bacteroides fragilis | 2 | 0.60% |
| Burkholderia species | 1 | 0.30% |
| Enterobacter cloacae | 5 | 1.51% |
| E. coli | 25 | 7.55% |
| Klebsiella oxytoca | 1 | 0.30% |
| Klebsiella pneumoniae | 7 | 2.11% |
| Morganella morganii | 1 | 0.30% |
| Proteus mirabilis | 10 | 3.02% |
| Pseudomonas aeruginosa | 11 | 3.32% |
| Serratia marcescens | 1 | 0.30% |
| Candida spp. | 5 | 1.51% |
References
- Fröschen, F.S.; Randau, T.M.; Franz, A.; Molitor, E.; Hoerauf, A.; Hischebeth, G.T.R. Microbiological Trends and Antibiotic Susceptibility Patterns in Patients with Periprosthetic Joint Infection of the Hip or Knee over 6 Years. Antibiotics 2022, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Diez-Escudero, A.; Hailer, N.P. The Role of Silver Coating for Arthroplasty Components. Bone Jt. J. 2021, 103-B, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.; Legnani, C.; Meani, E. A New Perspective on Current Prosthetic Joint Infection Classifications: Introducing Topography as a Key Factor Affecting Treatment Strategy. Arch. Orthop. Trauma Surg. 2019, 139, 317–322. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Infectious Diseases Society of America Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef]
- Hsieh, P.-H.; Huang, K.-C.; Shih, H.-N. Prosthetic Joint Infection in Patients with Rheumatoid Arthritis: An Outcome Analysis Compared with Controls. PLoS ONE 2013, 8, e71666. [Google Scholar] [CrossRef]
- Longo, U.G.; De Salvatore, S.; Bandini, B.; Lalli, A.; Barillà, B.; Budhiparama, N.C.; Lustig, S. Debridement, Antibiotics, and Implant Retention (DAIR) for the Early Prosthetic Joint Infection of Total Knee and Hip Arthroplasties: A Systematic Review. J. ISAKOS 2024, 9, 62–70. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic Joint Infection: Current Concepts and Outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, S.; Wang, Z.; Yan, X.; Luo, H. Systematic Review and Meta-Analysis of Single-Stage vs Two-Stage Revision for Periprosthetic Joint Infection: A Call for a Prospective Randomized Trial. BMC Musculoskelet. Disord. 2024, 25, 153. [Google Scholar] [CrossRef]
- EPRD Deutsche Endoprothesenregister gGmbH. Jahresbericht 2024: Mit Sicherheit Mehr Qualität/Endoprothesenregister Deutschland (EPRD); EPRD Deutsche Endoprothesenregister gGmbH: Berlin, Germany, 2024; ISBN 978-3-949872-04-4. [Google Scholar]
- Alt, V.; Rupp, M.; Langer, M.; Baumann, F.; Trampuz, A. Can the Oncology Classification System Be Used for Prosthetic Joint Infection?: The PJI-TNM System. Bone Jt. Res. 2020, 9, 79–81. [Google Scholar] [CrossRef]
- Rupp, M.; Kerschbaum, M.; Freigang, V.; Bärtl, S.; Baumann, F.; Trampuz, A.; Alt, V. PJI-TNM als neues Klassifikationssystem für Endoprotheseninfektionen: Eine Evaluation von 20 Fällen. Orthopäde 2021, 50, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2019, 34, S337–S342. [Google Scholar] [CrossRef] [PubMed]
- Lum, Z.C.; Natsuhara, K.M.; Shelton, T.J.; Giordani, M.; Pereira, G.C.; Meehan, J.P. Mortality During Total Knee Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 3783–3788. [Google Scholar] [CrossRef] [PubMed]
- Baertl, S.; Rupp, M.; Kerschbaum, M.; Morgenstern, M.; Baumann, F.; Pfeifer, C.; Worlicek, M.; Popp, D.; Amanatullah, D.F.; Alt, V. The PJI-TNM Classification for Periprosthetic Joint Infections. Bone Jt. Res. 2024, 13, 19–27. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Tsukayama, D.T.; Estrada, R.; Gustilo, R.B. Infection after Total Hip Arthroplasty. A Study of the Treatment of One Hundred and Six Infections. J. Bone Jt. Surg. 1996, 78, 512–523. [Google Scholar] [CrossRef]
- Alt, V.; Walter, N.; Rupp, M.; Baertl, S. Comment on Lunz et al. Impact and Modification of the New PJI-TNM Classification for Periprosthetic Joint Infections. J. Clin. Med. 2023, 12, 1262. J. Clin. Med. 2023, 12, 6073. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Romanò, C.L.; Manzi, G.; Logoluso, N.; Romanò, D. Value of Debridement and Irrigation for the Treatment of Peri-Prosthetic Infections. A Systematic Review. HIP Int. 2012, 22, 19–24. [Google Scholar] [CrossRef]
- Qasim, S.N.; Swann, A.; Ashford, R. The DAIR (Debridement, Antibiotics and Implant Retention) Procedure for Infected Total Knee Replacement—A Literature Review. SICOT J. 2017, 3, 2. [Google Scholar] [CrossRef]
- Patel, D.; Shannon, V.; Sharma, S.; Liu, J.; Skie, M. A Meta-Analysis of Success Rates of One-Stage Versus Two-Stage Revisions in Knee Prosthetic Joint Infections. Cureus 2024, 16, e57533. [Google Scholar] [CrossRef] [PubMed]
- McPherson, E.J.; Woodson, C.; Holtom, P.; Roidis, N.; Shufelt, C.; Patzakis, M. Periprosthetic Total Hip Infection: Outcomes Using a Staging System. Clin. Orthop. Relat. Res. 2002, 403, 8–15. [Google Scholar] [CrossRef]
- McPherson, E.J.; Tontz, W.; Patzakis, M.; Woodsome, C.; Holtom, P.; Norris, L.; Shufelt, C. Outcome of Infected Total Knee Utilizing a Staging System for Prosthetic Joint Infection. Am. J. Orthop. 1999, 28, 161–165. [Google Scholar] [PubMed]
- Hegde, V.; Bracey, D.N.; Johnson, R.M.; Dennis, D.A.; Jennings, J.M. Increased Prevalence of Depressive Symptoms in Patients Undergoing Revision for Periprosthetic Joint Infection. Arthroplast. Today 2022, 13, 69–75. [Google Scholar] [CrossRef]
- Vasarhelyi, E.M.; Somerville, L.; Barton, K.I.; Howard, J.L.; Lanting, B.A.; Naudie, D.D.R.; McCalden, R.W.; MacDonald, S.J. Survivorship and Outcomes of 2-Stage Revision for Infected Total Hip Arthroplasty at a Mean of 7-Year Follow-Up. J. Arthroplast. 2024, 39, S243–S247. [Google Scholar] [CrossRef]
- Lim, C.T.; Amanatullah, D.F.; Huddleston, J.I.; Harris, A.H.S.; Hwang, K.L.; Maloney, W.J.; Goodman, S.B. Reconstruction of Disrupted Extensor Mechanism After Total Knee Arthroplasty. J. Arthroplast. 2017, 32, 3134–3140. [Google Scholar] [CrossRef]
- Chalmers, B.P.; Mabry, T.M.; Abdel, M.P.; Berry, D.J.; Hanssen, A.D.; Perry, K.I. Two-Stage Revision Total Hip Arthroplasty with a Specific Articulating Antibiotic Spacer Design: Reliable Periprosthetic Joint Infection Eradication and Functional Improvement. J. Arthroplast. 2018, 33, 3746–3753. [Google Scholar] [CrossRef]
- Eka, A.; Chen, A.F. Patient-Related Medical Risk Factors for Periprosthetic Joint Infection of the Hip and Knee. Ann. Transl. Med. 2015, 3, 233. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kamath, A.F.; Ong, K.; Lau, E.; Kurtz, S.; Chan, V.; Vail, T.P.; Rubash, H.; Berry, D.J. Comparative Epidemiology of Revision Arthroplasty: Failed THA Poses Greater Clinical and Economic Burdens Than Failed TKA. Clin. Orthop. Relat. Res. 2015, 473, 2131–2138. [Google Scholar] [CrossRef]
- Tsai, Y.; Chang, C.-H.; Lin, Y.-C.; Lee, S.-H.; Hsieh, P.-H.; Chang, Y. Different Microbiological Profiles between Hip and Knee Prosthetic Joint Infections. J. Orthop. Surg. 2019, 27, 230949901984776. [Google Scholar] [CrossRef]
- Hu, L.; Fu, J.; Zhou, Y.; Chai, W.; Zhang, G.; Hao, L.; Chen, J. Trends in Microbiological Profiles and Antibiotic Resistance in Periprosthetic Joint Infections. J. Int. Med. Res. 2021, 49, 030006052110027. [Google Scholar] [CrossRef] [PubMed]
- Bjerke-Kroll, B.T.; Christ, A.B.; McLawhorn, A.S.; Sculco, P.K.; Jules-Elysée, K.M.; Sculco, T.P. Periprosthetic Joint Infections Treated with Two-Stage Revision over 14Years: An Evolving Microbiology Profile. J. Arthroplast. 2014, 29, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Fröschen, F.S.; Randau, T.M.; Franz, A.; Molitor, E.; Hischebeth, G.T.R. Microbiological Profiles of Patients with Periprosthetic Joint Infection of the Hip or Knee. Diagnostics 2022, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Akgün, D.; Perka, C.; Trampuz, A.; Renz, N. Outcome of Hip and Knee Periprosthetic Joint Infections Caused by Pathogens Resistant to Biofilm-Active Antibiotics: Results from a Prospective Cohort Study. Arch. Orthop. Trauma Surg. 2018, 138, 635–642. [Google Scholar] [CrossRef]
- Ull, C.; Yilmaz, E.; Baecker, H.; Schildhauer, T.A.; Waydhas, C.; Hamsen, U. Microbial Findings and the Role of Difficult-to-Treat Pathogens in Patients with Periprosthetic Infection Admitted to the Intensive Care Unit. Orthop. Rev. 2020, 12, 8867. [Google Scholar] [CrossRef]
- Yilmaz, E.; Poell, A.; Baecker, H.; Frieler, S.; Waydhas, C.; Schildhauer, T.A.; Hamsen, U. Poor Outcome of Octogenarians Admitted to ICU due to Periprosthetic Joint Infections: A Retrospective Cohort Study. BMC Musculoskelet. Disord. 2020, 21, 304. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Klika, A.K.; Lu, Q.; Higuera-Rueda, C.A.; Stappenbeck, T.; Visperas, A. Periprosthetic Joint Infection and Immunity: Current Understanding of Host–Microbe Interplay. J. Orthop. Res. 2024, 42, 7–20. [Google Scholar] [CrossRef]
- Yapp, L.Z.; Clement, N.D.; Moran, M.; Clarke, J.V.; Simpson, A.H.R.W.; Scott, C.E.H. Long-Term Mortality Rates and Associated Risk Factors Following Primary and Revision Knee Arthroplasty: 107,121 Patients from the Scottish Arthroplasty Project. Bone Jt. J. 2022, 104-B, 45–52. [Google Scholar] [CrossRef]

| T-tissue and implant conditions | |||
| T0 | a | Stable standard implant without important soft tissue defect | |
| b | Stable revision implant without important soft tissue defect | ||
| T1 | a | Loosened standard implant without important soft tissue defect | |
| b | Loosened revision implant without important soft tissue defect | ||
| T2 | a | Severe soft tissue defect with standard implant | |
| b | Severe soft tissue defect with revision implant | ||
| N-non-human cells | |||
| N0 | a | No mature biofilm formation (former: acute), directly postoperatively | |
| b | No mature biofilm formation (former: acute), late hematogenous | ||
| N1 | a | Mature biofilm formation (former: chronic) without “difficult-to-treat bacteria” | |
| b | Mature biofilm formation (former: chronic) with culture-negative infection | ||
| N2 | a | Mature biofilm formation (former: chronic) with “difficult-to-treat bacteria” | |
| b | Mature biofilm formation (former: chronic) with polymicrobial infection | ||
| c | Mature biofilm formation (former: chronic) with fungi | ||
| M-morbidity of the patient | |||
| M0 | Not or only mildly compromised (Charlson comorbidity index: 0–1) | ||
| M1 | Moderately compromised patient (Charlson comorbidity index: 2–3) | ||
| M2 | Severely compromised patient (Charlson comorbidity index 4–5) | ||
| M3 | a | Patient refuses surgical treatment | |
| b | Patient does not benefit from surgical treatment | ||
| c | Patient does not survive surgical treatment | ||
| r—reinfection | If the infection involves a previously infected implant, the situation is considered to be “reinfection” and an “r” is placed in front of the classification | ||
| Clinical Features | 2015–2019 |
|---|---|
| Number of patients | 443 (222 M; 221 F) |
| Age (range) [years] | 70.5 ± 11.9 (21–98) |
| BMI (range) [kg/m2] | 29.5 ± 6.9 (14.2–60.6) |
| Hospital stay (range) [days] | 26.1 ± 25.5 (2–354) |
| Infected joint: hip/knee | 247 (55.8%)/196 (44.2%) |
| Preoperative C-reactive protein [mg/L] | 71.3 ± 87.1 (0.5–557.6) |
| Preoperative leukocyte count [G/L] | 9.4 ± 5.0 (2.7–56.2) |
| Preoperative haemoglobin [g/dL] | 11.5 ± 2.2 (6.7–18.7) |
| Preoperative Creatinine [mg/dL] | 1.0 ± 0.5 (0.2–5.3) |
Preoperative synovial cell count [cells/µL] (n = 180)
| 67,942.2 ± 122,785.9 66,216.3 ± 119,306.6 48,439.4 ± 79,964.9 |
| PJI-TNM Classification | 2015–2019 |
|---|---|
| Inlying implant ad first admission: standard implant/revision implant | 150 (33.9%)/293 (66.1%) |
| Standing time implant at PJI [months] | 23.7 ± 33.1 (0–155) |
Performed operative procedure:
| 138 (31%) 29 (7%) 272 (61%) 4 (1%) |
| Incision to suture time [min] | 149.9 ± 63.5 (26–348) |
| Comorbidities | Hip (n/%) | Knee (n/%) | Total (n) |
|---|---|---|---|
| Myocardial infarction | 29 (11.7%) | 24 (12.2%) | 53 (23.9%) |
| Congestive heart failure | 39 (15.8%) | 42 (21.4%) | 81 (37.2%) |
| Peripheral vascular | 9 (3.6%) | 9 (4.6%) | 18 (8.2%) |
| Deep vein thrombosis/pulmonary embolism | 21 (8.5%) | 20 (10.2%) | 41 (18.7%) |
| Cerebrovascular | 15 (6.1%) | 16 (8.2%) | 31 (14.3%) |
| Hemiplegia | 2 (0.8%) | 0 (0.0%) | 2 (0.8%) |
| Dementia | 5 (2.0%) | 5 (2.6%) | 10 (4.6%) |
| Pulmonary | 40 (16.2%) | 43 (21.9%) | 83 (38.1%) |
| Collagenosis | 28 (11.3%) | 19 (9.7%) | 47 (21%) |
| Peptic ulcer | 22 (8.9%) | 17 (8.7%) | 39 (17.6%) |
| Diabetes with/without end organ damage | 47 (19.0%) | 48 (24.5%) | 95 (43.5%) |
| Liver damage | 14 (5.6%) | 8 (4.0%) | 22 (9.6%) |
| Cancer | 20 (8.1%) | 13 (6.6%) | 33 (14.7%) |
| AIDS | 1 (0.4%) | 1 (0.5%) | 2 (0.9%) |
| PJI-TNM Classification | 2015–2019 |
|---|---|
| Tissue and implant conditions | |
| T0a/T0b | 87 (19.6%)/183 (41.3%) |
| T1a/T1b | 60 (13.5%)/99 (22.3%) |
| T2a/T2b | 5 (1.1%)/9 (2.0%) |
| Non-human cells | |
| N0a/N0b | 29 (6.5%)/38 (8.6%) |
| N1a/N1b | 195 (44.0%)/76 (17.2%) |
| N2a/N2b/N2c | 33 (7.4%)/65 (14.7%)/7 (1.6%) |
| Morbidity | |
| M0 | 262 (59.1%) |
| M1 | 114 (25.7%) |
| M2 | 58 (13.1%) |
| M3a/M3b/M3c | 4 (0.9%)/0 (0%)/5 (1.1%) |
| Tissue and Implant Conditions | 12 Months | 24 Months | 48 Months |
|---|---|---|---|
| T0 | 56% | 53% | 41% |
| T1 | 70% | 67% | 55% |
| T2 | 69% | 54% | 46% |
| Non-human cells | |||
| N0 | 66% | 61% | 40% |
| N1 | 60% | 58% | 50% |
| N2 | 61% | 55% | 45% |
| Morbidity | |||
| M0 | 66.1% | 62.0% | 56.5% |
| M1 | 57.5% | 52.7% | 51.4% |
| M2 | 52.5% | 50.5% | 50% |
| M3 | 22.2% | 22.2% | 11.1% |
| Overall | |||
| rT0bN1aM0 | 58% | 52% | 41% |
| rT0bN1aM1 | 75% | 67% | 42% |
| rT1bN1aM0 | 52% | 48% | 39% |
| Reinfection | |||
| “r” | 53% | 50% | 46% |
| Non-“r” | 70% | 66% | 61% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröschen, F.S.; Greber, L.; Molitor, E.; Hischebeth, G.T.R.; Franz, A.; Randau, T.M. The PJI-TNM Classification as Predictor for Revision-Free Implant Survival Rates in Patients with Periprosthetic Joint Infection of the Hip or Knee Joint. Infect. Dis. Rep. 2025, 17, 54. https://doi.org/10.3390/idr17030054
Fröschen FS, Greber L, Molitor E, Hischebeth GTR, Franz A, Randau TM. The PJI-TNM Classification as Predictor for Revision-Free Implant Survival Rates in Patients with Periprosthetic Joint Infection of the Hip or Knee Joint. Infectious Disease Reports. 2025; 17(3):54. https://doi.org/10.3390/idr17030054
Chicago/Turabian StyleFröschen, Frank Sebastian, Lisa Greber, Ernst Molitor, Gunnar Thorben Rembert Hischebeth, Alexander Franz, and Thomas Martin Randau. 2025. "The PJI-TNM Classification as Predictor for Revision-Free Implant Survival Rates in Patients with Periprosthetic Joint Infection of the Hip or Knee Joint" Infectious Disease Reports 17, no. 3: 54. https://doi.org/10.3390/idr17030054
APA StyleFröschen, F. S., Greber, L., Molitor, E., Hischebeth, G. T. R., Franz, A., & Randau, T. M. (2025). The PJI-TNM Classification as Predictor for Revision-Free Implant Survival Rates in Patients with Periprosthetic Joint Infection of the Hip or Knee Joint. Infectious Disease Reports, 17(3), 54. https://doi.org/10.3390/idr17030054






